Effect of Dietary Composite Probiotic Supplementation on the Microbiota of Different Oral Sites in Cats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Sample Collection
2.4. Oral Microbiota Analysis
2.5. Statistical Analysis
3. Results
3.1. Oral Microbial Composition in Cats at Baseline
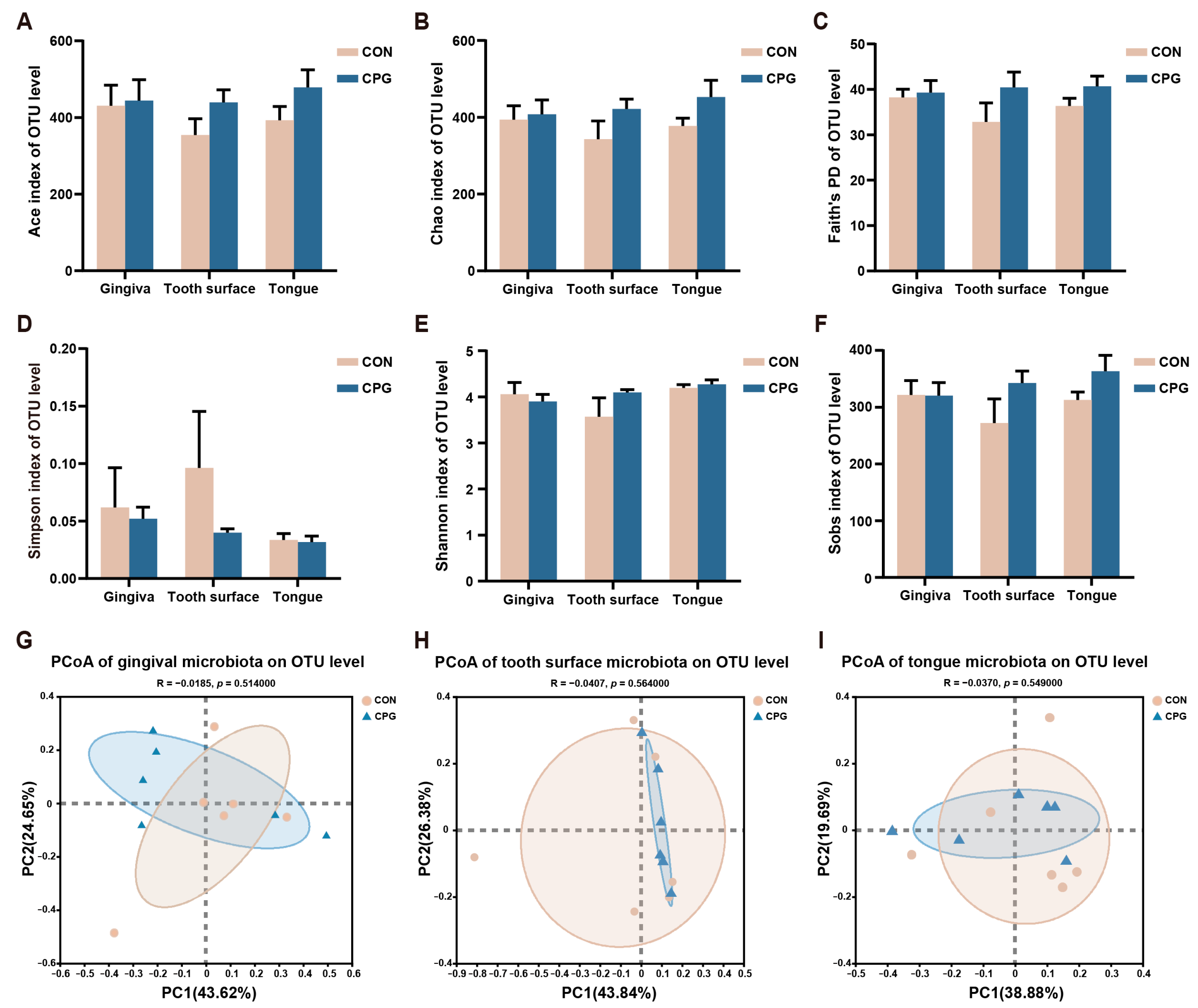
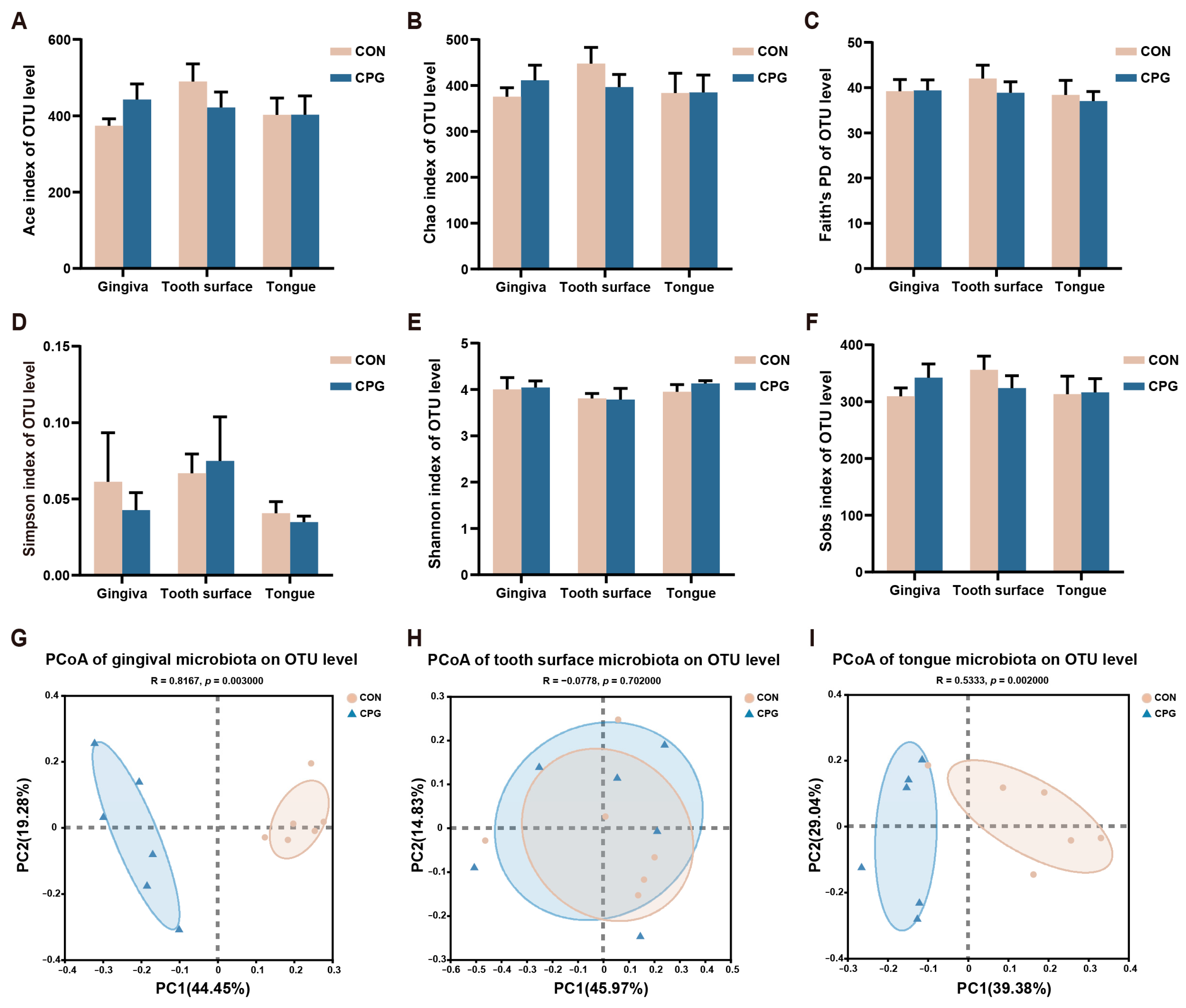
3.2. Effects of Composite Probiotics on α- and β-Diversity of Oral Microbiota in Cats
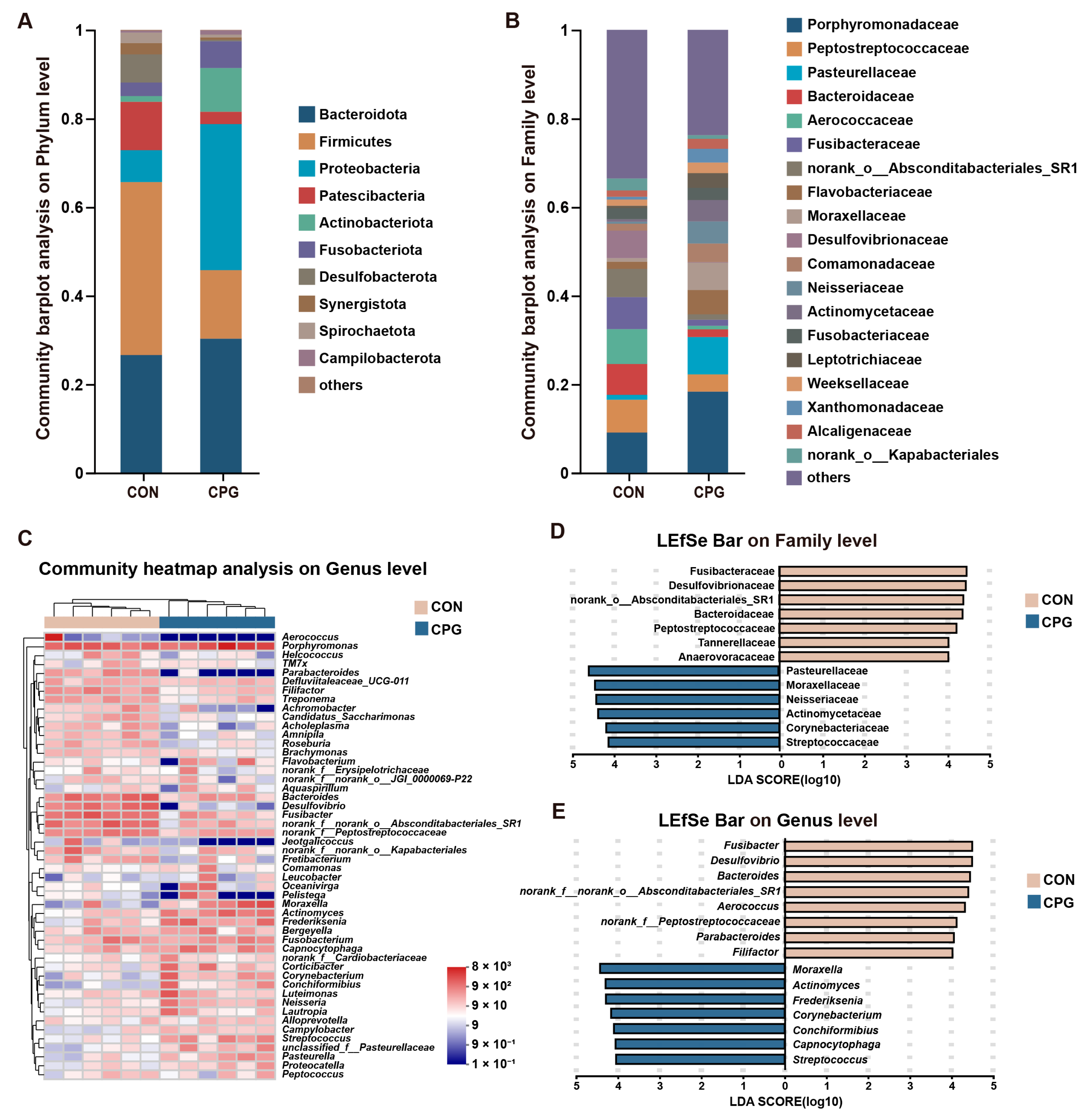
3.3. Effects of Composite Probiotics on the Composition of Gingival Microbiota in Cats
3.4. Effects of Composite Probiotics on the Composition of Tooth Surface Microbiota in Cats
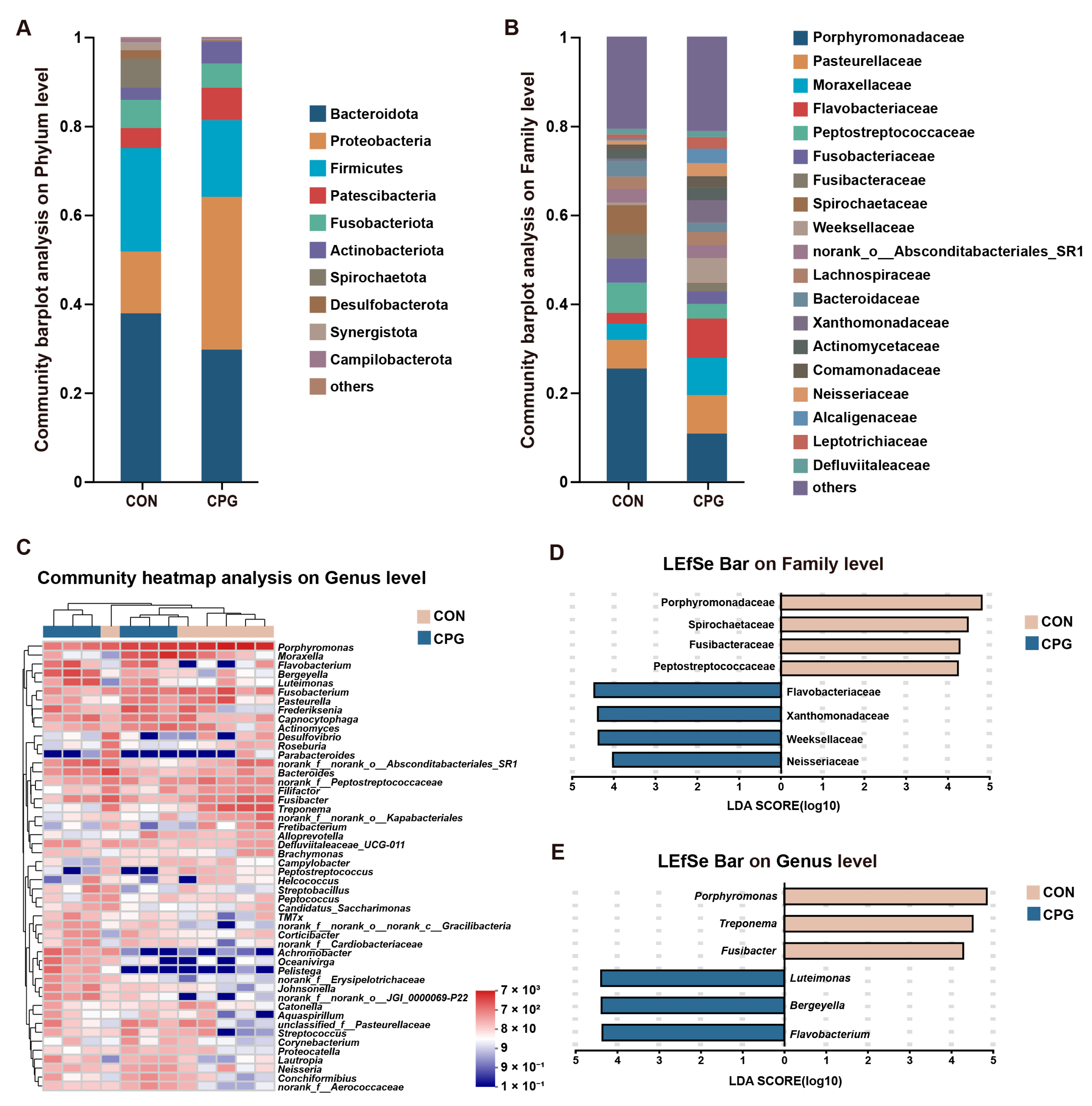
3.5. Effects of Composite Probiotics on the Composition of Tongue Microbiota in Cats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, R.; Tutt, C. Periodontal disease in cats: Back to basics—With an eye on the future. J. Feline Med. Surg. 2015, 17, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.B.; Verstraete, F.J.M.; Arzi, B. An Update on Feline Chronic Gingivostomatitis. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.X.; Bicalho, R.C.; Fiani, N.; Lima, S.F.; Peralta, S. The subgingival microbial community of feline periodontitis and gingivostomatitis: Characterization and comparison between diseased and healthy cats. Sci. Rep. 2019, 9, 12340. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.G.; Rojas, C.A.; Scarsella, E.; Entrolezo, Z.; Jospin, G.; Hoffman, S.L.; Force, J.; MacLellan, R.H.; Peak, M.; Shope, B.H.; et al. The Oral Microbiome across Oral Sites in Cats with Chronic Gingivostomatitis, Periodontal Disease, and Tooth Resorption Compared with Healthy Cats. Animals 2023, 13, 3544. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6 (Suppl. S1), S14. [Google Scholar] [CrossRef] [PubMed]
- Logan, E.I. Dietary influences on periodontal health in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 1385–1401. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.Y.; Steinbach-Rankins, J.M.; Demuth, D.R. Functional assessment of peptide-modified PLGA nanoparticles against oral biofilms in a murine model of periodontitis. J. Control. Release 2019, 297, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Wan, X.; Tao, H.; Yang, Q.; Zhao, X.; Liu, H.; Hu, J.; Luo, Y.; Shu, T.; Geng, R.; et al. Multi-function screening of probiotics to improve oral health and evaluating their efficacy in a rat periodontitis model. Front. Cell. Infect. Microbiol. 2023, 13, 1261189. [Google Scholar] [CrossRef]
- Van Holm, W.; Carvalho, R.; Delanghe, L.; Eilers, T.; Zayed, N.; Mermans, F.; Bernaerts, K.; Boon, N.; Claes, I.; Lebeer, S.; et al. Antimicrobial potential of known and novel probiotics on in vitro periodontitis biofilms. NPJ Biofilms Microbiomes 2023, 9, 3. [Google Scholar] [CrossRef]
- Araujo, L.D.C.; Furlaneto, F.A.C.; da Silva, L.A.B.; Kapila, Y.L. Use of the Probiotic Bifidobacterium animalis subsp. lactis HN019 in Oral Diseases. Int. J. Mol. Sci. 2022, 23, 9334. [Google Scholar] [CrossRef] [PubMed]
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; Novaes, A.B., Jr.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Cosme-Silva, L.; Dal-Fabbro, R.; Cintra, L.T.A.; Dos Santos, V.R.; Duque, C.; Ervolino, E.; Mogami Bomfim, S.; Gomes-Filho, J.E. Systemic administration of probiotics reduces the severity of apical periodontitis. Int. Endod. J. 2019, 52, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Korte, F.; Dörfer, C.E.; Kneist, S.; Fawzy El-Sayed, K.; Paris, S. Inhibition of Streptococcus mutans Growth and Biofilm Formation by Probiotics in vitro. Caries Res. 2017, 51, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The oral microbiome: Diversity, biogeography and human health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Mark Welch, J.L.; Ramírez-Puebla, S.T.; Borisy, G.G. Oral Microbiome Geography: Micron-Scale Habitat and Niche. Cell Host Microbe 2020, 28, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef]
- Ruparell, A.; Inui, T.; Staunton, R.; Wallis, C.; Deusch, O.; Holcombe, L.J. The canine oral microbiome: Variation in bacterial populations across different niches. BMC Microbiol. 2020, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Oba, P.M.; Carroll, M.Q.; Alexander, C.; Somrak, A.J.; Keating, S.C.J.; Sage, A.M.; Swanson, K.S. Dental chews positively shift the oral microbiota of adult dogs. J. Anim. Sci. 2021, 99, skab100. [Google Scholar] [CrossRef]
- Rober, M.; Quirynen, M.; Haffajee, A.D.; Schepers, E.; Teughels, W. Intra-oral microbial profiles of beagle dogs assessed by checkerboard DNA-DNA hybridization using human probes. Vet. Microbiol. 2008, 127, 79–88. [Google Scholar] [CrossRef][Green Version]
- Horwitz, W. (Ed.) Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Floyd, M.R. The modified Triadan system: Nomenclature for veterinary dentistry. J. Vet. Dent. 1991, 8, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mo, R.; Wang, H.; Liu, T.; Zhang, G.; Wu, Y. Grape seed proanthocyanidin improves intestinal inflammation in canine through regulating gut microbiota and bile acid compositions. FASEB J. 2023, 37, e23285. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, T.G.B.; de Oliveira, A.M.; Tsute Chen, G.; Colombo, A.P.V. Oral-gut bacterial profiles discriminate between periodontal health and diseases. J. Periodontal Res. 2022, 57, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xiao, L.; Shen, D.; Hao, Y. Competition between yogurt probiotics and periodontal pathogens in vitro. Acta Odontol. Scand. 2010, 68, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Cosme-Silva, L.; Dal-Fabbro, R.; Cintra, L.T.A.; Ervolino, E.; Plazza, F.; Mogami Bomfim, S.; Duarte, P.C.T.; Junior, V.; Gomes-Filho, J.E. Reduced bone resorption and inflammation in apical periodontitis evoked by dietary supplementation with probiotics in rats. Int. Endod. J. 2020, 53, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.G.; Carvalho, E.B.; Tinoco, E.M.B. Clinical effect of Lactobacillus on the treatment of severe periodontitis and halitosis: A double-blinded, placebo-controlled, randomized clinical trial. Am. J. Dent. 2019, 32, 9–13. [Google Scholar] [PubMed]
- Mayanagi, G.; Kimura, M.; Nakaya, S.; Hirata, H.; Sakamoto, M.; Benno, Y.; Shimauchi, H. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: A double-blinded, placebo-controlled, randomized clinical trial. J. Clin. Periodontol. 2009, 36, 506–513. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, W.; Ma, J.; Zhang, M.; Lu, X.; Liu, J.; Kou, Y. The rationale and potential for using Lactobacillus in the management of periodontitis. J. Microbiol. 2022, 60, 355–363. [Google Scholar] [CrossRef]
- Ishikawa, K.H.; Bueno, M.R.; Kawamoto, D.; Simionato, M.R.L.; Mayer, M.P.A. Lactobacilli postbiotics reduce biofilm formation and alter transcription of virulence genes of Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2021, 36, 92–102. [Google Scholar] [CrossRef]
- Imran, F.; Das, S.; Padmanabhan, S.; Rao, R.; Suresh, A.; Bharath, D. Evaluation of the efficacy of a probiotic drink containing Lactobacillus casei on the levels of periodontopathic bacteria in periodontitis: A clinico-microbiologic study. Indian J. Dent. Res. 2015, 26, 462–468. [Google Scholar] [CrossRef]
- Older, C.E.; Gomes, M.O.S.; Hoffmann, A.R.; Policano, M.D.; Reis, C.; Carregaro, A.B.; Ambrósio, C.E.; Carregaro, V.M.L. Influence of the FIV Status and Chronic Gingivitis on Feline Oral Microbiota. Pathogens 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Pyysalo, M.J.; Mishra, P.P.; Sundström, K.; Lehtimäki, T.; Karhunen, P.J.; Pessi, T. Increased tooth brushing frequency is associated with reduced gingival pocket bacterial diversity in patients with intracranial aneurysms. PeerJ 2019, 7, e6316. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.J.; Malik, R.; Browne, G.V.; Norris, J.M. Diet may influence the oral microbiome composition in cats. Microbiome 2016, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, A.; Pinder, S.L.; Costa, M.C.; Weese, J.S. Characterization of the oral microbiota of healthy cats using next-generation sequencing. Vet. J. 2014, 201, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, L.; Leylabadlo, H.E.; Pourlak, T.; Eslami, H.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Kafil, H.S. Oral spirochetes: Pathogenic mechanisms in periodontal disease. Microb. Pathog. 2020, 144, 104193. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hemme, C.; Beleno, J.; Shi, Z.J.; Ning, D.; Qin, Y.; Tu, Q.; Jorgensen, M.; He, Z.; Wu, L.; et al. Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. ISME J. 2018, 12, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, G.; Chen, M.; He, Y.; Yu, W.; Chen, X.; Mao, W.; Liu, N.; Zhang, Y.; Chang, Q.; et al. Oral microbial dysbiosis in patients with periodontitis and chronic obstructive pulmonary disease. Front. Cell. Infect. Microbiol. 2023, 13, 1121399. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Salcedo, L.; Herrera, D.; Esteban-Saltiveri, D.; León, R.; Jeusette, I.; Torre, C.; O’Connor, A.; González, I.; González, I. Isolation and identification of Porphyromonas spp. and other putative pathogens from cats with periodontal disease. J. Vet. Dent. 2013, 30, 208–213. [Google Scholar] [CrossRef]
- Lamont, R.J.; Fitzsimonds, Z.R.; Wang, H.; Gao, S. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontology 2000 2022, 89, 154–165. [Google Scholar] [CrossRef]
- Rafiei, M.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Sheikhi, A.; Zamanian Azodi, M. Study of Porphyromonas gingivalis in periodontal diseases: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran 2017, 31, 62. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Van Dyke, T.E. Periodontitis: A host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontology 2000 2013, 62, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Aruni, A.W.; Dou, Y.; Mishra, A.; Fletcher, H.M. The Biofilm Community-Rebels with a Cause. Curr. Oral Health Rep. 2015, 2, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Sisk-Hackworth, L.; Ortiz-Velez, A.; Reed, M.B.; Kelley, S.T. Compositional Data Analysis of Periodontal Disease Microbial Communities. Front. Microbiol. 2021, 12, 617949. [Google Scholar] [CrossRef] [PubMed]
- Oba, P.M.; Sieja, K.M.; Schauwecker, A.; Somrak, A.J.; Hristova, T.S.; Keating, S.C.J.; Swanson, K.S. Effects of a novel dental chew on oral health outcomes, halitosis, and microbiota of adult dogs. J. Anim. Sci. 2024, 102, skae071. [Google Scholar] [CrossRef] [PubMed]
- Dolieslager, S.M.; Riggio, M.P.; Lennon, A.; Lappin, D.F.; Johnston, N.; Taylor, D.; Bennett, D. Identification of bacteria associated with feline chronic gingivostomatitis using culture-dependent and culture-independent methods. Vet. Microbiol. 2011, 148, 93–98. [Google Scholar] [CrossRef]
- Thomas, S.; Lappin, D.F.; Nile, C.J.; Spears, J.; Bennett, D.; Brandt, B.W.; Riggio, M.P. Microbiome analysis of feline odontoclastic resorptive lesion (FORL) and feline oral health. J. Med. Microbiol. 2021, 70, 001353. [Google Scholar] [CrossRef]

| Diet Composition | % | Nutrient Content | % |
|---|---|---|---|
| Chicken meal | 54.50 | Moisture | 7.12 |
| Chicken fat | 8.00 | Crude protein | 41.65 |
| Fish oil | 2.00 | Crude fat | 20.28 |
| Tapioca | 3.00 | Crude fiber | 1.82 |
| Potato starch | 19.00 | Ash | 7.87 |
| Rice | 4.00 | ||
| Chicken liver powder | 5.00 | ||
| Alfalfa meal | 3.00 | ||
| Choline chloride | 0.30 | ||
| Salt | 0.50 | ||
| Taurine | 0.20 | ||
| Mineral complexes and vitamins 1 | 0.50 |
| Items | CON | CPG | p-Value |
|---|---|---|---|
| Gingiva | |||
| Desulfomicrobiaceae | 0.011 ± 0.004 | 0.096 ± 0.046 | 0.03 |
| Caulobacteraceae | 0 | 0.022 ± 0.013 | 0.03 |
| Parabacteroides | 1.743 ± 1.295 | 0.044 ± 0.028 | 0.03 |
| Granulicatella | 0.201 ± 0.156 | 0 | 0.01 |
| Desulfomicrobium | 0.011 ± 0.004 | 0.096 ± 0.046 | 0.03 |
| unclassified_f_Anaerovoracaceae | 0.071 ± 0.046 | 0 | 0.03 |
| norank_f_Propionibacteriaceae | 0.004 ± 0.004 | 0.031 ± 0.011 | 0.03 |
| Tooth surface | |||
| Campilobacterota | 0.326 ± 0.284 | 0.839 ± 0.276 | 0.04 |
| unclassified_c_Gammaproteobacteria | 0.030 ± 0.015 | 0.209 ± 0.083 | 0.008 |
| Frederiksenia | 1.423 ± 0.644 | 4.214 ± 0.797 | 0.03 |
| unclassified_c_Gammaproteobacteria | 0.030 ± 0.015 | 0.209 ± 0.083 | 0.008 |
| norank_f_Pasteurellaceae | 0.004 ± 0.002 | 0.018 ± 0.005 | 0.03 |
| Tongue | |||
| Synergistota | 0.770 ± 0.424 | 0.177 ± 0.058 | 0.04 |
| Synergistaceae | 0.770 ± 0.424 | 0.177 ± 0.058 | 0.04 |
| Fretibacterium | 0.767 ± 0.423 | 0.176 ± 0.059 | 0.04 |
| unclassified_f_Lachnospiraceae | 0.042 ± 0.018 | 0.145 ± 0.026 | 0.03 |
| Prevotellaceae_UCG-003 | 0.079 ± 0.038 | 0.003 ± 0.003 | 0.004 |
| Items | CON | CPG | p-Value |
|---|---|---|---|
| Firmicutes | 39.074 ± 5.236 | 15.495 ± 2.310 | 0.005 |
| Proteobacteria | 7.181 ± 1.415 | 32.968 ± 6.508 | 0.008 |
| Patescibacteria | 10.917 ± 1.415 | 2.783 ± 0.804 | 0.005 |
| Actinobacteriota | 1.307 ± 0.252 | 9.877 ± 2.152 | 0.005 |
| Desulfobacterota | 6.355 ± 1.197 | 0.322 ± 0.112 | 0.005 |
| Spirochaetota | 2.344 ± 0.282 | 0.644 ± 0.230 | 0.008 |
| Peptostreptococcaceae | 7.414 ± 0.652 | 3.926 ± 0.886 | 0.02 |
| Pasteurellaceae | 1.107 ± 0.411 | 8.409 ± 1.961 | 0.005 |
| Bacteroidaceae | 6.970 ± 1.566 | 1.727 ± 0.511 | 0.02 |
| Fusibacteraceae | 7.240 ± 1.177 | 1.369 ± 0.549 | 0.005 |
| norank_o_Absconditabacteriales_SR1 | 6.380 ± 1.326 | 1.222 ± 0.418 | 0.008 |
| Moraxellaceae | 0.822 ± 0.788 | 6.179 ± 2.563 | 0.03 |
| Desulfovibrionaceae | 6.192 ± 1.214 | 0.111 ± 0.081 | 0.005 |
| Neisseriaceae | 0.430 ± 0.148 | 5.002 ± 2.589 | 0.005 |
| Actinomycetaceae | 0.565 ± 0.156 | 4.832 ± 1.068 | 0.005 |
| Corynebacteriaceae | 0.218 ± 0.108 | 2.822 ± 1.379 | 0.008 |
| Streptococcaceae | 0.257 ± 0.090 | 2.764 ± 0.652 | 0.01 |
| Anaerovoracaceae | 2.690 ± 0.624 | 0.300 ± 0.101 | 0.005 |
| Tannerellaceae | 2.641 ± 0.496 | 0.330 ± 0.137 | 0.005 |
| Bacteroides | 6.970 ± 1.566 | 1.727 ± 0.511 | 0.02 |
| Fusibacter | 7.240 ± 1.177 | 1.369 ± 0.549 | 0.005 |
| Aerococcus | 7.636 ± 7.616 | 0 | 0.002 |
| norank_f_norank_o_ Absconditabacteriales_SR1 | 6.380 ± 1.326 | 1.222 ± 0.418 | 0.008 |
| Moraxella | 0.818 ± 0.788 | 6.086 ± 2.582 | 0.03 |
| Desulfovibrio | 6.187 ± 1.214 | 0.111 ± 0.081 | 0.005 |
| norank_f_Peptostreptococcaceae | 4.206 ± 0.521 | 1.558 ± 0.267 | 0.005 |
| Actinomyces | 0.565 ± 0.156 | 4.832 ± 1.068 | 0.005 |
| Frederiksenia | 0.688 ± 0.319 | 4.675 ± 1.473 | 0.02 |
| Capnocytophaga | 1.212 ± 0.406 | 3.532 ± 0.967 | 0.04 |
| Filifactor | 2.722 ± 0.540 | 0.719 ± 0.161 | 0.005 |
| Corynebacterium | 0.218 ± 0.108 | 2.822 ± 1.379 | 0.008 |
| Streptococcus | 0.257 ± 0.090 | 2.764 ± 0.652 | 0.01 |
| Parabacteroides | 2.362 ± 0.468 | 0.038 ± 0.038 | 0.004 |
| Conchiformibius | 0.057 ± 0.024 | 2.266 ± 1.236 | 0.005 |
| Items | CON | CPG | p-Value |
|---|---|---|---|
| Weeksellaceae | 1.499 ± 0.822 | 4.919 ± 1.207 | 0.04 |
| Lachnospiraceae | 1.626 ± 0.182 | 0.904 ± 0.184 | 0.04 |
| Streptococcaceae | 0.170 ± 0.053 | 0.675 ± 0.220 | 0.04 |
| Campylobacteraceae | 0.600 ± 0.183 | 0.152 ± 0.090 | 0.04 |
| Lentimicrobiaceae | 0.037 ± 0.015 | 0.001 ± 0.001 | 0.02 |
| Bergeyella | 1.379 ± 0.837 | 4.612 ± 1.282 | 0.04 |
| Catonella | 0.887 ± 0.227 | 0.227 ± 0.057 | 0.02 |
| Helcococcus | 0.632 ± 0.138 | 0.259 ± 0.081 | 0.04 |
| Streptococcus | 0.167 ± 0.052 | 0.673 ± 0.221 | 0.04 |
| Campylobacter | 0.600 ± 0.183 | 0.152 ± 0.090 | 0.04 |
| Lentimicrobium | 0.037 ± 0.015 | 0.001 ± 0.001 | 0.02 |
| Items | CON | CPG | p-Value |
|---|---|---|---|
| Proteobacteria | 13.900 ± 4.604 | 34.358 ± 2.901 | 0.01 |
| Spirochaetota | 6.544 ± 1.704 | 0.186 ± 0.036 | 0.005 |
| Porphyromonadaceae | 25.328 ± 4.148 | 10.671 ± 2.892 | 0.02 |
| Flavobacteriaceae | 2.465 ± 0.984 | 8.788 ± 1.762 | 0.01 |
| Peptostreptococcaceae | 6.767 ± 0.824 | 3.280 ± 0.797 | 0.03 |
| Fusibacteraceae | 5.448 ± 1.175 | 1.739 ± 0.477 | 0.03 |
| Spirochaetaceae | 6.536 ± 1.704 | 0.185 ± 0.036 | 0.005 |
| Weeksellaceae | 0.595 ± 0.261 | 5.599 ± 2.121 | 0.01 |
| Xanthomonadaceae | 0.439 ± 0.293 | 5.057 ± 1.765 | 0.01 |
| Neisseriaceae | 0.916 ± 0.324 | 2.909 ± 0.545 | 0.02 |
| Porphyromonas | 25.328 ± 4.148 | 10.671 ± 2.892 | 0.02 |
| Fusibacter | 5.448 ± 1.175 | 1.739 ± 0.477 | 0.03 |
| Treponema | 6.504 ± 1.696 | 0.176 ± 0.036 | 0.005 |
| Bergeyella | 0.413 ± 0.229 | 5.222 ± 2.128 | 0.03 |
| Flavobacterium | 0.422 ± 0.367 | 5.058 ± 1.697 | 0.01 |
| Luteimonas | 0.419 ± 0.294 | 4.718 ± 1.790 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Cui, Y.; Mei, X.; Li, L.; Wang, H.; Li, Y.; Wu, Y. Effect of Dietary Composite Probiotic Supplementation on the Microbiota of Different Oral Sites in Cats. Vet. Sci. 2024, 11, 351. https://doi.org/10.3390/vetsci11080351
Zhang M, Cui Y, Mei X, Li L, Wang H, Li Y, Wu Y. Effect of Dietary Composite Probiotic Supplementation on the Microbiota of Different Oral Sites in Cats. Veterinary Sciences. 2024; 11(8):351. https://doi.org/10.3390/vetsci11080351
Chicago/Turabian StyleZhang, Mingrui, Yingyue Cui, Xiaoying Mei, Longxian Li, Haotian Wang, Yingying Li, and Yi Wu. 2024. "Effect of Dietary Composite Probiotic Supplementation on the Microbiota of Different Oral Sites in Cats" Veterinary Sciences 11, no. 8: 351. https://doi.org/10.3390/vetsci11080351
APA StyleZhang, M., Cui, Y., Mei, X., Li, L., Wang, H., Li, Y., & Wu, Y. (2024). Effect of Dietary Composite Probiotic Supplementation on the Microbiota of Different Oral Sites in Cats. Veterinary Sciences, 11(8), 351. https://doi.org/10.3390/vetsci11080351









