Pathogen Detection in Early Phases of Experimental Bovine Tuberculosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Inoculum, and Mycobacterium bovis Aerosol Challenge
2.2. Nasal Swabs and Saliva Collection
2.3. Blood Collection and PCR
2.4. Interferon Gamma Release Assay
2.5. IDEXX M. bovis ELISA
2.6. Necropsy and Lesion Scoring
2.7. Statistical Analysis
3. Results
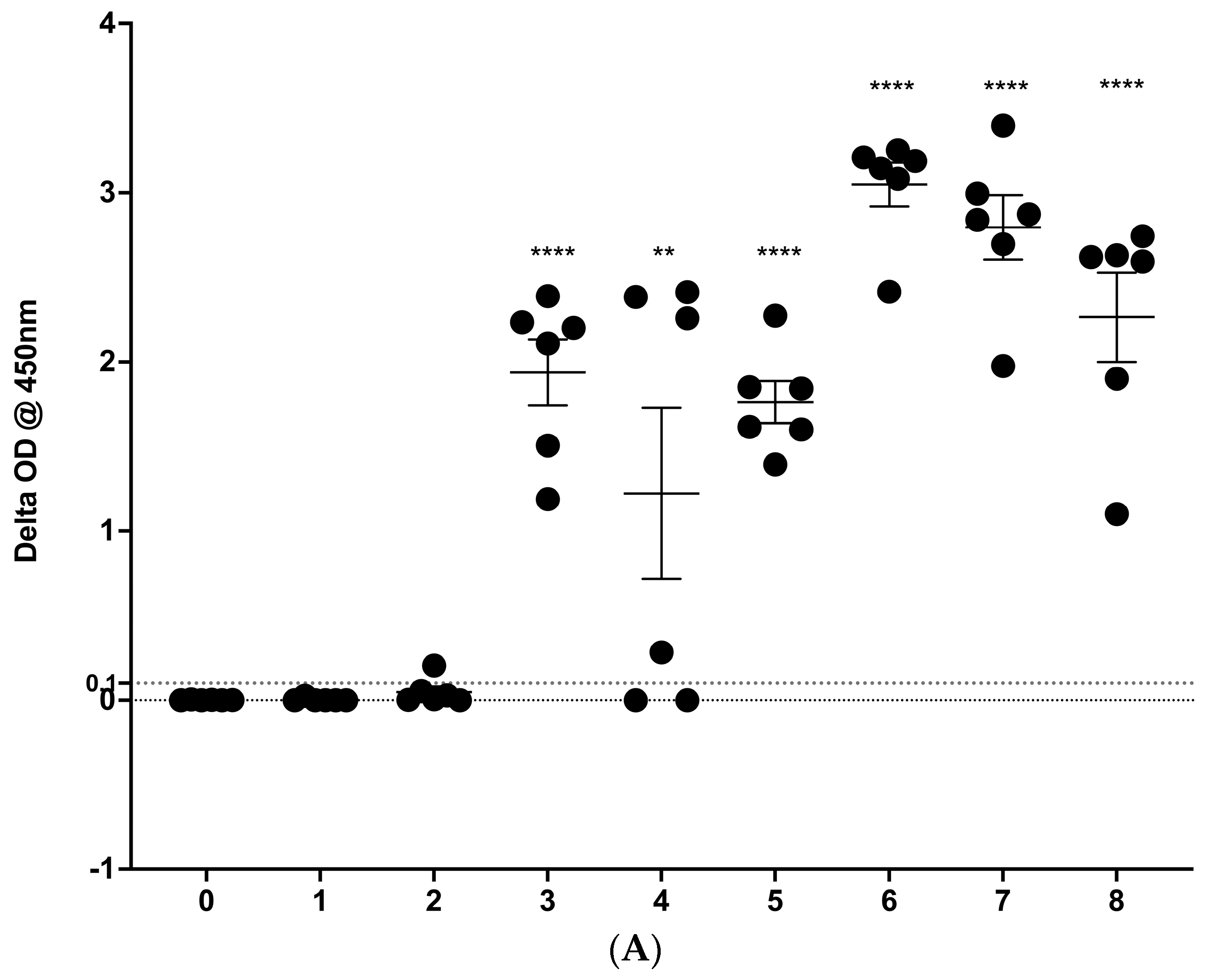
3.1. IGRA Results
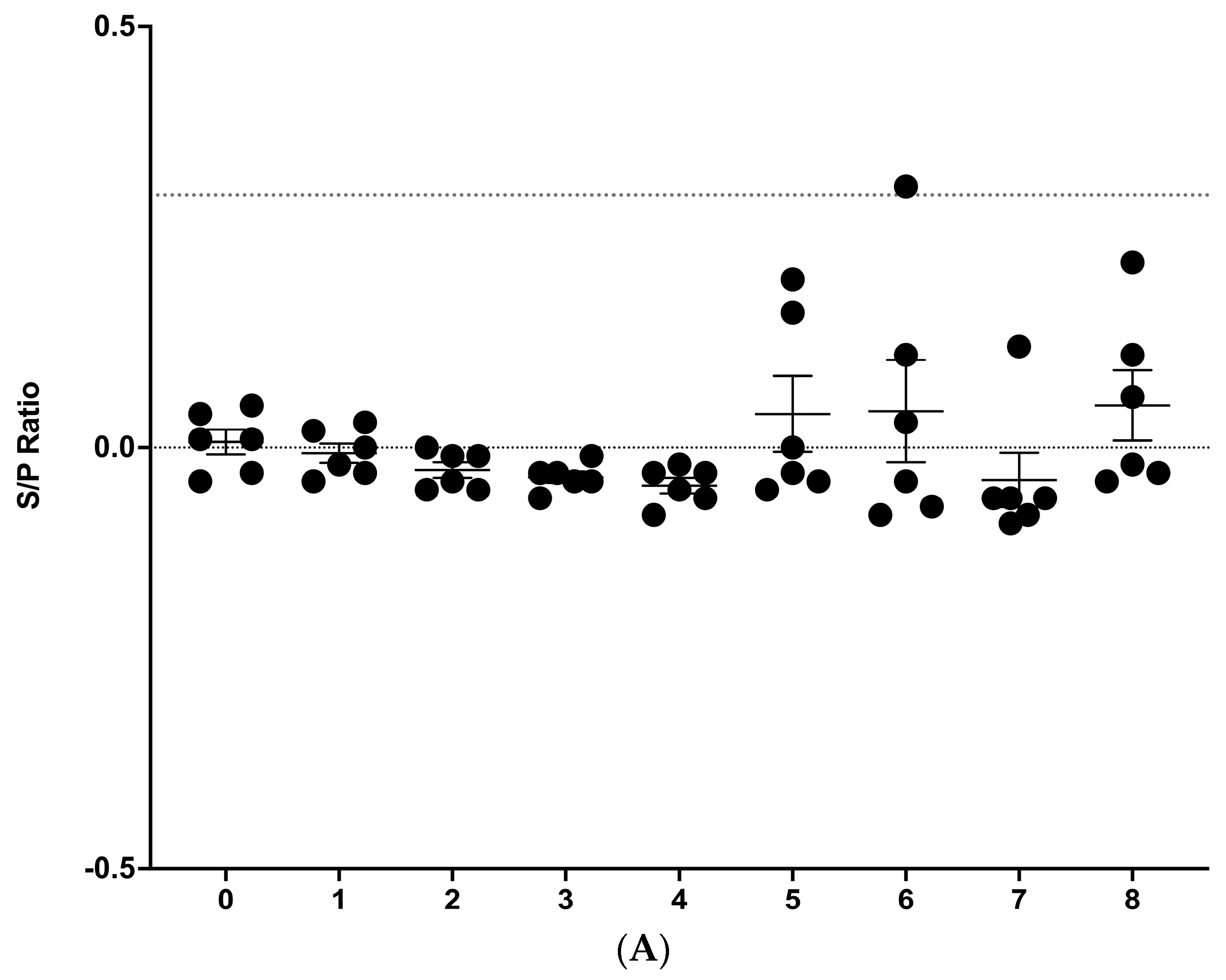
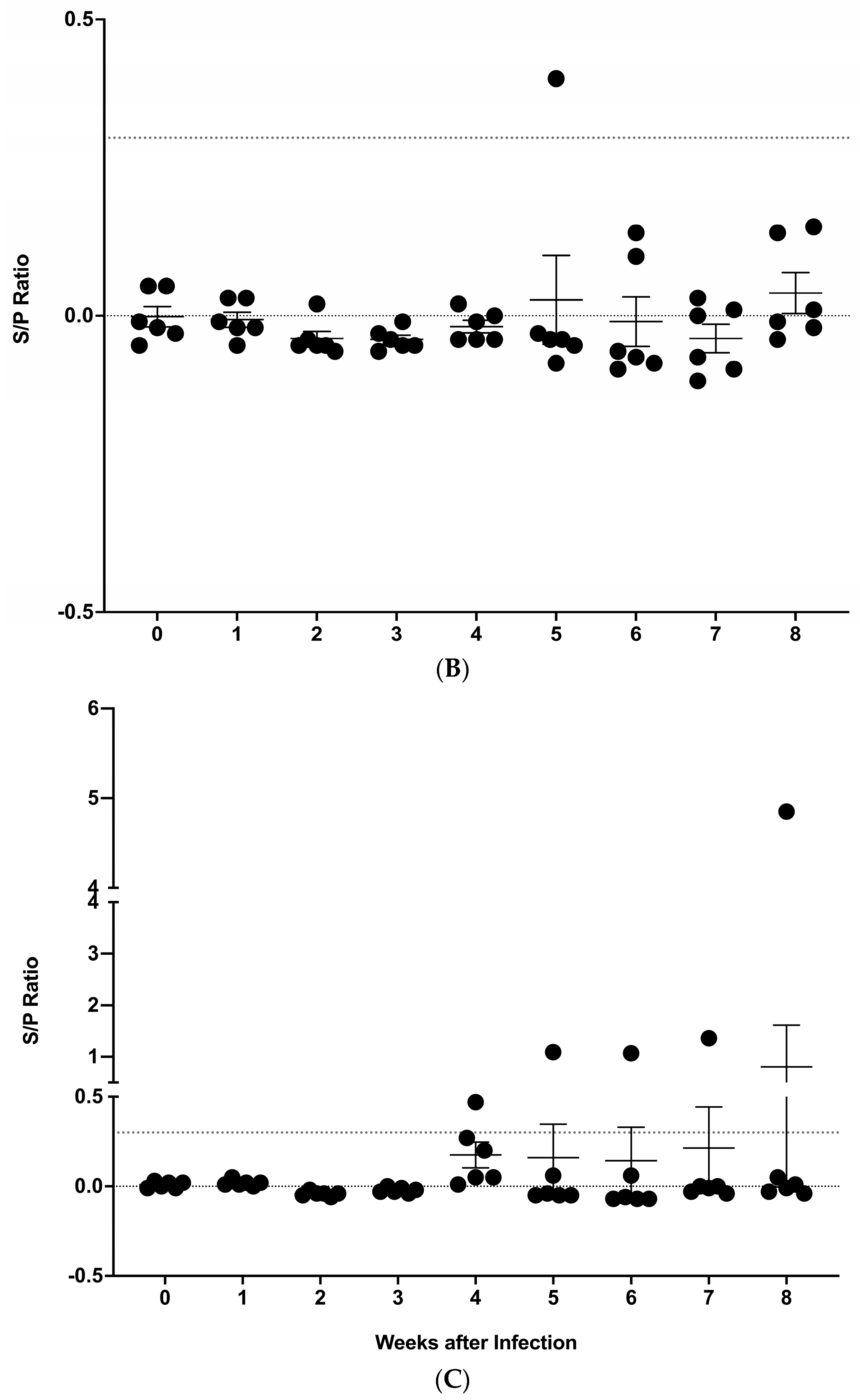
3.2. IDEXX M. bovis ELISA
3.3. PCR Results from Nasal Swabs, Saliva and Blood
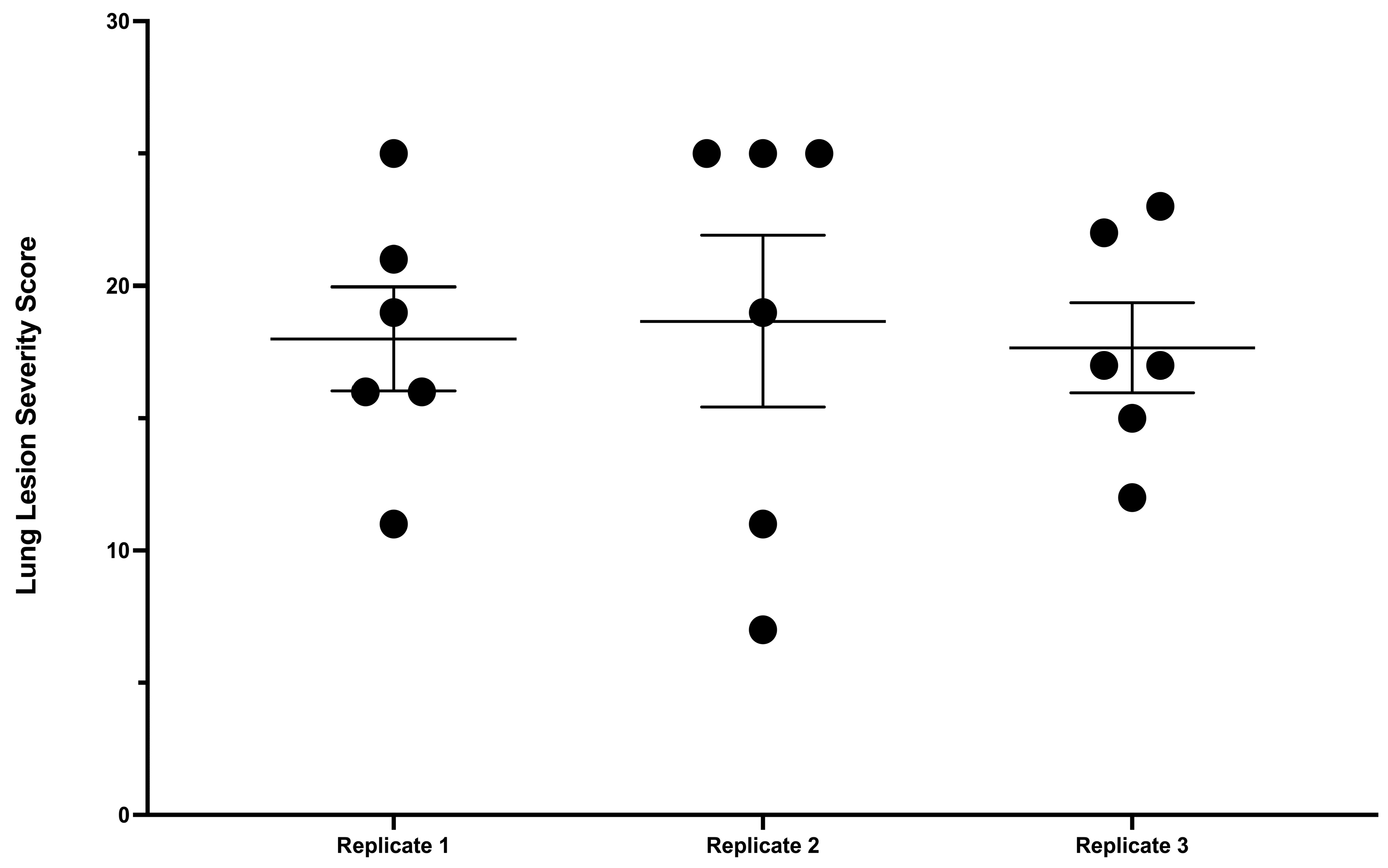
3.4. Lesion Scoring and Quantitative Culture
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sizemore, C.; Lacourciere, K.; Parker, T. The Many Hosts of Mycobacteria: An Interdisciplinary Approach to Understanding Mycobacterial Diseases. In Tuberculosis, Leprosy and Mycobacterial Diseases of Man and Animals; Mukundan, H., Chambers, M.A., Waters, R.W., Larsen, M.H., Eds.; CABI: Boston, MA, USA, 2015; pp. xv–xvii. [Google Scholar]
- Langer, A.; LoBue, P.A. Public health significance of zoonotic tuberculosis in animals and humans. In Zoonotic Tuberculosis: Mycobacterium bovis and Other Pathogenic Mycobacteria, 3rd ed.; Thoen, C.O., Steele, J.H., Kaneene, J.B., Eds.; Wiley Blackwell: Ames, IA, USA, 2014; pp. 21–34. [Google Scholar]
- Olmstead, A.; Rhode, P. An impossible undertaking the eradication of bovine TB from the US. J. Econ. Hist. 2004, 64, 734–772. [Google Scholar] [CrossRef]
- Rodwell, T.C.; Kapasi, A.J.; Moore, M.; Milian-Suazo, F.; Harris, B.; Guerrero, L.P.; Moser, K.; Strathdee, S.A.; Garfein, R.S. Tracing the origins of Mycobacterium bovis tuberculosis in humans in the USA to cattle in Mexico using spoligotyping. Int. J. Infect. Dis. 2010, 14, e129–e135. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Singhal, A.; Chauhan, D.S.; Katoch, V.M.; Srivastava, K.; Thakral, S.S.; Bharadwaj, S.S.; Sreenivas, V.; Prasad, H.K. Direct detection and identification of Mycobacterium tuberculosis and Mycobacterium bovis in bovine samples by a novel nested PCR assay: Correlation with conventional techniques. J. Clin. Microbiol. 2005, 43, 5670–5678. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.J.; Foster, C.R.; Morris, P.A.; Teverson, R. The transmission of Mycobacterium bovis infection to cattle. Res. Vet. Sci. 2003, 74, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.P.; Bryson, D.G.; Pollock, J.M.; Evans, R.T.; Forster, F.; Neill, S.D. Early lesion formation in cattle experimentally infected with Mycobacterium bovis. J. Comp. Pathol. 1998, 119, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.P.; Bryson, D.G.; Pollock, J.M.; Evans, R.T.; Forster, F.; Neill, S.D. Lesions in cattle exposed to Mycobacterium bovis-inoculated calves. J. Comp. Pathol. 1999, 121, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.D.; Hanna, J.; O’Brien, J.J.; McCracken, R.M. Transmission of tuberculosis from experimentally infected cattle to in-contact calves. Vet. Rec. 1989, 124, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.D.; Hanna, J.; O’Brien, J.J.; McCracken, R.M. Excretion of Mycobacterium bovis by experimentally infected cattle. Vet. Rec. 1988, 123, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.D.; O’Brien, J.J.; Hanna, J. A mathematical model for Mycobacterium bovis excretion from tuberculous cattle. Vet. Microbiol. 1991, 28, 103–109. [Google Scholar] [CrossRef]
- Palmer, M.V.; Thacker, T.C.; Waters, W.R. Analysis of cytokine gene expression using a novel chromogenic in-situ hybridization method in pulmonary granulomas of cattle infected experimentally by aerosolized Mycobacterium bovis. J. Comp. Pathol. 2015, 153, 150–159. [Google Scholar] [CrossRef]
- Waters, W.R.; Thacker, T.C.; Nelson, J.T.; DiCarlo, D.M.; Maggioli, M.F.; Greenwald, R.; Esfandiari, J.; Lyashchenko, K.P.; Palmer, M.V. Virulence of two strains of Mycobacterium bovis in cattle following aerosol infection. J. Comp. Pathol. 2014, 151, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.V.; Waters, W.R.; Whipple, D.L. Aerosol delivery of virulent Mycobacterium bovis to cattle. Tuberculosis 2002, 82, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.H.; Biermann, K.; Jacobs, W.R., Jr. Laboratory maintenance of Mycobacterium tuberculosis. Curr. Protoc. Microbiol. 2007, 6, 10A.1.1–10A.1.8. [Google Scholar] [CrossRef] [PubMed]
- Garber, J.C. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; p. 220. [Google Scholar]
- FASS. Guide for the Care and Use of Agricultural Animals in Research and Teaching, 3rd ed.; Federation of Animal Science Societies: Champaign, IL, USA, 2010; p. 164. [Google Scholar]
- Palmer, M.V.; Thacker, T.C.; Waters, W.R.; Robbe-Austerman, S.; Harris, B. Investigations on deer to deer and deer to cattle transmission of the vaccine Mycobacterium bovis Bacillus Calmette-Guerin (BCG). J. Vaccines Vaccin. 2010, 1, 104. [Google Scholar] [CrossRef]
- Hines, N.; Payeur, J.B.; Hoffman, L.J. Comparison of the recovery of Mycobacterium bovis isolates using the BACTEC MGIT 960 system, BACTEC 460 system, and Middlebrook 7H10 and 7H11 solid media. J. Vet. Diagn. Investig. 2006, 18, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Eisenach, K.D.; Cave, M.D.; Bates, J.H.; Crawford, J.T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J. Infect. Dis. 1990, 161, 977–981. [Google Scholar] [CrossRef]
- Roth, A.; Reischl, U.; Streubel, A.; Naumann, L.; Kroppenstedt, R.M.; Habicht, M.; Fischer, M.; Mauch, H. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S-23S rRNA gene spacer and restriction endonucleases. J. Clin. Microbiol. 2000, 38, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Thacker, T.C.; Harris, B.; Palmer, M.V.; Waters, W.R. Improved specificity for detection of Mycobacterium bovis in fresh tissues using IS6110 real-time PCR. BMC Vet. Res. 2011, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, S.G.; Neill, S.D.; McCracken, R.M. Pulmonary lesions and Mycobacterium bovis excretion from the respiratory tract of tuberculin reacting cattle. Vet. Rec. 1986, 118, 718–721. [Google Scholar] [CrossRef]
- Neill, S.D.; O’Brien, J.J.; McCracken, R.M. Mycobacterium bovis in the anterior respiratory tracts in the heads of tuberculin-reacting cattle. Vet. Rec. 1988, 122, 184–186. [Google Scholar] [CrossRef]
- Holder, T.; Srinivasan, S.; McGoldrick, A.; Williams, G.A.; Palmer, S.; Clarke, J.; O’Brien, A.; Conlan, A.J.K.; Juleff, N.; Vordermeier, H.M.; et al. Temporal dynamics of the early immune response following Mycobacterium bovis infection of cattle. Sci. Rep. 2024, 14, 2600. [Google Scholar] [CrossRef] [PubMed]
- Vordermeier, H.M.; Chambers, M.A.; Cockle, P.J.; Whelan, A.O.; Simmons, J.; Hewinson, R.G. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 2002, 70, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Ghebremariam, M.K.; Rutten, V.P.; Vernooij, J.C.; Uqbazghi, K.; Tesfaalem, T.; Butsuamlak, T.; Idris, A.M.; Nielen, M.; Michel, A.L. Prevalence and risk factors of bovine tuberculosis in dairy cattle in Eritrea. BMC Vet. Res. 2016, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Kazwala, R.R.; Kambarage, D.M.; Daborn, C.J.; Nyange, J.F.; Jiwa, S.F.H.; Sharp, J.M. Risk factors associated with the occurrence of bovine tuberculosis in cattle in the Southern Highlands of Tanzania. Vet. Res. Commun. 2001, 25, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.K.S.; Rumi, T.B.; Kabir, S.M.L.; van der Zanden, A.G.M.; Kapur, V.; Rahman, A.; Ward, M.P.; Bakker, D.; Ross, A.G.; Rahim, Z. Bovine tuberculosis prevalence and risk factors in selected districts of Bangladesh. PLoS ONE 2020, 15, e0241717. [Google Scholar] [CrossRef] [PubMed]
- Smith, T. Certain aspects of natural and acquired resistance to tuberculosis. J. Am. Med. Assoc. 1917, 68, 669–674. [Google Scholar] [CrossRef]
- Oliver, S.P.; Sordillo, L.M. Udder health in the periparturient period. J. Dairy Sci. 1988, 71, 2584–2606. [Google Scholar] [CrossRef] [PubMed]
- Burvenich, C.; Bannerman, D.D.; Lippolis, J.D.; Peelman, L.; Nonnecke, B.J.; Kehrli, M.E., Jr.; Paape, M.J. Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period. J. Dairy Sci. 2007, 90 (Suppl. 1), E39–E54. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.F. Stress and immunity: A unifying concept. Vet. Immunol. Immunopathol. 1989, 20, 263–312. [Google Scholar] [CrossRef]
- Maggioli, M.F. A bloody evidence: Is Mycobacterium bovis bacteraemia frequent in cattle? Virulence 2016, 7, 748–750. [Google Scholar] [CrossRef]
- Encinas, M.; Muniz, X.F.; Sammarruco, R.A.; Menna, V.R.; Garro, C.J.; Delgado, F.; Macias, A.; Magnano, G.; Zumarraga, M.; Garbaccio, S.; et al. Limited usefulness of the IS6110 touchdown-PCR in blood for tuberculin skin test false-negative cattle with serological response to Mycobacterium bovis. Front. Vet. Sci. 2024, 11, 1359205. [Google Scholar] [CrossRef] [PubMed]
- Lepper, A.W.; Corner, L.A.; Pearson, C.W. Serological responses in experimental bovine tuberculosis. Aust. Vet. J. 1977, 53, 301–305. [Google Scholar] [CrossRef]
- Srivastava, K.; Chauhan, D.S.; Gupta, P.; Singh, H.B.; Sharma, V.D.; Yadav, V.S.; Sreekumaran; Thakral, S.S.; Dharamdheeran, J.S.; Nigam, P.; et al. Isolation of Mycobacterium bovis & M. tuberculosis from cattle of some farms in north India-possible relevance in human health. Indian J. Med. Res. 2008, 128, 26–31. [Google Scholar] [PubMed]
- Elsohaby, I.; Ahmed, H.A.; El-Diasty, M.M.; Elgedawy, A.A.; Mahrous, E.; El Hofy, F.I. Serological and molecular evidence of Mycobacterium bovis in dairy cattle and dairy farm workers under the intensive dairy production system in Egypt. J. Appl. Microbiol. 2020, 129, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Elsohaby, I.; Mahmmod, Y.S.; Mweu, M.M.; Ahmed, H.A.; El-Diasty, M.M.; Elgedawy, A.A.; Mahrous, E.; El Hofy, F.I. Accuracy of PCR, mycobacterial culture and interferon-gamma assays for detection of Mycobacterium bovis in blood and milk samples from Egyptian dairy cows using Bayesian modelling. Prev. Vet. Med. 2020, 181, 105054. [Google Scholar] [CrossRef] [PubMed]
- Cezar, R.D.; Lucena-Silva, N.; Filho, A.F.; Borges Jde, M.; de Oliveira, P.R.; Lucio, E.C.; Arruda-Lima, M.; Santana, V.L.; Pinheiro Junior, J.W. Molecular detection of Mycobacterium bovis in cattle herds of the state of Pernambuco, Brazil. BMC Vet. Res. 2016, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Brahma, D.; Narang, D.; Chandra, M.; Singh, S.T. Comparison of multiplex and ordinary PCR for diagnosis of paratuberculosis and tuberculosis in blood samples of cattle and buffaloes. Iran. J. Vet. Res. 2020, 21, 52–56. [Google Scholar] [PubMed]
- Swift, B.M.; Convery, T.W.; Rees, C.E. Evidence of Mycobacterium tuberculosis complex bacteraemia in intradermal skin test positive cattle detected using phage-RPA. Virulence 2016, 7, 779–788. [Google Scholar] [CrossRef]
- Ritacco, V.; de Kantor, I.N.; Barrera, L.; Nader, A.; Bernardelli, A.; Torrea, G.; Errico, F.; Fliess, E. Assessment of the sensitivity and specificity of enzyme-linked immunosorbent assay (ELISA) for the detection of mycobacterial antibodies in bovine tuberculosis. Zentralbl Vet. B 1987, 34, 119–125. [Google Scholar] [CrossRef]


| Animal | Infection Status | Replicate | Weeks Post-Infection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| 68917 | Infected | 2 | ND | ND | ND | ND | ND | ND | ND | ND | 36.26 |
| 68211 | Infected | 2 | ND | ND | ND | ND | ND | 36.19 | ND | ND | ND |
| 69261 | Infected | 1 | ND | ND | ND | ND | ND | ND | 36.15 | ND | ND |
| 68779 | Infected | 3 | ND | ND | ND | ND | ND | ND | 36.25 | ND | ND |
| Animal | Infection Status | Replicate | Weeks Post-Infection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| 68392 | Infected | 3 | ND | 37.49 | ND | ND | ND | ND | ND | ND | ND |
| 68299 | Infected | 1 | ND | ND | ND | ND | ND | 37.14 | ND | ND | ND |
| 66429 | Infected | 1 | ND | ND | ND | ND | ND | ND | ND | 34.67 | ND |
| 68917 | Infected | 2 | ND | ND | ND | ND | ND | ND | ND | ND | 36.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmer, M.V.; Kanipe, C.; Hwang, S.; Thacker, T.C.; Lehman, K.A.; Ledesma, N.A.; Gustafson, K.K.; Boggiatto, P.M. Pathogen Detection in Early Phases of Experimental Bovine Tuberculosis. Vet. Sci. 2024, 11, 357. https://doi.org/10.3390/vetsci11080357
Palmer MV, Kanipe C, Hwang S, Thacker TC, Lehman KA, Ledesma NA, Gustafson KK, Boggiatto PM. Pathogen Detection in Early Phases of Experimental Bovine Tuberculosis. Veterinary Sciences. 2024; 11(8):357. https://doi.org/10.3390/vetsci11080357
Chicago/Turabian StylePalmer, Mitchell V., Carly Kanipe, Soyoun Hwang, Tyler C. Thacker, Kimberly A. Lehman, Nicholas A. Ledesma, Kristophor K. Gustafson, and Paola M. Boggiatto. 2024. "Pathogen Detection in Early Phases of Experimental Bovine Tuberculosis" Veterinary Sciences 11, no. 8: 357. https://doi.org/10.3390/vetsci11080357





