Simple Summary
Simple Summary: The female reproductive tract has a complex mix of microorganisms that are important for keeping the reproductive system healthy. When the balance of these microorganisms is disrupted, it may predispose to diseases. One such disease, endometritis, commonly causes fertility problems in mares and is difficult to diagnose and treat using standard tests. Although advanced DNA sequencing provides useful information about the microorganisms in the reproductive tract of mares, it is still challenging to define what a “normal” microbiota looks like. This review aims to summarize current knowledge about the microorganisms in the reproductive tract of mares, including the vagina, cervix, and uterus. It also explores factors that can affect these microorganisms, such as the stage of the estrous cycle, the types of bacteria, the season, and geographic location.
Abstract
The female reproductive tract microbiota is a complex community of microorganisms that might be crucial in maintaining a healthy reproductive environment. Imbalances in the bacterial community (dysbiosis) and the reduction of beneficial organisms and pathogen proliferation are associated with disease. Endometritis is a common cause of fertility problems in mares, and it is still challenging to diagnose and treat based on routine culture results of certain microorganisms. Although high-throughput sequencing studies provide helpful information regarding the composition of the reproductive tract microbiota in mares, there are still challenges in defining a “normal” microbiota. The primary objective of this literature review is to summarize the current knowledge regarding the microbiota present in the reproductive tract of mares, including the vagina, cervix, and uterus. The second objective is to describe the relevant factors that can impact the reproductive microbiota of mares, including the estrous cycle stage, the type of species (genera) investigated, season, and geographic location. The rationality of identifying the normal microbiota in the reproductive tract of a mare will likely aid in understanding the impact of the microbiota on the host’s reproductive health and contribute to the treatment and prevention of equine sub and infertility issues.
1. Introduction
Research on the microbiota of the female reproductive tract in horses using high-throughput sequencing is a developing area of study, and little is known about it compared to other mammals. This niche has received limited attention in the equine species, particularly in comparison with other body regions, such as the gastrointestinal tract [,,,]. However, there is increasing evidence that the reproductive tracts of mammals harbour distinctive bacterial communities [,,]. High-throughput sequencing studies have supported those using culture-dependent methods, suggesting that the uterus may not be a sterile environment []. However, the results of the culture-dependent method studies were limited, particularly in underestimating the abundance of certain bacteria, but the newer sequencing technologies allow researchers a broader identification of the bacteria present in this body system []. Despite this, few studies have characterized the microbiota of the uterus and vagina of healthy mares, particularly comparing both organs within the same animal. Most published studies currently use 16s rRNA sequencing to identify the bacterial microbiota, technical limitations or confounding factors can lead to discrepancies in the results, especially when determining which bacterial communities and at what proportion they are present in the uterus and vagina. It is difficult to establish a consensus on the normal microbiota of the reproductive tract in healthy mares unless sequencing is performed at a very deep level (shotgun sequencing), which is yet to be described in mares. In addition, determining a normal or core microbiota present in the reproductive tract of mares can be challenging because relevant factors such as the sampling site, the stage of the estrous cycle, age, nutritional regime, systemic disease, previous administration of antimicrobial drugs, season, and geographic location among other factors may impact the reproductive host’s health and therefore the microbiota. Therefore, this narrative literature review aims to summarize the current information regarding the microbial communities present in the reproductive tract of mares, the factors associated with their development and establishment, and their association with fertility, health, and disease.
2. Materials and Methods
This narrative literature review summarizes the current information regarding the microbial communities described in the reproductive tract of mares and their association with health and disease. The following keywords were used in an electronic search: mares, equine, metritis, microbiota, vaginal, uterine, and microbiota and microbiome. Articles published until January 2024 were considered for this review. Articles were collected from the following online databases: Google Scholar (https://scholar.google.ca/ (last accessed 30 January 2024), PubMed (https://pubmed.ncbi.nlm.nih.gov/) (last accessed 30 January 2024, and ScienceDirect (https://www.sciencedirect.com/) (last accessed 30 January 2024. Information from each reference was included in this review if they discussed bacterial communities present in the reproductive tract of mares, methods for detection of those bacteria, factors associated with the development and establishment of bacteria in the reproductive tract, the pathophysiology of reproductive diseases, and the relationship between microbiota and fertility.
3. Results and Discussion
3.1. Methods and Sampling Techniques to Characterize Uterine and Vaginal Microbiota
Initial studies investigating the microbial communities present in the uterus and vagina of mares were conducted using culture-based methodologies [,,]. These studies reported positive cultures in 30% to 50% of the uterine samples and 40% to 95% of the vaginal swabs. In recent decades, there has been a growing interest in studying the female reproductive microbiota in horses using culture-independent methods. This approach overcomes the intrinsic limitation of culture-dependent methods in that it can detect bacteria populations that cannot be cultured and, therefore, cannot be unaccounted for otherwise []. For instance, the presence of dormant bacteria located deep within the endometrium (Streptococcus zooepidemicus) [] and biofilm-producing bacteria (Escherichia coli and Klebsiella pneumoniae) [] are known to promote high false negative endometritis results in routinely equine uterine cultures [,], due to their ability to remain in a latent state in the uterine glands of the stratum compacting [] or live in bacterial aggregates embedded within a complex matrix capsule (extracellular polymeric substances) acquiring a layer of protection against the host immune system [,], challenging routine culture diagnostic [] and treatment [].
Additionally, conventional aerobic cultivation methods fail to detect known genital pathogens such as E. coli and P. aeruginosa, which have been detected using 16S ribosomal RNA sequences in mares []. Similarly, sequence analysis has demonstrated the presence of bacteria from both negative and positive cultures in mares during estrus and early pregnancy [], highlighting the potential existence of bacterial microbiota in the uterus. High-throughput sequencing and bioinformatic analysis can accurately identify microbial populations in the equine reproductive tract, including live, dead, and fragmented bacterial DNA. However, experimental [] and computational challenges can lead to high variability in sequencing results, including study design, sample handling, nucleic preparation, sequencing, quality control, assembly, binning, and functional classification []. Similarly, sequencing studies may have difficulty detecting bacteria beyond the family level unless sequencing is at a very deep level (shotgun sequencing) [,] as well as when selecting primer sets for amplification of the 16S rRNA gene, certain bacterial species may be over- or under-represented []. Additionally, due to the costly technique expenses, limited practitioner accessibility, and a high number of mares with reproductive problems per season, sequencing is not commonly performed in clinical settings []. Therefore, clinicians use relatively faster, cheaper, and less complex culture-dependent techniques for diagnosis and treatment. However, despite the limitations of both microbial detection methods, high-throughput sequencing-based methods are a robust technique that can be used to define the reproductive tract microbiota for high-quality research [,,].
Most samples from the mare’s uterus for bacterial culture are obtained using guarded sterile swabs. A study by Blanchard et al. [] compared the bacterial culture yield of samples from the endometria of 39 mares obtained using a guard with a double cannula and a distal Teflon plug, and an unguarded swab with a single, open cannula. This study showed that 38.5% and 61.5% of the samples obtained with the guarded and unguarded swabs yielded growth in blood agar at 48 h of incubation, respectively. When samples were incubated for 48 h in MacConkey’s agar, the guarded swab yielded growth in <1% of the samples, whereas 20.5% using an unguarded swab. These studies highlight the impact of different sample methods and the media on bacterial cultures from uterine samples of healthy mares.
Similarly, the impact of sampling techniques to characterize the uterine microbiota of healthy mares has been investigated. Double-guarded sterile swabs, low-volume lavage (LVL), and endometrial biopsy produced similar results when used to characterize the uterine microbiota of 16 mares during estrous []. Regardless of the endometrial sampling technique, similarities in composition and relative abundance occur at the level of phyla (Proteobacteria, Firmicutes, and Bacteroidota) and genus (Klebsiella, Mycoplasma, and Aeromonas) level. However, these results suggested that LVL is more effective for identifying low-abundance or rare taxa than endometrial biopsy [].
3.2. Uterine Microbiota
Historically, the uterus was considered a sterile environment []. However, studies in the 1970s and 1980s using culture-dependent approaches challenged this assumption, but conflicting results based on positive or negative bacterial cultures made it historically difficult to deduce the sterility of the uterine environment reliably. An early study by Scott et al. [] showed that, on aerobic cultures, 33% of uterine swab specimens collected from 100 mares at slaughterhouses yielded a positive result. The most commonly identified bacteria were β-hemolytic Streptococcus. A later study by Hinrichs et al. [] documented that approximately 30 and 40% of the samples from the uterus and vagina of 48 Thoroughbred and Standardbred mares with healthy reproductive tracts confirmed by endometrial biopsy and without a history of reproductive diseases yielded a positive culture on aerobic conditions, respectively. Samples were obtained using a double-guarded, occluded swab. The most common bacteria identified were Arcanobacterium (formerly Corynebacterium, n = 6), Staphylococcus (n = 7), and Streptococcus (n = 4). Purswell et al. [] investigated the longitudinal changes in the mare’s uterus after foaling. Thirteen mares were examined at foaling and at foal heat, and seven of these mares were sampled at the second estrus using a guarded swab to obtain samples. Cultures were performed in aerobic and anaerobic conditions. During the immediate postpartum, 54% (n = 7) and 23% (n = 3) mares showed bacterial growth in aerobic and anaerobic conditions, respectively. At foal heat, 23% (n = 3) of the mares had a positive anaerobic bacterial culture, and no growth was reported in anaerobic conditions. At the second postpartum estrus, all 13 mares were negative in bacterial cultures from the uterus. Streptococcus (n = 14) and Arcanobacterium (formerly Corynebacterium, n = 4) were the most frequently isolated bacteria. These studies consistently identified Arcanobacterium and Streptococcus in the uterus of the mares suggesting that these bacteria might inhabit at different periods the uterus of healthy mares. However, the experimental design of these studies prevents determining whether those bacteria reside in the uterus and are transient visitors or invaders.
Newer technology and results from sequencing studies have presented convincing evidence to challenge the “sterile uterus” dogma []. In one study, two predominant phyla (Bacteroidetes and Proteobacteria) were identified in uterine flush samples from healthy mares, suggesting that the equine uterine environment was not sterile during or after estrus []. The metagenomic sequencing used in this study identifies a complex population of bacteria residing within the equine uterus, regardless of the culture outcome. This study offered an initial valuable insight into the importance of implementing sophisticated diagnostic methods and sampling techniques to increase the sensitivity of the bacteriological results. The existing literature primarily reports the reproductive microbiota of healthy mares. However, despite consistently healthy sampling groups, study variations are evident. Table 1 and Figure 1 summarize the main bacterial taxa described in the uterus and vagina of healthy mares. The most abundant phyla within the equine uterus and vagina are Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria [,,]. A study by Holyoak et al. [] showed that Firmicutes were the most abundant phylum (52%) identified in uterine samples from mares located in Louisiana, while in mares from Oklahoma, Proteobacteria (36%) and Firmicutes (36%) were the most abundant phyla detected. Proteobacteria was also the more abundant phylum detected in uterine samples from mares in Australia (40%) and from different locations from the Southern Midwestern states of the US (80%). Additionally, the study by Heil et al. [] reported Proteobacteria (>50%) to be the most abundant phyla in the uterus of healthy mares, followed by Firmicutes and Bacteroidota regardless of the sampling method used to characterize the microbiota. Thomson et al. (2022) reported that Proteobacteria (69%), Firmicutes (21%), Bacteroidetes (7.8%), and Actinobacteria (1%) accounted for 99.6% of the total phyla abundance.

Table 1.
Summary of main findings obtained from studies describing the microbiota of the reproductive tract in mares.
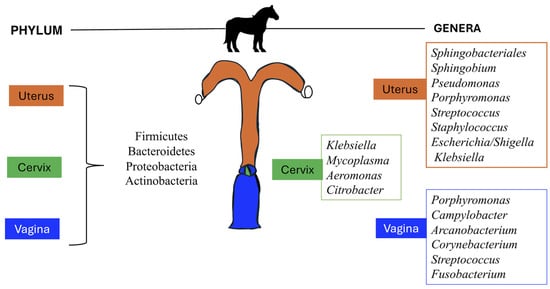
Figure 1.
Vaginal, cervical, and uterine microbiota identified in healthy mares.
In contrast, at the genus level, any microbe has no marked dominance. Streptococcus, Campylobacter, Klebsiella, Pseudomonas, Mycoplasma, and Aeromonas are some of the genera that are consistently present within the equine reproductive microbiota, although they vary in abundance across studies [,,]. Holyoak et al. [] found that Pseudomonas was the most abundant (27%) genus cumulative across all samples from mares at 4 different locations, followed by Lonsdalea (8%), Lactobacillus (7.5%), Escherichia/Shigella (4.5%) and Prevotella (3%). However, Lonsdalea was only detected in Australian samples but not in samples of mares from Oklahoma or Louisiana. Lactobacillus and Escherichia/Shigella were dominant genera in Oklahoma or Louisiana, but their abundance in Australian mares was low. The study by Heil et al. [] found that Klebsiella, Mycoplasma, Aeromonas, and Citrobacter were the most abundant genera present in the cervical swabs, endometrial biopsy, and low-volume lavage of healthy mares, accounting for approximately 50% of analyzed sequences. Thomson et al. reported that Staphylococcus (19%), Pseudomonas (18%), Escherichia/Shigella (10%), and Klebsiella (10%) were the most abundant genera identified in uterine biopsy of healthy mares. The reasons for these differences remain unclear because most studies focus on assessing the reproductive microbiota of a single group of animals from a single facility, all under the same environmental, housing, and nutritional conditions. Consequently, the influence of management practices cannot be evaluated within the statistical model. Similarly, studies examining the reproductive microbiota across various geographic locations with different management practices failed to report the specific conditions under which the animals were managed, preventing an assessment of these practices’ impact within the statistical model [].
3.3. Vaginal Microbiota
Vaginal microbiota has also been investigated using culture-dependent methodologies. The study by Scott et al. [] showed that 95% (95/100) of the mare’s vaginal swabs obtained at the slaughterhouse grew a bacterium in aerobic conditions. The most common bacteria identified were β-hemolytic streptococci and coliforms. The study by Hinrichs et al. [] reported that approximately 40% (19/48) of the samples from the vagina yielded a positive culture on aerobic conditions. The most common bacteria isolated from vagina samples were Arcanobacterium (n = 10), Streptococcus (n = 4), and Staphylococcus (n = 4), while no coliforms were identified. A more recent study by Malaluang et al. [] cultured vaginal swabs from maiden mares and mares not bred in the previous 10 years and reported that Escherichia coli, Staphylococcus capitis, Streptococcus equisimilis, Streptococcus thoraltensis, and S. zooepidemicus were the most common isolated bacteria with E. coli being dominant (40% of the samples). Malaluang et al. [] investigated the mare’s vaginal microbiota throughout the estrous cycle from ovulation the following 3, 7, and 14 days. Samples were collected from the cranial floor of the vagina using a double-guarded occluded swab. Bacterial growth was observed in all aerobic and anaerobic cultures from the vagina of all mares on all sampling days. The number of positive cultures was higher on Days 3 and 7 (beginning and middle of diestrus), with E. coli and S. zooepidemicus being the most frequently isolated bacteria. In maiden mares, E. coli was particularly dominant compared to those that had foaled.
The vaginal microbiota of healthy mares has also been studied using high-throughput sequencing approaches. Barba et al. [] characterized the vaginal microbiota of 8 healthy Arabian mares on estrous and diestrus. Firmicutes and Bacteroides were the most abundant phylum identified in all samples regardless of the estrous cycle phase, with an abundance accounting for 32% of the sequences each, followed by Epsilonbacteraeota (9%), Actinobacteria (8%), Kiritimatiellaeota (6.5%), Proteobacteria (3.5%) and Fusobacteria (2.7%). The most abundant genera were identified at similar abundances in estrous and diestrus, with Porphyromonas accounting for approximately 15% of the sequences, followed by Campylobacter (approximately 10% of the sequences). Corynebacterium, Streptococcus, Fusobacterium, and Akkermansia were also identified in high abundance in the vagina. Lactobacillus only accounted for 0.18% of the abundance in estrus and 0.37% in diestrus.
The results of studies employing both culture-dependent methods and high-throughput sequencing reveal that bacteria can be detected in the uterus and vagina of healthy mares throughout the estrous cycle. Although the same bacterial phyla are identified in the uterus of healthy mares, their abundance varies among studies. At the genus level, there are significant differences between studies investigating either uterine or vaginal microbiota. These discrepancies are attributed to true differences among healthy groups, influenced by factors such as geographic location, health status, diet, body mass index, and hormone homeostasis, which can affect bacterial colonization and establishment in the mare’s uterus and vagina. Additionally, variations in the methodologies used to collect (e.g., swabs, low-volume lavage, or biopsy) and process samples may also account for differences among studies. While most current studies utilize 16S rRNA sequencing, potential technical limitations or confounding factors inherent to metagenomic analysis may further contribute to the lack of concordance, especially at lower taxonomic levels.
3.4. Factors Associated with Colonization and Establishment of the Reproductive Microbiota: Site, Diet, Parity, Stage of Estrous, and Species
In mares [,], cattle [], women [], female dogs [], minipigs [], and giant pandas [], the vaginal and endometrial microbiota have been characterized. These studies reveal unique site-specific microbiotas. Both the canine and human endometrial microbiota demonstrate higher bacterial diversity when compared with the vaginal microbiota. However, in women, the endometrial microbiota has a higher richness than the vaginal microbiota, whereas in dogs, it is lower [,]. In cattle during the postpartum period, there is a shared community in the vagina and uterus []. The bacterial populations present in the vagina and uterus of healthy mares were presented earlier in the text, summarized in Table 1, and depicted in Figure 1. However, those studies are limited for the sample size, and more importantly, no published studies have compared the uterus and vagina microbiota within the same animal. Characterizing the vaginal and uterine microbiota in the same animal can aid in understanding how microbiota can reach the uterus []. Traditionally, it has been suggested that the cervical mucus plug prevents vaginal bacteria from reaching the uterus. However, this mucus plug appears to be permeable to vaginal bacteria, which may allow bacterial translocation from the proximity to the vagina [,]. Additionally, in women, uterine peristaltic contractions, such as those that aid in sperm transport and intensify during the follicular phase of the menstrual cycle, can also move particles into the uterus, potentially seeding the uterus with bacteria during specific phases of the estrous cycle [,]. Therefore, studies examining the uterine and vaginal microbiota throughout the estrous cycle in the same animal can help to identify the factors influencing and the dynamics of bacterial communities in the reproductive tract of mares. These types of studies can also aid in determining whether bacteria detected in the uterus of the mares are residents, transient invaders, or potential pathogens causing disease.
Hormonal fluctuations and diet are some of the factors thought to influence the reproductive microbiota in cattle and humans [,,]. High progesterone level is linked to a high abundance of Proteobacteria, while low progesterone concentration is associated with low Firmicutes abundance in the vagina of cattle []. In contrast, estrogen tends to have little influence on the vaginal microbiota of beef heifers []. Additionally, low glycemic index, low-fat, and nutrient-dense diets lower human bacterial vaginosis risk []. Other factors include ethnicity, pregnancy, hygiene, sexual exposure, and contraceptives in women []. Parity affects microbiota in cows, with multiparous cows having different microbiota than primiparous cows, but primiparous cows have more bacterial diversity in the uterus than their multiparous ones []. Little is known about the influence of these factors on the taxonomic composition of the mares’ reproductive tract.
There is an evident gap concerning the understanding of the microbiota present in the reproductive tract at different stages of the estrus cycle. While extensive research has been conducted on humans [,,], there is little knowledge of mares. Hormonal fluctuations during the menstrual cycle are one of the factors responsible for shifts in the reproductive microbiota in women []. The effect of the estrous cycle stage is less clear in horses. A study on Arabian mares found a consistent vaginal microbiota in both the follicular and luteal phase []. The uterine microbiota of mares during anestrus appears to have a higher microbial diversity and richness than during estrus []. High estrogen concentration in the endometrium of mares in estrus may stimulate the local immune response, resulting in a less diverse microbiome during this phase of the reproductive cycle [].
The bacteria present in the reproductive tract in healthy conditions are influenced by the type of bacteria species being studied. Lactobacillus has been detected in equine [], porcine [], bovine and ovine [] vaginal samples. Furthermore, Lactobacillus dominates the healthy vagina in women []. However, in the vaginal microbiota of healthy mares, a predominance of Lactobacilli is not evident either through culture or metagenomics analyses []. Similarly, a low abundance of Lactobacillus is common in the vaginal samples of cows and ewes []. This marked difference suggests that Lactobacillus have a different role in the reproductive tracts of mares and other investigated species in comparison to humans, where Lactobacillus provides a barrier to opportunistic pathogen invasion and are considered a biomarker of vaginal health []. Furthermore, this discrepancy emphasizes the species-specific nature of certain aspects of the reproductive microbiota. Clearly, although there is a certain overlap, unique differences between mammals and reproductive tract sites necessitate species and site-specific studies regarding the female reproductive system.
3.5. The Reproductive Microbiota and Disease
It is widely accepted that the presence of commensal bacteria in the reproductive tract has many benefits, including enhancing the barrier function, modulating the immune response, promoting healthy microbiota, and preventing colonization by pathogenic organisms []. In women, the acidic environment is generated within the vagina by the commensal bacterial species Lactobacillus limits colonization of the reproductive tract by opportunistic pathogens []. Lactobacilli also compete for a niche with other opportunistic bacteria, thus avoiding the overgrowth of other bacteria, which could create a problematic environment within the vagina [,]. In women, vulvovaginal candidiasis (vaginal yeast infection) is a common disease caused by an overgrowth of yeast within the vagina, and it is associated with alterations in the population of Lactobacillus and Megasphaera []. The reproductive tract of cows, including the uterus and vagina, host distinct populations of microbiota [,], and vaginal reductions in diversity can predispose individual animals to uterine infections [,]. Also, shifts in the uterine bacterial community to one characterized by a low bacterial diversity dominated by Bacteroides, Porphyromonas, and Fusobacterum [] predispose the cows to disease []. These studies exemplified the role of bacterial communities present in the vagina and uterus in maintaining the health of the reproductive tract in different species. However, studies investigating the association between vaginal and uterine microbiota and the development of inflammatory diseases of the endometrium are lacking.
Endometritis is a common disease in mares that causes subfertility, reduced pregnancy rates, and economic losses [,]. The disease can be triggered by various factors, with two primary causes being persistent breeding-induced endometritis (PBIE) and chronic inflammation associated with Streptococcus zooepidemicus and E. coli infections (CIE) []. PBIE occurs as an inflammatory response of the endometrium more than 48 h after mating or insemination. In healthy mares, both culture-based and culture-independent techniques frequently detect Streptococcus and E. coli in uterine and vaginal samples [,,,], suggesting that these bacteria may be normal inhabitants of the reproductive tract. However, factors that disrupt the normal balance of the vaginal and uterine microbiota (dysbiosis) could facilitate the proliferation of certain bacteria, leading to endometrial inflammation []. Therefore, studies evaluating the association between vaginal and uterine dysbiosis, and the development of endometritis could provide valuable insights into the pathophysiology of the disease. This knowledge could inform potential therapeutic and preventive strategies for managing endometritis beyond the use of antimicrobial drugs.
3.6. The Reproductive Microbiota and Fertility
In humans and other mammalian species, dysbiosis or a non-balanced state of the vaginal microbiome is consistently associated with poor reproductive outcomes, including repeated insemination frequency, low pregnancy rates, and negative in vitro fertilization rates []. Alterations of the normal microbiota, synergistic effects with co-existing bacteria, and microbial composition imbalances in the uterus are thought to predispose to infections known as endometritis or metritis, which affect different layers of the uterus and are a major cause of infertility in most mammalian females [,,,].
In healthy women, the normal vaginal microbiota is primarily composed of Firmicutes, with Lactobacillus being the most prevalent genera. The uterus has a more diverse microbiota, including Lactobacillus, Bacteroides, Gardnerella, and Prevotella. Both the vaginal and uterine microbiota normally share some phyla, such as Firmicutes and Actinobacteria, observed in both culture-dependent and independent research [,,,,,]. In cattle, the dominant phyla within the vagina and uterus mainly include Firmicutes, Proteobacteria, Bacteroides, and Actinobacteria []. Streptococcus spp., Staphylococcus spp., and Bacillus spp. have been isolated in the uterus of healthy cattle [].
In both women and cattle, having a healthy and normal uterine microbiome has been linked to positive reproductive outcomes such as high implantation rates and fertility success [,]. However, the impact of microbiota within the reproductive tract on disease and fertility in mares is not well understood and requires further investigation. This is an important area of study as it will likely affect the reproductive health of mares, as seen in other species.
3.7. Limitations in Current Equine Reproductive Microbiome Literature
Few studies account for various environmental effects, including diet and management practices or recent medical conditions, before the sample collection, which could impact study results. Oral-uterine translocation has been reported in women with preterm delivery and neonatal sepsis [], and gut-uterine translocation via the bloodstream has also been proposed in cows []. However, associations between reproductive tract or digestive microbiota and reproductive outcomes have not yet been made in horses and are still controversial in humans []. Microbiota translocation from other parts of the body might predispose horses to detrimental reproductive outcomes, as reported in other species. Furthermore, many studies attempting to characterize the equine reproductive microbiota still need to publish their data. This limits interpretation and research validity, challenging the consolidation of knowledge on this topic. Additionally, most equine reproductive microbiota studies are descriptive in nature. Finally, the current understanding of the commensal reproductive microbiota in mares is limited, as “pathogenic” bacteria have also been reported in healthy uterine samples. Determining the reproductive microbiota in healthy mares during estrus is paramount for understanding the role of the resident microorganisms in different parts of the reproductive tract and how dysbiosis can affect fertility and the overall host’s health [].
4. Conclusions
In conclusion, there remains a significant amount of ambiguity surrounding the composition, function, and impact of the microbiota inhabiting the female equine reproductive tract. More research on the female reproductive microbiota has been conducted in other mammals. However, the species-specific nature of this niche environment demands caution when trying to generalize results. Several considerations, including sampling site, reproductive phase, and likely confounding factors, including season and geographic location, should be considered in future studies. The microbiota within the reproductive tract likely impacts disease and fertility status. Therefore, this area of equine research urgently requires more attention.
Author Contributions
Conceptualization, A.G.-M., D.O.-G., J.C.S. and D.E.G.; methodology, A.G.-M., J.M., and D.E.G.; writing—original draft preparation, A.G.-M.; writing—review and editing, J.M., D.O.-G., J.C.S. and D.E.G.; visualization, A.G.-M.; supervision, D.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data contained within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gomez, D.; Toribio, R.; Caddey, B.; Costa, M.; Vijan, S.; Dembek, K. Longitudinal Effects of Oral Administration of Antimicrobial Drugs on Fecal Microbiota of Horses. J. Vet. Intern. Med. 2023, 37, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.E.; Wong, D.; MacNicol, J.; Dembek, K. The Fecal Bacterial Microbiota of Healthy and Sick Newborn Foals. J. Vet. Intern. Med. 2023, 37, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, C.; Arroyo, L.G.; MacNicol, J.L.; Renaud, D.; Weese, J.S.; Gomez, D.E. Fecal Microbiota of Horses with Colitis and Its Association with Laminitis and Survival during Hospitalization. J. Vet. Intern. Med. 2022, 36, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, C.; Arroyo, L.G.; Renaud, D.; Weese, J.S.; Gomez, D.E. Fecal Microbiota Comparison Between Healthy Teaching Horses and Client-Owned Horses. J. Equine Vet. Sci. 2022, 118, 104105. [Google Scholar] [CrossRef] [PubMed]
- Heil, B.A.; Paccamonti, D.L.; Sones, J.L. Role for the Mammalian Female Reproductive Tract Microbiome in Pregnancy Outcomes. Physiol. Genom. 2019, 51, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef] [PubMed]
- Liptáková, A.; Čurová, K.; Záhumenský, J.; Visnyaiová, K.; Varga, I. Microbiota of Female Genital Tract-Functional Overview of Microbial Flora from Vagina to Uterine Tubes and Placenta. Physiol. Res. 2022, 71, S21–S33. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.; Hastie, P.; Murray, J.-A. Factors Influencing Equine Gut Microbiota: Current Knowledge. J. Equine Vet. Sci. 2020, 88, 102943. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Daley, P.; Baird, G.G.; Sturgess, S.; Frost, A.J. The Aerobic Bacterial Flora of the Reproductive Tract of the Mare. Vet. Rec. 1971, 88, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, K.; Cummings, M.R.; Sertich, P.L.; Kenney, R.M. Clinical Significance of Aerobic Bacterial Flora of the Uterus, Vagina, Vestibule, and Clitoral Fossa of Clinically Normal Mares. J. Am. Vet. Med. Assoc. 1988, 193, 72–75. [Google Scholar]
- Purswell, B.J.; Ley, W.B.; Sriranganathan, N.; Bowen, J.M. Aerobic and Anaerobic Bacterial Flora in the Postpartum Mare. J. Equine Vet. Sci. 1989, 9, 141–144. [Google Scholar] [CrossRef]
- Ferris, R.A.; McCue, P.M.; Borlee, G.I.; Glapa, K.E.; Martin, K.H.; Mangalea, M.R.; Hennet, M.L.; Wolfe, L.M.; Broeckling, C.D.; Borlee, B.R. Model of Chronic Equine Endometritis Involving a Pseudomonas Aeruginosa Biofilm. Infect. Immun. 2017, 85, e00332-17. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.R.; Skive, B.; Christoffersen, M.; Lu, K.; Nielsen, J.M.; Troedsson, M.H.T.; Bojesen, A.M. Activation of Persistent Streptococcus Equi Subspecies Zooepidemicus in Mares with Subclinical Endometritis. Vet. Microbiol. 2015, 179, 119–125. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.M.; Causey, R.C. Clinical and Subclinical Endometritis in the Mare: Both Threats to Fertility. Reprod. Domest. Anim. 2009, 44 (Suppl. S3), 10–22. [Google Scholar] [CrossRef] [PubMed]
- Heil, B.A.; van Heule, M.; Thompson, S.K.; Kearns, T.A.; Oberhaus, E.L.; King, G.; Daels, P.; Dini, P.; Sones, J.L. Effect of Sampling Method on Detection of the Equine Uterine Microbiome during Estrus. Vet. Sci. 2023, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.H.A.; McCue, P.M.; Aurich, C. Equine Endometritis: A Review of Challenges and New Approaches. Reproduction 2020, 160, R95–R110. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus Aureus Biofilms Prevent Macrophage Phagocytosis and Attenuate Inflammation in Vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.; O’Toole, G.A. Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Schnobrich, M.; Atwood, K.; Barr, B.; Bradecamp, E.; Scoggin, C.; Hospital, R. Next Generation DNA Sequencing, Culture and Cytology Results in 10 Clinically Normal Mares. Clin. Theriogenology 2017, 9, 443. [Google Scholar]
- Sathe, S.; Leiken, A.; Plummer, P. Metagenomic Sequencing of the Uterine Microbial Environment during Estrus and Early Pregnancy in Mares. Clin. Theriogenology 2017, 9, 453. [Google Scholar]
- Bharti, R.; Grimm, D.G. Current Challenges and Best-Practice Protocols for Microbiome Analysis. Brief. Bioinform. 2021, 22, 178–193. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S RRNA Gene Sequencing for Bacterial Identification in the Diagnostic Laboratory: Pluses, Perils, and Pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef] [PubMed]
- Kauter, A.; Epping, L.; Semmler, T.; Antao, E.-M.; Kannapin, D.; Stoeckle, S.D.; Gehlen, H.; Lübke-Becker, A.; Günther, S.; Wieler, L.H.; et al. The Gut Microbiome of Horses: Current Research on Equine Enteral Microbiota and Future Perspectives. Anim. Microbiome 2019, 1, 14. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Holyoak, G.R.; Premathilake, H.U.; Lyman, C.C.; Sones, J.L.; Gunn, A.; Wieneke, X.; DeSilva, U. The Healthy Equine Uterus Harbors a Distinct Core Microbiome plus a Rich and Diverse Microbiome That Varies with Geographical Location. Sci. Rep. 2022, 12, 14790. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, T.L.; Garcia, M.C.; Hurtgen, J.P.; Kenney, R.M. Comparison of Two Techniques for Obtaining Endometrial Bacteriologic Cultures in the Mare. Theriogenology 1981, 16, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ansbacher, R.; Boyson, W.A.; Morris, J.A. Sterility of the Uterine Cavity. Am. J. Obstet. Gynecol. 1967, 99, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Barba, M.; Martínez-Boví, R.; Quereda, J.J.; Mocé, M.L.; Plaza-Dávila, M.; Jiménez-Trigos, E.; Gómez-Martín, Á.; González-Torres, P.; Carbonetto, B.; García-Roselló, E. Vaginal Microbiota Is Stable throughout the Estrous Cycle in Arabian Maress. Animals 2020, 10, 2020. [Google Scholar] [CrossRef] [PubMed]
- Jones, E. Characterization of the Equine Microbiome during Late Gestation and the Early Postpartum Period, and at Various Times during the Estrous Cycle in Mares Being Bred with Raw or Extended Semen. Master’s Thesis, Kansas City University, Kansas City, MO, USA, 2019. [Google Scholar]
- Beckers, K.F.; Gomes, V.C.L.; Crissman, K.R.; Liu, C.-C.; Schulz, C.J.; Childers, G.W.; Sones, J.L. Metagenetic Analysis of the Pregnant Microbiome in Horses. Animals 2023, 13, 1999. [Google Scholar] [CrossRef] [PubMed]
- Holyoak, R.; Premathilake, H.; Guo, M.; DeSilva, U. The conundrum of the uterine microbiome. Clin. Theriogenology 2022, 14, 247–251. [Google Scholar] [CrossRef]
- Thomson, P.; Pareja, J.; Núñez, A.; Santibáñez, R.; Castro, R. Characterization of Microbial Communities and Predicted Metabolic Pathways in the Uterus of Healthy Mares. Open Vet. J. 2022, 12, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Malaluang, P.; Wilén, E.; Frosth, S.; Lindahl, J.; Hansson, I.; Morrell, J.M. Vaginal Bacteria in Mares and the Occurrence of Antimicrobial Resistance. Microorganisms 2022, 10, 2204. [Google Scholar] [CrossRef] [PubMed]
- Malaluang, P.; Åkerholm, T.; Nyman, G.; Lindahl, J.; Hansson, I.; Morrell, J.M. Bacteria in the Healthy Equine Vagina during the Estrous Cycle. Theriogenology 2024, 213, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Virendra, A.; Gulavane, S.U.; Ahmed, Z.A.; Reddy, R.; Chaudhari, R.J.; Gaikwad, S.M.; Shelar, R.R.; Ingole, S.D.; Thorat, V.D.; Khanam, A.; et al. Metagenomic analysis unravels novel taxonomic differences in the uterine microbiome between healthy mares and mares with endometritis. Vet. Med. Sci. 2024, 10, e1369. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.G.; Ericsson, A.C.; Poock, S.E.; Melendez, P.; Lucy, M.C. Hot Topic: 16S RRNA Gene Sequencing Reveals the Microbiome of the Virgin and Pregnant Bovine Uterus. J. Dairy. Sci. 2017, 100, 4953–4960. [Google Scholar] [CrossRef]
- Moreno, I.; Simon, C. Deciphering the Effect of Reproductive Tract Microbiota on Human Reproduction. Reprod. Med. Biol. 2019, 18, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Lyman, C.C.; Holyoak, G.R.; Meinkoth, K.; Wieneke, X.; Chillemi, K.A.; DeSilva, U. Canine Endometrial and Vaginal Microbiomes Reveal Distinct and Complex Ecosystems. PLoS ONE 2019, 14, e0210157. [Google Scholar] [CrossRef]
- Lorenzen, E.; Kudirkiene, E.; Gutman, N.; Grossi, A.B.; Agerholm, J.S.; Erneholm, K.; Skytte, C.; Dalgaard, M.D.; Bojesen, A.M. The Vaginal Microbiome Is Stable in Prepubertal and Sexually Mature Ellegaard Göttingen Minipigs throughout an Estrous Cycle. Vet. Res. 2015, 46, 125. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, G.; Li, C.; Yang, J.; Li, J.; Chen, D.; Zou, W.; Jin, S.; Zhang, H.; Li, D.; et al. The Normal Vaginal and Uterine Bacterial Microbiome in Giant Pandas (Ailuropoda melanoleuca). Microbiol. Res. 2017, 199, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miranda-CasoLuengo, R.; Lu, J.; Williams, E.J.; Miranda-CasoLuengo, A.A.; Carrington, S.D.; Evans, A.C.O.; Meijer, W.G. Delayed Differentiation of Vaginal and Uterine Microbiomes in Dairy Cows Developing Postpartum Endometritis. PLoS ONE 2019, 14, e0200974. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.K.; Becher, N.; Bastholm, S.; Glavind, J.; Ramsing, M.; Kim, C.J.; Romero, R.; Jensen, J.S.; Uldbjerg, N. The Cervical Mucus Plug Inhibits, but Does Not Block, the Passage of Ascending Bacteria from the Vagina during Pregnancy. Acta Obstet. Gynecol. Scand. 2014, 93, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kunz, G.; Leyendecker, G. Uterine Peristaltic Activity during the Menstrual Cycle: Characterization, Regulation, Function and Dysfunction. Reprod. Biomed. Online 2002, 4 (Suppl. S3), 5–9. [Google Scholar] [CrossRef] [PubMed]
- Kunz, G.; Beil, D.; Deiniger, H.; Einspanier, A.; Mall, G.; Leyendecker, G. The Uterine Peristaltic Pump. Normal and Impeded Sperm Transport within the Female Genital Tract. Adv. Exp. Med. Biol. 1997, 424, 267–277. [Google Scholar] [PubMed]
- Lehtoranta, L.; Ala-Jaakkola, R.; Laitila, A.; Maukonen, J. Healthy Vaginal Microbiota and Influence of Probiotics Across the Female Life Span. Front. Microbiol. 2022, 13, 819958. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.B.; Clemmons, B.A.; Reese, S.T.; Dantas, F.G.; Franco, G.A.; Smith, T.P.L.; Edwards, J.L.; Myer, P.R.; Pohler, K.G. Bacterial Taxonomic Composition of the Postpartum Cow Uterus and Vagina Prior to Artificial Insemination1. J. Anim. Sci. 2019, 97, 4305–4313. [Google Scholar] [CrossRef] [PubMed]
- Quereda, J.J.; Barba, M.; Mocé, M.L.; Gomis, J.; Jiménez-Trigos, E.; García-Muñoz, Á.; Gómez-Martín, Á.; González-Torres, P.; Carbonetto, B.; García-Roselló, E. Vaginal Microbiota Changes During Estrous Cycle in Dairy Heifers. Front. Vet. Sci. 2020, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Messman, R.D.; Contreras-Correa, Z.E.; Paz, H.A.; Perry, G.; Lemley, C.O. Vaginal Bacterial Community Composition and Concentrations of Estradiol at the Time of Artificial Insemination in Brangus Heifers. J. Anim. Sci. 2020, 98, skaa178. [Google Scholar] [CrossRef] [PubMed]
- Neggers, Y.H.; Nansel, T.R.; Andrews, W.W.; Schwebke, J.R.; Yu, K.; Goldenberg, R.L.; Klebanoff, M.A. Dietary Intake of Selected Nutrients Affects Bacterial Vaginosis in Women. J. Nutr. 2007, 137, 2128–2133. [Google Scholar] [CrossRef] [PubMed]
- Punzón-Jiménez, P.; Labarta, E. The Impact of the Female Genital Tract Microbiome in Women Health and Reproduction: A Review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef] [PubMed]
- Bogado Pascottini, O.; Spricigo, J.F.W.; Van Schyndel, S.J.; Mion, B.; Rousseau, J.; Weese, J.S.; LeBlanc, S.J. Effects of Parity, Blood Progesterone, and Non-Steroidal Anti-Inflammatory Treatment on the Dynamics of the Uterine Microbiota of Healthy Postpartum Dairy Cows. PLoS ONE 2021, 16, e0233943. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological Dynamics of the Vaginal Microbiome in Relation to Health and Disease. Am. J. Obstet. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.; Perelmuter, K.; Delucchi, L.; Cidade, E.; Zunino, P. Vaginal Lactic Acid Bacteria in the Mare: Evaluation of the Probiotic Potential of Native Lactobacillus Spp. and Enterococcus Spp. Strains. Antonie Van. Leeuwenhoek 2008, 93, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Swartz, J.D.; Lachman, M.; Westveer, K.; O’Neill, T.; Geary, T.; Kott, R.W.; Berardinelli, J.G.; Hatfield, P.G.; Thomson, J.M.; Roberts, A.; et al. Characterization of the Vaginal Microbiota of Ewes and Cows Reveals a Unique Microbiota with Low Levels of Lactobacilli and Near-Neutral PH. Front. Vet. Sci. 2014, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus Species as Biomarkers and Agents That Can Promote Various Aspects of Vaginal Health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.E.; Galvão, K.N.; Rodriguez-Lecompte, J.C.; Costa, M.C. The Cattle Microbiota and the Immune System: An Evolving Field. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Scott, R.T.J. Reproductive Tract Microbiome in Assisted Reproductive Technologies. Fertil. Steril. 2015, 104, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Witkin, S.S.; Linhares, I.M. Why Do Lactobacilli Dominate the Human Vaginal Microbiota? BJOG Int. J. Obstet. Gynaecol. 2017, 124, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.E.; Schwartz, J.A.; Robinson, C.K.; OʼHanlon, D.E.; Bradford, L.L.; He, X.; Mark, K.S.; Bruno, V.M.; Ravel, J.; Brotman, R.M. The Vaginal Microbiota and Behavioral Factors Associated With Genital Candida Albicans Detection in Reproductive-Age Women. Sex. Transm. Dis. 2019, 46, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Clemmons, B.A.; Reese, S.T.; Dantas, F.G.; Franco, G.A.; Smith, T.P.L.; Adeyosoye, O.I.; Pohler, K.G.; Myer, P.R. Vaginal and Uterine Bacterial Communities in Postpartum Lactating Cows. Front. Microbiol. 2017, 8, 1047. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.M.; Holman, D.B.; Schmidt, K.N.; Pun, B.; Sedivec, K.K.; Hurlbert, J.L.; Bochantin, K.A.; Ward, A.K.; Dahlen, C.R.; Amat, S. Sequencing and Culture-Based Characterization of the Vaginal and Uterine Microbiota in Beef Cattle That Became Pregnant or Remained Open Following Artificial Insemination. Microbiol. Spectr. 2023, 11, e0273223. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, M.L.S.; Santin, T.; Rodrigues, M.X.; Marques, C.E.; Lima, S.F.; Bicalho, R.C. Dynamics of the Microbiota Found in the Vaginas of Dairy Cows during the Transition Period: Associations with Uterine Diseases and Reproductive Outcome. J. Dairy. Sci. 2017, 100, 3043–3058. [Google Scholar] [CrossRef]
- Galvão, K.N.; Bicalho, R.C.; Jeon, S.J. Symposium Review: The Uterine Microbiome Associated with the Development of Uterine Disease in Dairy Cows. J. Dairy. Sci. 2019, 102, 11786–11797. [Google Scholar] [CrossRef] [PubMed]
- Traub-Dargatz, J.L.; Salman, M.D.; Voss, J.L. Medical Problems of Adult Horses, as Ranked by Equine Practitioners. J. Am. Vet. Med. Assoc. 1991, 198, 1745–1747. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Segabinazzi, L.G.T.M.; Fedorka, C.E. Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-L.; Liu, M.-C.; Xu, J.; An, L.-G.; Wang, J.-F.; Zhu, Y.-H. Uterine Microbiota of Dairy Cows With Clinical and Subclinical Endometritis. Front. Microbiol. 2018, 9, 2691. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Y.; Mi, J.; Zhao, Y.; Holyoak, G.R.; Yi, Z.; Wu, R.; Wang, Z.; Zeng, S. Endometrial and Vaginal Microbiome in Donkeys with and without Clinical Endometritis. Front. Microbiol. 2022, 13, 884574. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Franasiak, J.M. Endometrial Microbiota-New Player in Town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.K.; Soffa, D.R.; McAnally, B.E.; Smith, M.S.; Hickman-Brown, K.J.; Stockland, E.L. Reproductive Microbiomes in Domestic Livestock: Insights Utilizing 16S RRNA Gene Amplicon Community Sequencing. Animals 2023, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How Uterine Microbiota Might Be Responsible for a Receptive, Fertile Endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Ikegami, A.; Bissada, N.F.; Herbst, M.; Redline, R.W.; Ashmead, G.G. Transmission of an Uncultivated Bergeyella Strain from the Oral Cavity to Amniotic Fluid in a Case of Preterm Birth. J. Clin. Microbiol. 2006, 44, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Cunha, F.; Vieira-Neto, A.; Bicalho, R.C.; Lima, S.; Bicalho, M.L.; Galvão, K.N. Blood as a Route of Transmission of Uterine Pathogens from the Gut to the Uterus in Cows. Microbiome 2017, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, R.M.; Arias-Álvarez, M.; Jordán-Rodríguez, D.; Rebollar, P.G.; Lorenzo, P.L.; Herranz, C.; Rodríguez, J.M. Female Reproduction and the Microbiota in Mammals: Where Are We? Theriogenology 2022, 194, 144–153. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).