Intra-Articular Surgical Reconstruction of a Canine Cranial Cruciate Ligament Using an Ultra-High-Molecular-Weight Polyethylene Ligament: Case Report with Six-Month Clinical Outcome
Abstract
Simple Summary
Abstract
1. Introduction
2. Case Presentation
2.1. Clinical History
2.2. General and Orthopedic Examination
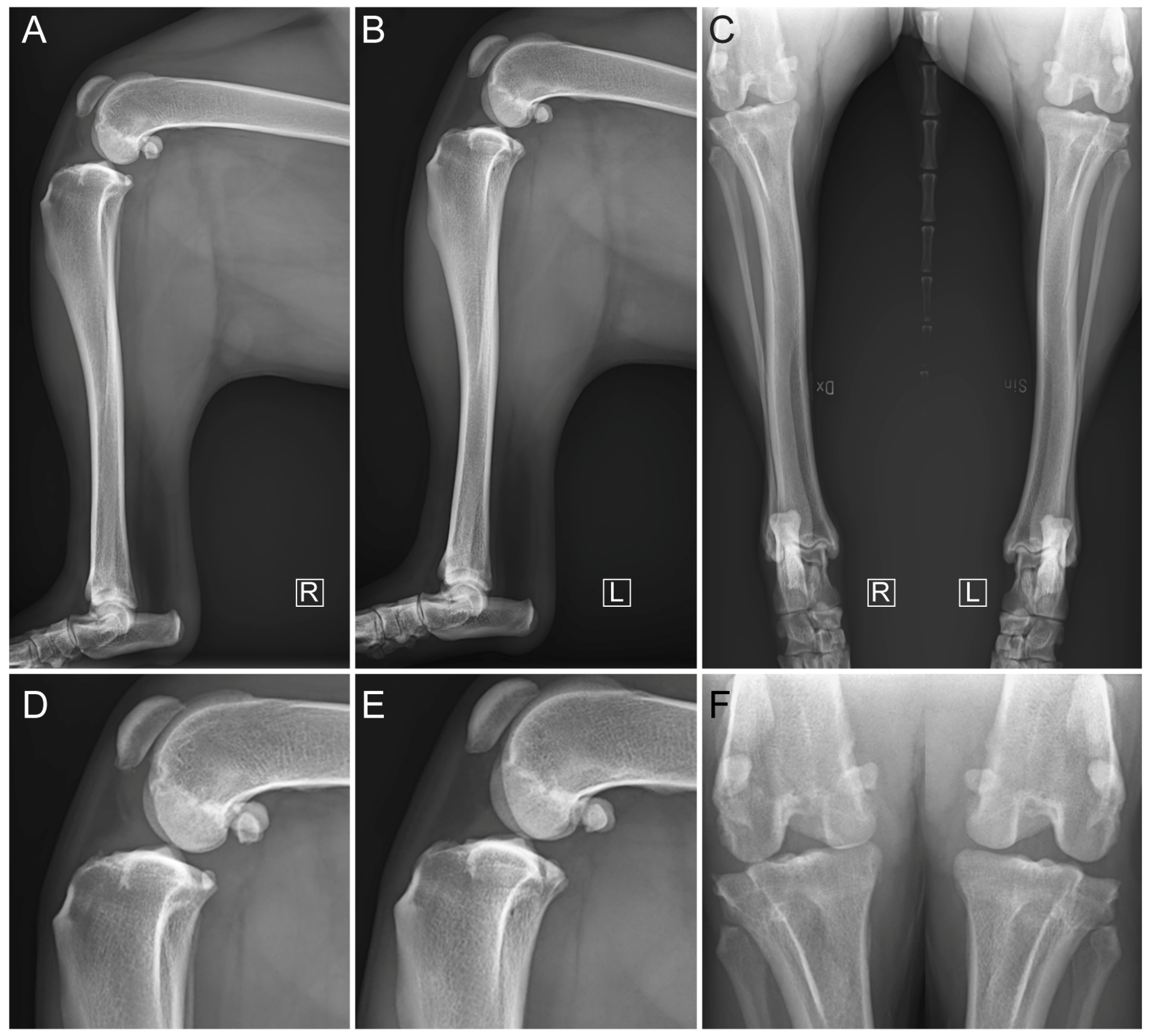
2.3. Diagnostic Imaging under Anesthesia
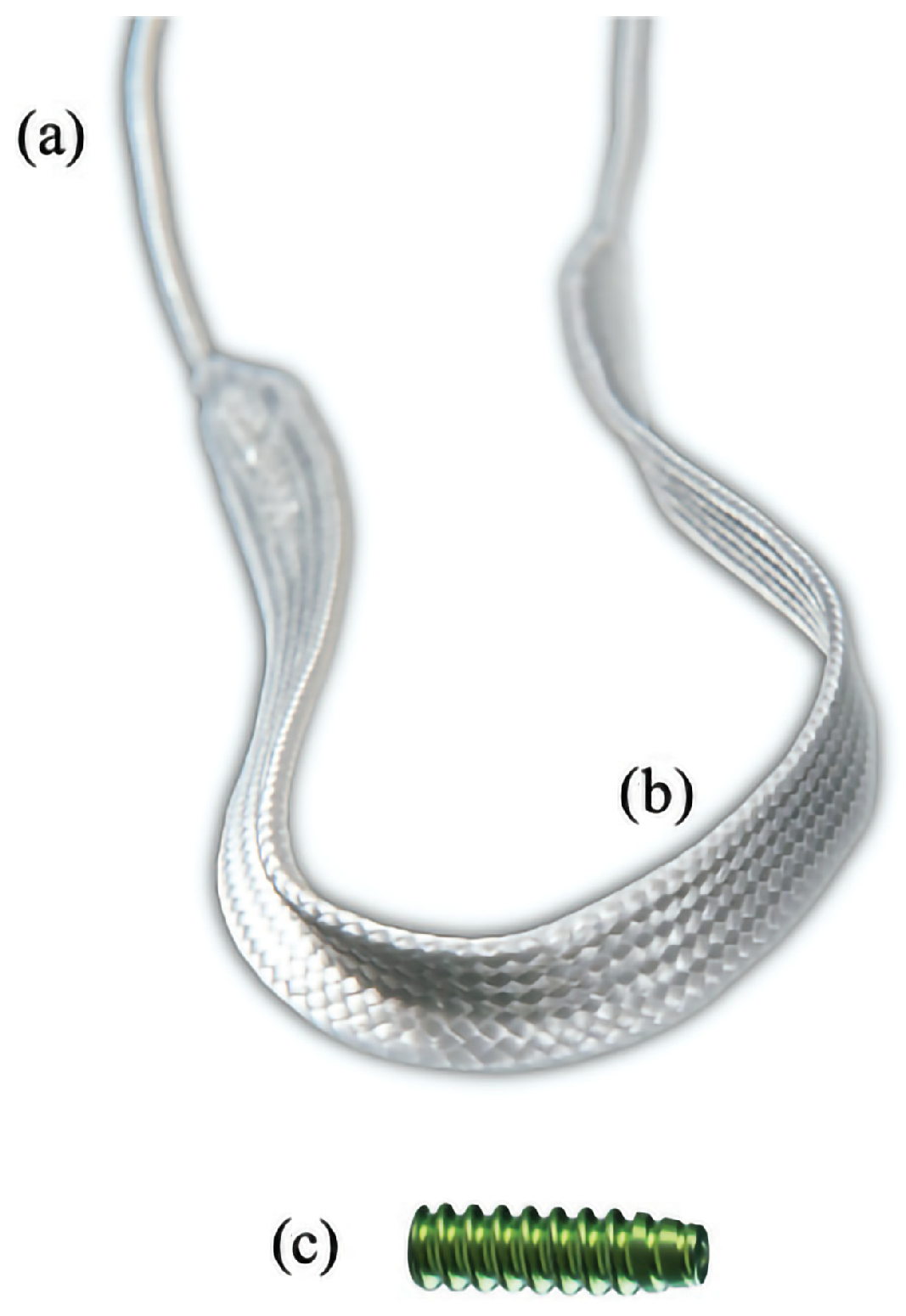
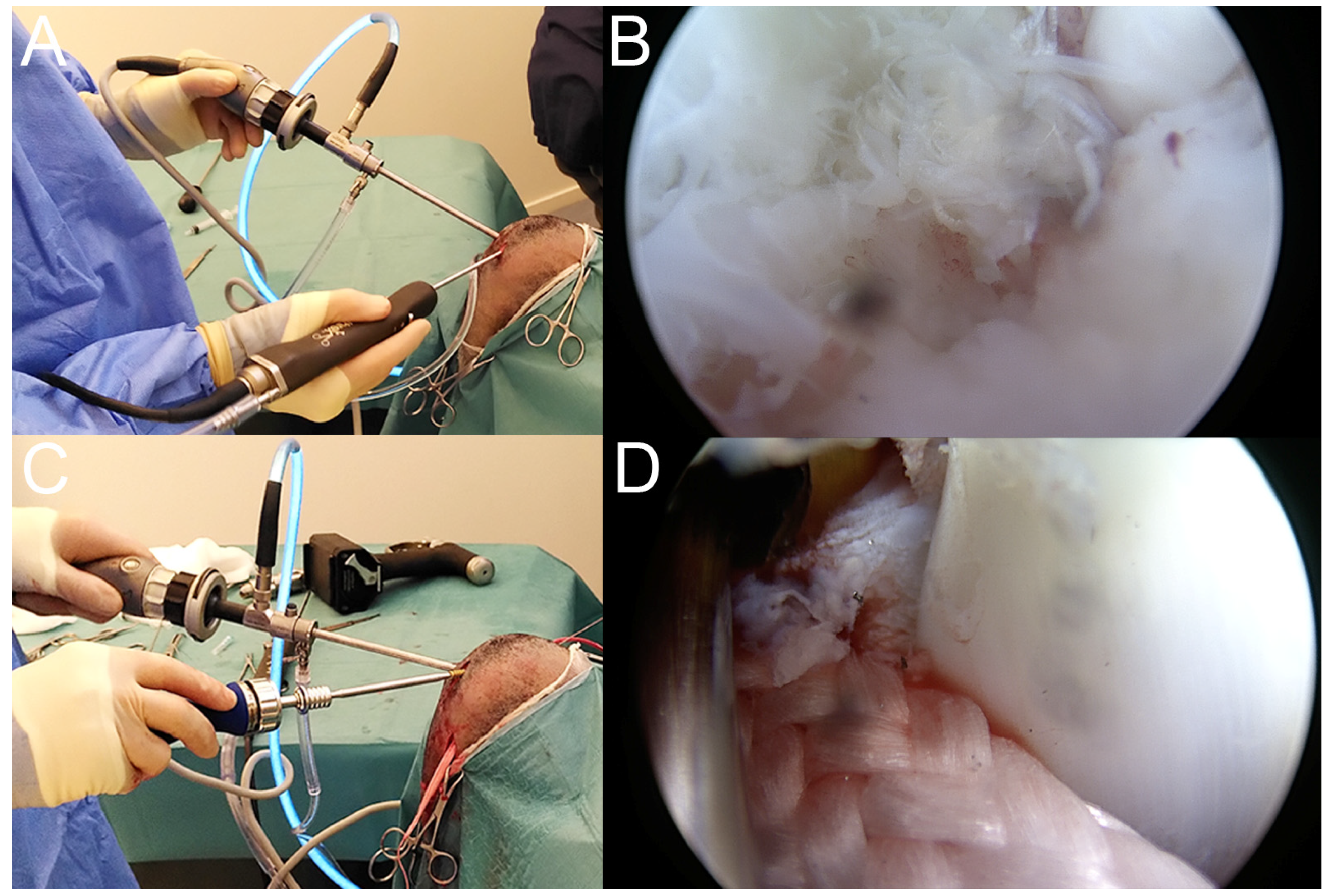
2.4. Surgical Treatment
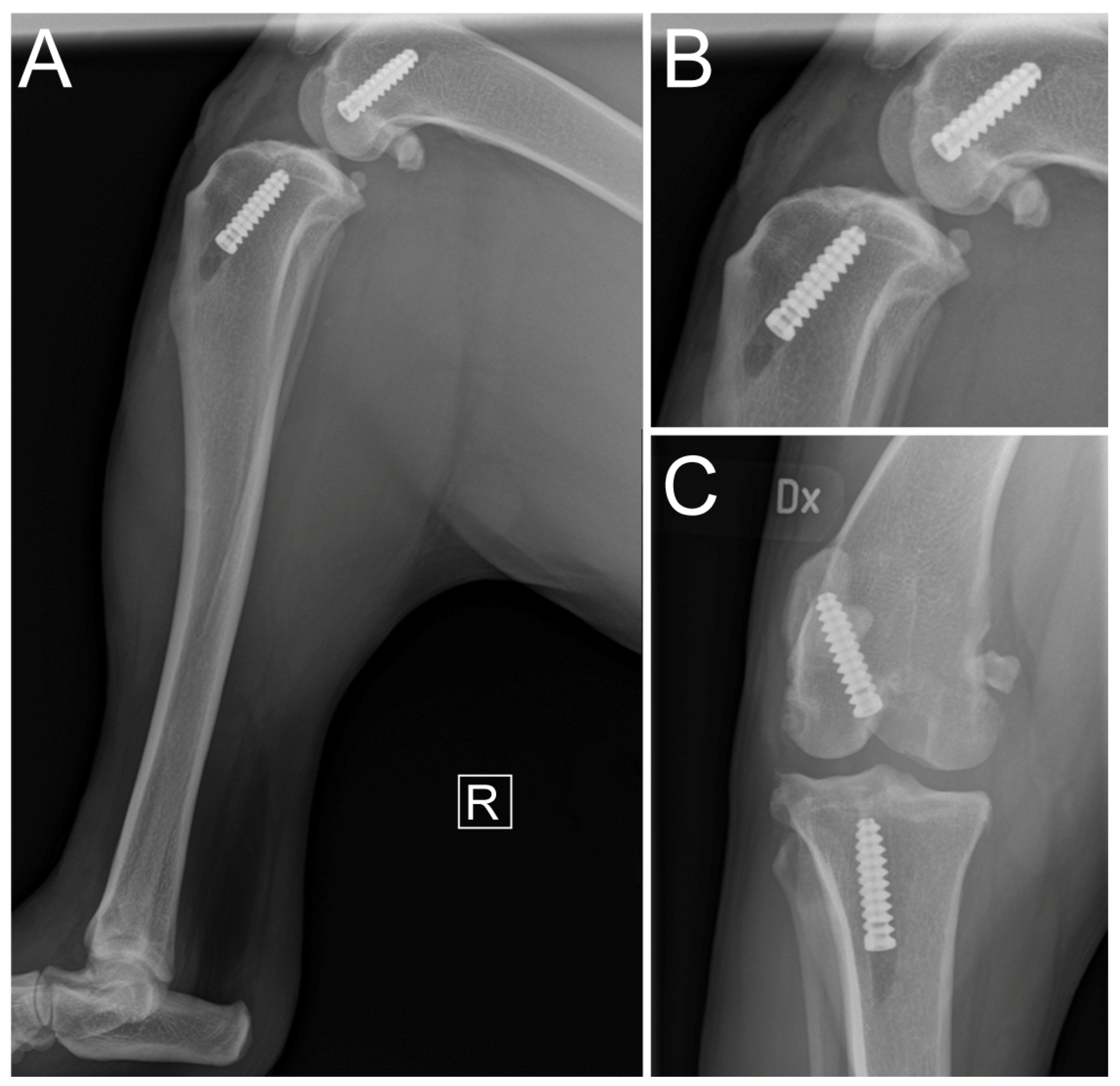
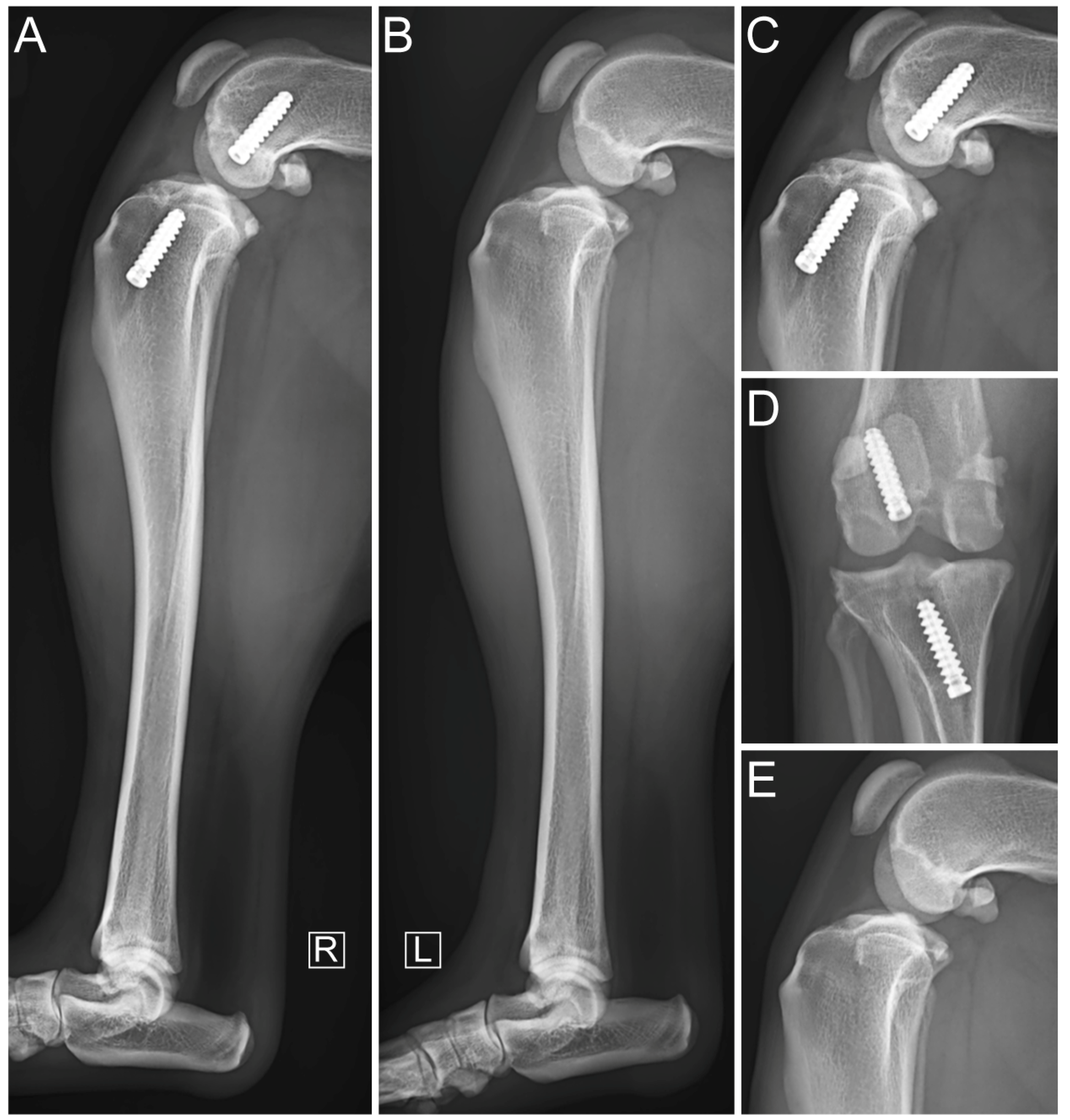
2.5. Immediate Postoperative Evaluation
2.6. Postoperative Management
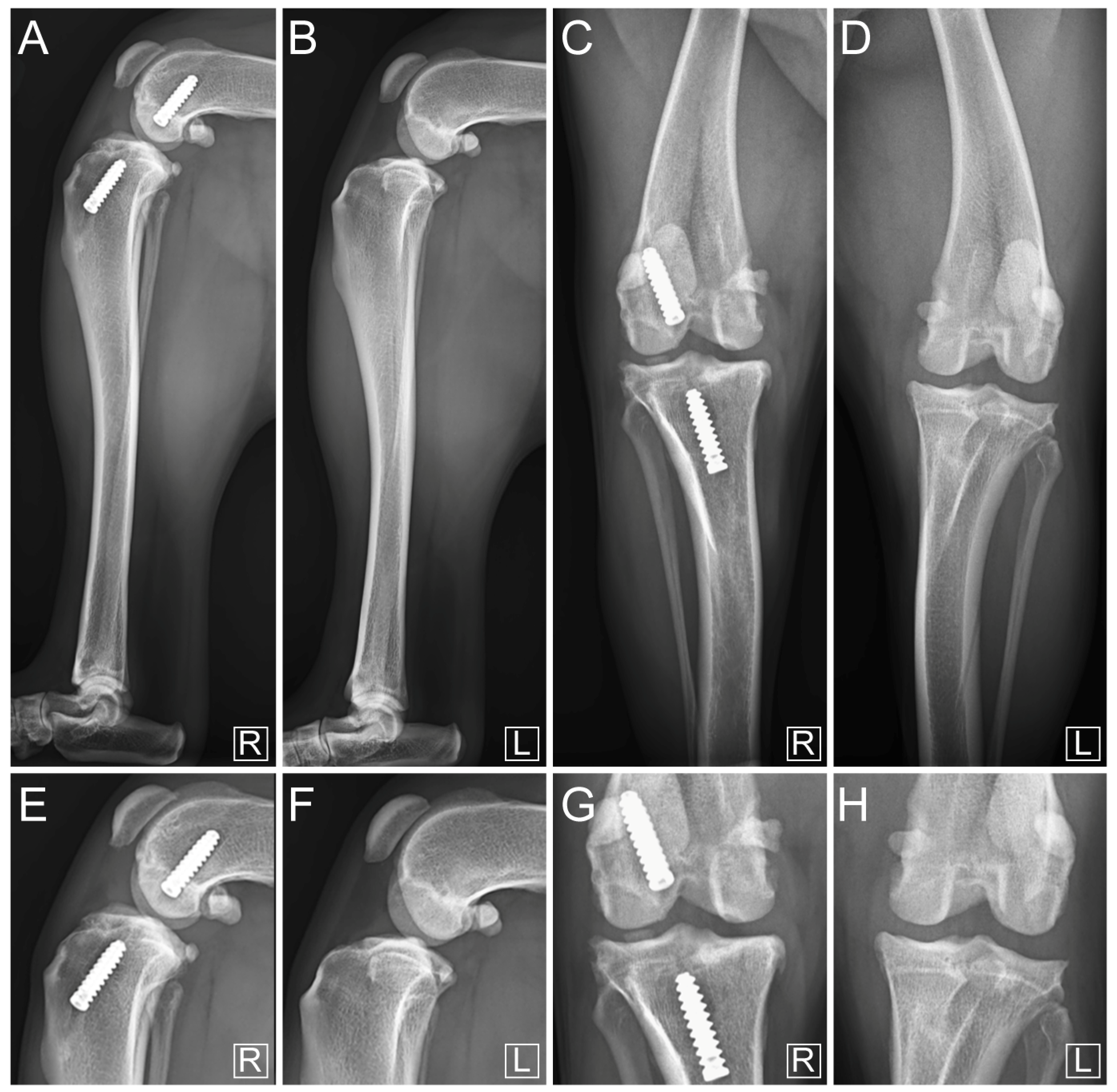
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witsberger, T.H.; Villamil, J.A.; Schultz, L.G.; Hahn, A.W.; Cook, J.L. Prevalence of and risk factors for hip dysplasia and cranial cruciate ligament deficiency in dogs. J. Am. Vet. Med. Assoc. 2008, 232, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, K.; Hanson, J.; Bergström, A.; Bonnett, B.; Höglund, O.; Emanuelson, U. The epidemiology of stifle joint disease in an insured Swedish dog population. Vet. Rec. 2021, 189, e197. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, K.; Emanuelson, U.; Höglund, O.; Bergström, A.; Hanson, J. The epidemiology of cruciate ligament rupture in an insured Swedish dog population. Sci. Rep. 2021, 11, 9546. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Brown, F.E.; Meeson, R.L.; Brodbelt, D.C.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; O’Neill, D.G. Epidemiology of Cranial Cruciate Ligament Disease Diagnosis in Dogs Attending Primary-Care Veterinary Practices in England. Vet. Surg. 2015, 44, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Jerram, R.M.; Walker, A.M. Cranial cruciate ligament injury in the dog: Pathophysiology, diagnosis and treatment. N. Z. Vet. J. 2003, 51, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Comerford, E.; Forster, K.; Gorton, K.; Maddox, T. Management of cranial cruciate ligament rupture in small dogs: A questionnaire study. Vet. Comp. Orthop. Traumatol. 2013, 26, 493–497. [Google Scholar] [CrossRef]
- von Pfeil, D.J.F.; Kowaleski, M.P.; Glassman, M.; Dejardin, L.M. Results of a survey of Veterinary Orthopedic Society members on the preferred method for treating cranial cruciate ligament rupture in dogs weighing more than 15 kilograms (33 pounds). J. Am. Vet. Med. Assoc. 2018, 253, 586–597. [Google Scholar] [CrossRef]
- Kim, S.E.; Pozzi, A.; Kowaleski, M.P.; Lewis, D.D. Tibial Osteotomies for Cranial Cruciate Ligament Insufficiency in Dogs. Vet. Surg. 2008, 37, 111–125. [Google Scholar] [CrossRef]
- Slocum, B.; Slocum, T.D. Tibial Plateau Leveling Osteotomy for Repair of Cranial Cruciate Ligament Rupture in the Canine. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 777–795. [Google Scholar] [CrossRef]
- Montavon, P.M.; Damur, D.M.; Tepic, S. Advancement of the tibial tuberosity for the treatment of cranial cruciate deficient canine stifle. In Proceedings of the 1st World Orthopaedic Veterinary Congress, Munich, Germany, 5–8 September 2002; p. 152. [Google Scholar]
- Raske, M.; Hulse, D.; Beale, B.; Saunders, W.B.; Kishi, E.; Kunze, C. Stabilization of the CORA Based Leveling Osteotomy for Treatment of Cranial Cruciate Ligament Injury Using a Bone Plate Augmented With a Headless Compression Screw. Vet. Surg. 2013, 42, 759–764. [Google Scholar] [CrossRef]
- Bruce, W.J.; Rose, A.; Tuke, J.; Robins, G.M. Evaluation of the Triple Tibial Osteotomy. A new technique for the management of the canine cruciate-deficient stifle. Vet. Comp. Orthop. Traumatol. 2007, 20, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Frederick, S.W.; Cross, A.R. Modified cranial closing wedge osteotomy for treatment of cranial cruciate ligament insufficiency in dogs with excessive tibial plateau angles: Technique and complications in 19 cases. Vet. Surg. 2017, 46, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Flo, G.L. Modification of the lateral retinacular imbrication technique for stabilizing cruciate ligament injuries. J. Am. Anim. Hosp. Assoc. 1975, 11, 570–576. [Google Scholar]
- Burgess, R.; Elder, S.; McLaughlin, R.O.N.; Constable, P. In Vitro Biomechanical Evaluation and Comparison of FiberWire, FiberTape, OrthoFiber, and Nylon Leader Line for Potential Use During Extraarticular Stabilization Of Canine Cruciate Deficient Stifles. Vet. Surg. 2010, 39, 208–215. [Google Scholar] [CrossRef]
- Sicard, G.K.; Hayashi, K.; Manley, P.A. Evaluation of 5 Types of Fishing Material, 2 Sterilization Methods, and a Crimp-Clamp System for Extra-Articular Stabilization of the Canine Stifle Joint. Vet. Surg. 2002, 31, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Luther, J.K.; Beetem, J.; Karnes, J.; Cook, C.R. Clinical Comparison of a Novel Extracapsular Stabilization Procedure and Tibial Plateau Leveling Osteotomy for Treatment of Cranial Cruciate Ligament Deficiency in Dogs. Vet. Surg. 2010, 39, 315–323. [Google Scholar] [CrossRef]
- Muro, N.M.; Lanz, O.I. Use of a novel extracapsular bone anchor system for stabilisation of cranial cruciate ligament insufficiency. J. Small Anim. Pract. 2017, 58, 284–292. [Google Scholar] [CrossRef]
- Krotscheck, U.; Nelson, S.A.; Todhunter, R.J.; Stone, M.; Zhang, Z. Long Term Functional Outcome of Tibial Tuberosity Advancement vs. Tibial Plateau Leveling Osteotomy and Extracapsular Repair in a Heterogeneous Population of Dogs. Vet. Surg. 2016, 45, 261–268. [Google Scholar] [CrossRef]
- Corse, M.; Holsworth, I.G. Complications Associated with Extracapsular Stabilization using Multifilament Material. In Complications in Canine Cranial Cruciate Ligament Surgery; Ben-Amotz, R., Dycus, D.L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2022; pp. 121–143. [Google Scholar]
- Paatsama, S. Ligament Injuries in the Canine Stifle Joint: A Clinical and Experimental Study; Royal Veterinary College: Helsinki, Finland, 1952. [Google Scholar]
- Mao, G.; Qin, Z.; Li, Z.; Li, X.; Qiu, Y.; Bian, W. A tricalcium phosphate/polyether ether ketone anchor bionic fixation device for anterior cruciate ligament reconstruction: Safety and efficacy in a beagle model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 554–563. [Google Scholar] [CrossRef]
- Biskup, J.J.; Balogh, D.G.; Haynes, K.H.; Freeman, A.L.; Conzemius, M.G. Mechanical strength of four allograft fixation techniques for ruptured cranial cruciate ligament repair in dogs. Am. J. Vet. Res. 2015, 76, 411–419. [Google Scholar] [CrossRef]
- Biskup, J.J.; Conzemius, M.G. Long-term arthroscopic assessment of intra-articular allografts for treatment of spontaneous cranial cruciate ligament rupture in the dog. Vet. Surg. 2020, 49, 764–771. [Google Scholar] [CrossRef]
- Cook, J.L.; Smith, P.; Stannard, J.P.; Pfeiffer, F.; Kuroki, K.; Bozynski, C.C.; Cook, C. A Canine Arthroscopic Anterior Cruciate Ligament Reconstruction Model for Study of Synthetic Augmentation of Tendon Allografts. J. Knee Surg. 2017, 30, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, M.D.; Getzy, D.; Gardiner, D.W. Histologic Analysis of Retrieved Synthetic Ligaments Implanted in Dogs for Treatment of Cranial Cruciate Ligament Disease. J. Vet. Sci. Med. Diagn. 2018, 7, 1000248. [Google Scholar] [CrossRef]
- Barnhart, M.D.; Maritato, K.; Schankereli, K.; Wotton, H.; Naber, S. Evaluation of an intra-articular synthetic ligament for treatment of cranial cruciate ligament disease in dogs: A six-month prospective clinical trial. Vet. Comp. Orthop. Traumatol. 2016, 29, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.A.; Conzemius, M.G. Outcome of cranial cruciate ligament replacement with an enhanced polyethylene terephthalate implant in the dog: A pilot clinical trial. Vet. Surg. 2022, 51, 1215–1222. [Google Scholar] [CrossRef]
- Soreide, E.; Denbeigh, J.M.; Lewallen, E.A.; Thaler, R.; Xu, W.; Berglund, L.; Yao, J.J.; Martinez, A.; Nordsletten, L.; van Wijnen, A.J.; et al. In vivo assessment of high-molecular-weight polyethylene core suture tape for intra-articular ligament reconstruction. Bone Jt. J. 2019, 101-B, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, X.-C.; Li, L.; Cao, X.-M.; Li, K.; Guo, L.; Lu, J.-G.; Duan, Z.-Q.; Xiang, C.; Wei, L. Autografts vs. Synthetics for Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Orthop. Surg. 2020, 12, 378–387. [Google Scholar] [CrossRef]
- Pinna, S.; Lanzi, F.; Tassani, C.; Mian, G. Intra-articular replacement of a ruptured cranial cruciate ligament using the Mini-TightRope in the dog: A preliminary study. J. Vet. Sci. 2020, 21, e53. [Google Scholar] [CrossRef]
- Carmagnani Prada, T.; Coutinho da Silva, A.; Watanabe Minto, B. Short-term evaluation of an intra-articular technique for cranial cruciate ligament rupture in dogs using nylon or polyester. Semin. Cienc. Agrar. 2018, 39, 593–604. [Google Scholar] [CrossRef]
- Blanc, Q.; Goin, B.; Rafael, P.; Moissonnier, P.; Carozzo, C.; Buttin, P.; Cachon, T.; Viguier, E. Effect of the number of interference screws for the fixation of an intra-articular cranial cruciate ligament prosthesis in dogs: Biomechanical study. Comput. Methods Biomech. Biomed. Eng. 2019, 22, S102–S104. [Google Scholar] [CrossRef]
- Goin, B.; Buttin, P.; Lafon, Y.; Massenzio, M.; Viguier, E.; Cachon, T. Biomechanical cyclic loading test of a synthetic ligament fixation system used for intra-articular stabilization of deficient canine stifles. Open Vet. J. 2022, 12, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Goin, B.; Rafael, P.; Blanc, Q.; Cachon, T.; Buttin, P.; Carozzo, C.; Chabrand, P.; Viguier, E. Biomechanical analysis of a ligament fixation system for CCL reconstruction in a canine cadaver model. Comput. Methods Biomech. Biomed. Eng. 2019, 22, S109–S111. [Google Scholar] [CrossRef]
- Rafael, P.; Goin, B.; Buttin, P.; Cachon, T.; Viguier, E. Comparison of two methods of fixation with interference screw for cranial cruciate ligament reconstruction in canine cadaver model. Comput. Methods Biomech. Biomed. Eng. 2020, 23, S247–S249. [Google Scholar] [CrossRef]
- Fauqueux, F.; Goin, B.; Agbalé, M.; Crumière, A.J.J.; Buttin, P.; Viguier, E.; Cachon, T. Intra-articular replacement of the caudal cruciate ligament using a UHMWPE ligament under arthroscopic guidance in a dog: A case report. Open Vet. J. 2023, 13, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Buttin, P.; Vallefuoco, R.; Le Pommellet, H. Stabilization of multiple ligament-injured stiffle using an ultra-high molecular weight polyethylen (UHMWPE) ligament in one dog and three cats. In Proceedings of the 21st Congress of the European Society of Veterinary Orthopaedics and Traumatology (ESVOT), Nice, France, 21–24 September 2022; pp. 420–421. [Google Scholar]
- McGreevy, P.D.; Thomson, P.C.; Pride, C.; Fawcett, A.; Grassi, T.; Jones, B. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet. Rec. 2005, 156, 695–702. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.A.; Millis, D.L.; Levine, D.; Weigel, J.P. Variables affecting thigh girth measurement and observer reliability in dogs. Front. Vet. Sci. 2018, 5, 203. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, K.L.; Maul, D.; French, E.; Hellyer, P.W.; Vandewoude, S. Species-Specific Assessment of Pain in Laboratory Animals. J. Am. Assoc. Lab. Anim. Sci. 2003, 42, 13–20. [Google Scholar]
- Bennett, D.; May, C. Meniscal damage associated with cruciate disease in the dog. J. Small Anim. Pract. 1991, 32, 111–117. [Google Scholar] [CrossRef]
- Winkels, P.; Werner, H.; Grevel, V.; Oechtering, G.; Böttcher, P. Development and In Situ Application of an Adjustable Aiming Device to Guide Extra- to Intraarticular Tibial Tunnel Drilling for the Insertion of the Cranial Cruciate Ligament in Dogs. Vet. Surg. 2010, 39, 324–333. [Google Scholar] [CrossRef]
- Biskup, J.J.; Balogh, D.G.; Scott, R.M.; Conzemius, M.G. Long-term outcome of an intra-articular allograft technique for treatment of spontaneous cranial cruciate ligament rupture in the dog. Vet. Surg. 2017, 46, 691–699. [Google Scholar] [CrossRef]
- Cook, J.L.; Smith, P.A.; Stannard, J.P.; Pfeiffer, F.M.; Kuroki, K.; Bozynski, C.C.; Cook, C.R. A canine hybrid double-bundle model for study of arthroscopic ACL reconstruction. J. Orthop. Res. 2015, 33, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Bozynski, C.C.; Kuroki, K.; Henrich, S.M.; Wijdicks, C.A.; Cook, J.L. Intra-Articular Biocompatibility of Multistranded, Long-Chain Polyethylene Suture Tape in a Canine ACL Model. J. Knee Surg. 2019, 32, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Lu, M.; Wang, S.-N.; Wu, C.-H. In vivo three-dimensional isometry analysis of suture attachment sites for extracapsular suture stabilization of the canine stifle. Vet. Rec. 2022, 190, e560. [Google Scholar] [CrossRef] [PubMed]
- Ho-Eckart, L.K.; Seki, M.; Luizza, L.; Kearney, M.T.; Lopez, M.J. Joint stability after canine cranial cruciate ligament graft reconstruction varies among femoral fixation sites. Vet. Surg. 2017, 46, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Roe, S.C.; Kue, J.; Gemma, J. Isometry of potential suture attachment sites for the cranial cruciate ligament deficient canine stifle. Vet. Comp. Orthop. Traumatol. 2008, 21, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Cherres, M.; Grevel, V.; Oechtering, G.; Böttcher, P. Effects of Attachment Sites and Joint Angle at the Time of Lateral Suture Fixation on Tension in the Suture for Stabilization of the Cranial Cruciate Ligament Deficient Stifle in Dogs. Vet. Surg. 2010, 39, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Bolia, A.; Böttcher, P. Arthroscopic assisted femoral tunnel drilling for the intra-articular anatomic cranial cruciate ligament reconstruction in dogs. Tierärztl. Prax. Ausg. K Kleintiere/Heimtiere 2015, 43, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Bolia, A.; Winkels, P.; Böttcher, P. Radiographic location of the femoral footprint of the cranial cruciate ligament in dogs. Tierärztliche Prax. Ausg. K Kleintiere/Heimtiere 2015, 43, 23–30. [Google Scholar] [CrossRef]
- Vergis, A.; Gillquist, J. Graft failure in intra-articular anterior cruciate ligament reconstructions: A review of the literature. Arthroscopy 1995, 11, 312–321. [Google Scholar] [CrossRef]
- Rafael, P.; Goin, B.; Blanc, Q.; Buttin, P.; Cachon, T.; Viguier, E. Ex-vivo biomechanical analysis of cranial cruciate ligament in Labrador breed dogs. Comput. Methods Biomech. Biomed. Eng. 2021, 24, S150–S152. [Google Scholar] [CrossRef]
- Goin, B.; Morvan, V.; Buttin, P.; Lafon, Y.; Massenzio, M.; Viguier, E.; Cachon, T. Biomechanical comparison of two femoral fixation methods for synthetic cranial cruciate ligament reconstruction in canine cadavers. Comput. Methods Biomech. Biomed. Eng. 2021, 24, S127–S129. [Google Scholar] [CrossRef]
- Barber, F.A.; Herbert, M.A.; Beavis, R.C. Cyclic Load and Failure Behavior of Arthroscopic Knots and High Strength Sutures. Arthroscopy 2009, 25, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.C.; Pienkowski, D.; Steenlage, E.; Hamilton, D.; Johnson, D.L.; Caborn, D.N.M. Interference Screw Fixation Strength of a Quadrupled Hamstring Tendon Graft Is Directly Related to Bone Mineral Density and Insertion Torque. Am. J. Sports Med. 2000, 28, 705–710. [Google Scholar] [CrossRef]
- Kandahari, A.M.; Yang, X.; Laroche, K.A.; Dighe, A.S.; Pan, D.; Cui, Q. A review of UHMWPE wear-induced osteolysis: The role for early detection of the immune response. Bone Res. 2016, 4, 16014. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.T.; Brodbelt, D.C.; Pegram, C.; Church, D.B.; O’Neill, D.G. Life tables of annual life expectancy and mortality for companion dogs in the United Kingdom. Sci. Rep. 2022, 12, 6415. [Google Scholar] [CrossRef]
- Brioschi, V.; Arthurs, G.I. Cranial cruciate ligament rupture in small dogs (<15 kg): A narrative literature review. J. Small Anim. Pract. 2021, 62, 1037–1050. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ödman, S.; Martenne-Duplan, A.; Finck, M.; Crumière, A.; Goin, B.; Buttin, P.; Viguier, E.; Cachon, T.; Julinder, K. Intra-Articular Surgical Reconstruction of a Canine Cranial Cruciate Ligament Using an Ultra-High-Molecular-Weight Polyethylene Ligament: Case Report with Six-Month Clinical Outcome. Vet. Sci. 2024, 11, 334. https://doi.org/10.3390/vetsci11080334
Ödman S, Martenne-Duplan A, Finck M, Crumière A, Goin B, Buttin P, Viguier E, Cachon T, Julinder K. Intra-Articular Surgical Reconstruction of a Canine Cranial Cruciate Ligament Using an Ultra-High-Molecular-Weight Polyethylene Ligament: Case Report with Six-Month Clinical Outcome. Veterinary Sciences. 2024; 11(8):334. https://doi.org/10.3390/vetsci11080334
Chicago/Turabian StyleÖdman, Sven, Antonin Martenne-Duplan, Marlène Finck, Antonin Crumière, Bastien Goin, Philippe Buttin, Eric Viguier, Thibaut Cachon, and Krister Julinder. 2024. "Intra-Articular Surgical Reconstruction of a Canine Cranial Cruciate Ligament Using an Ultra-High-Molecular-Weight Polyethylene Ligament: Case Report with Six-Month Clinical Outcome" Veterinary Sciences 11, no. 8: 334. https://doi.org/10.3390/vetsci11080334
APA StyleÖdman, S., Martenne-Duplan, A., Finck, M., Crumière, A., Goin, B., Buttin, P., Viguier, E., Cachon, T., & Julinder, K. (2024). Intra-Articular Surgical Reconstruction of a Canine Cranial Cruciate Ligament Using an Ultra-High-Molecular-Weight Polyethylene Ligament: Case Report with Six-Month Clinical Outcome. Veterinary Sciences, 11(8), 334. https://doi.org/10.3390/vetsci11080334





