Simple Summary
In fish farming, diseases have commonly been fought with the abusive use of antibiotics, which have caused antibiotic (multi)resistances in bacteria. Consequently, it is necessary to explore safe and environmentally friendly alternative approaches to improve the fish health and avoid the treatment of bacterial diseases with antibiotics such as the use of probiotics. This study focuses on bioinformatic and functional analyses of the genome sequences of Streptococcus salivarius MDI13 and Latilactobacillus sakei MEI5, two Lactic Acid Bacteria (LAB) isolated from the gut of European hakes (Merluccius merluccius, L.), a highly valued marine fish for Spanish gastronomy. The potential probiotic characteristics of both bacteria, and the lack of antibiotic resistance genes and virulence were confirmed. In addition, genes encoding three antimicrobial peptides (known as bacteriocins) were identified in the genome of S. salivarius MDI13. One of these in vitro-synthesized bacteriocins (BlpK) showed antimicrobial activity against two fish pathogens (namely Lactococcus garvieae and Streptococcus parauberis). Altogether, our results suggest that S. salivarius MDI13 and L. sakei MEI5 have a strong potential as probiotics to prevent bacterial diseases in fish farming.
Abstract
Frequently, diseases in aquaculture have been fought indiscriminately with the use of antibiotics, which has led to the development and dissemination of (multiple) antibiotic resistances in bacteria. Consequently, it is necessary to look for alternative and complementary approaches to chemotheraphy that are safe for humans, animals, and the environment, such as the use of probiotics in fish farming. The objective of this work was the Whole-Genome Sequencing (WGS) and bioinformatic and functional analyses of S. salivarius MDI13 and L. sakei MEI5, two LAB strains isolated from the gut of commercial European hakes (M. merluccius, L.) caught in the Northeast Atlantic Ocean. The WGS and bioinformatic and functional analyses confirmed the lack of transferable antibiotic resistance genes, the lack of virulence and pathogenicity issues, and their potentially probiotic characteristics. Specifically, genes involved in adhesion and aggregation, vitamin biosynthesis, and amino acid metabolism were detected in both strains. In addition, genes related to lactic acid production, active metabolism, and/or adaptation to stress and adverse conditions in the host gastrointestinal tract were detected in L. sakei MEI5. Moreover, a gene cluster encoding three bacteriocins (SlvV, BlpK, and BlpE) was identified in the genome of S. salivarius MDI13. The in vitro-synthesized bacteriocin BlpK showed antimicrobial activity against the ichthyopathogens Lc. garvieae and S. parauberis. Altogether, our results suggest that S. salivarius MDI13 and L. sakei MEI5 have a strong potential as probiotics to prevent fish diseases in aquaculture as an appropriate alternative/complementary strategy to the use of antibiotics.
1. Introduction
Aquaculture is defined by the Food and Agriculture Organization of the United Nations (FAO) as the farming of aquatic animals, including fish, crustaceans, mollusks, and aquatic plants. In 2022, global aquatic animal production was approximately 223.2 million tons, reaching a historic record. Compared to 2020, aquaculture increased by 6.6%. Of the total production of aquatic animals, 89% was intended for human consumption, making them one of the most traded food products globally. Today, the challenge of feeding a growing population without depleting natural resources continues to grow. In this sense, aquatic food systems have great potential to meet the nutritious food needs of humanity [1]. One of the main issues in fish farming is the fish stress, which can be minimized by using the appropriate husbandry methods. However, stress can never be completely eliminated, which increases the susceptibility to disease occurrence and leads to massive mortalities. Bacteria are the primary pathogens affecting marine species, accounting for 54.9% of all infections [2]. Common pathogenic bacteria responsible of severe infections in numerous economically valuable fish species include Vibrio spp. (e.g., Vibrio harveyi, Vibrio vulnificus, Vibrio parahaemolyticus, and Vibrio alginolyticus), Listonella anguillarum, Tenacibacullum maritimum, Aeromonas salmonicida, Streptococcus parauberis, and Lactococcus garvieae, amongst others. For example, diseases such as tenacibaculosis (caused by T. maritimum), which affects many marine fish such as turbot and sea bass, can have very high mortality rates. There is also vibriosis, which can cause the death of 50% of infected fish and shellfish. In addition, Aeromonas infections, such as A. salmonicida, are quite common and can be lethal, especially in cold-water salmonids. Regarding Gram-positive bacteria, S. parauberis is the main bacterial agent that causes a significant economic loss to the mariculture industry, and Lc. garvieae is a common agent that can provoke significant economic losses not only in several fresh fish species but also in saltwater species [2,3]. Traditionally, diseases in aquaculture have been fought with the indiscriminate use of antibiotics, which has led to the development and spread of (multiple) antibiotic resistances in bacteria. For this reason, it is necessary to search for new alternative/complementary strategies safe for humans, animals, and the environment, such as the use of probiotics in fish farming [4,5].
Probiotics are defined by the World Health Organization (WHO) and FAO as live microorganisms that, when supplied in sufficient quantity, enhance the health of the host [6]. In a broader definition, probiotics for aquaculture are considered as live microbial cultures that exert a beneficial effect on the host by (i) modifying its microbiota and/or that of its environment; (ii) enhancing the efficiency of feed assimilation and/or its nutritional value; (iii) improving the host response to disease; and/or (iv) increasing the quality of the aquatic environment in which the host grows [7,8,9].
Currently, only one microorganism is internationally authorized for use as a zootechnical additive in the aquaculture of all fish and crustacean species in the European Union (EU), namely Pediococcus acidilactici CNCM I-42622 (formerly MA18/5M), a strain registered under the trade name Bactocell® [10]. Microorganisms proposed as probiotics in fish farming must display antimicrobial activity and must be considered safe not only for aquatic animals, but also for the surrounding environment and humans [11]. Most of the studies conducted in the field of probiotics for aquaculture focus on Lactic Acid Bacteria (LAB), awarded the Generally Recognized As Safe (GRAS) status by the Food and Drug Administration (FDA) and included in the Qualified Presumption of Safety (QPS) list established by the European Food Safety Authority (EFSA) due to their universal presence in food and their contribution to the healthy microbiota of the human gut [12,13,14,15,16]. LAB are the most studied group of microorganisms as probiotics for aquaculture and there are many studies evaluating their application in fish production. The main mechanisms by which probiotics can exert benefits in aquaculture include the following: (i) the modulation of the host microbiota and/or its environment; (ii) the improvement of feed utilization and/or its nutritional value; (iii) the reduction of host susceptibility to diseases; (iv) the improvement of host response to stress; (v) the enhancement of reprouctive function; and/or (vi) the improvement of water characteristics [9,17,18,19,20,21].
In general, in the process of the selection and evaluation of probiotic strains, their full and comprehensive characterization is essential to demonstrate their safety and beneficial effects on the host health. Recently, the EFSA has included Whole-Genome Sequencing (WGS) as a requisite for the characterization of bacteria and yeasts proposed as products or as enzyme-producing strains in food and feed [22]. Therefore, the study of the complete genome of probiotic strains, using Next-Generation Sequencing (NGS) techniques, has great relevance in the detection of genes responsible for their probiotic characteristics (e.g., production of antimicrobial compounds) and in the evaluation of their safety by studying the presence of antibiotic resistance and other virulence factor genes [23]. Among the desirable antimicrobial compounds for probiotic strains are bacteriocins, which are defined as ribosomally synthesized peptides produced and secreted by Gram-positive and Gram-negative bacteria that have a variable antimicrobial spectrum against bacterial pathogens [23].
Therefore, the overall objective of this work was the WGS, bioinformatic, and functional analyses and safety characterization of two strains (S. salivarius MDI13 and L. sakei MEI5) isolated from the gut of European hakes (Merluccius merluccius, L.) from the Northeast Atlantic Ocean for their potential application as probiotics in aquaculture.
2. Materials and Methods
2.1. Growth Conditions and Isolation of Genomic DNA
S. salivarius MDI13 and L. sakei MEI5 were isolated from non-eviscerated commercial European hakes (Merluccius merluccius, L.) caught from the Northeast Atlantic Ocean (Southwest of Ireland), provided by a Galician skipper dedicated to professional fishing. Both strains were grown in Man, Rogosa and Sharpe (MRS) agar (1.5%, w/v) plates (Oxoid, Basingstoke, UK) at 30 °C overnight.
The genomic DNA of the two strains was first extracted and purified using the DNeasy Blood & Tissue kit (Qiagen, Hilden, Alemania) following the manufacturer’s instruction, and then quantified (ng/µL) in a Qubit 4 fluorometer (Invitrogen, Waltham, MA, USA).
2.2. Whole-Genome Sequencing (WGS), Assembly, and Mapping
The WGS of both strains was carried out at the SeqCenter (Pittsburgh, PA, USA). In brief, the Illumina DNA Prep kit and 10 bp unique dual index (UDI) from Integrated DNA Technologies (IDT) were used to prepare libraries. Afterward, they were sequenced on an Illumina NextSeq 2000 (Illumina, San Diego, CA, USA), producing 2 × 150 bp reads. The BCL Convert software v3.9.3 (Illumina) was used to perform the demultiplexing, quality control, and adapter trimming. The Unicycler v0.4.8 program was used to assemble the resulting sequence reads into contigs [24]. Rounds of assembly polishing were conducted with the Pilon program (Oxford Nanopore Technologies, Oxford, UK). Assembly annotation was performed with the Rapid Annotations using Subsystems Technology (RAST) server [25]. In addition, genome mapping of the two strains was created by the Proksee web server (https://proksee.ca/ accessed on 6 August 2024) [26].
2.3. Bioinformatic (In Silico) Analyses
2.3.1. Identification of Bacterial Species
Although the two probiotic candidates were formerly identified by DNA sequencing of the gene that encodes the 16S rRNA subunit (16S rDNA) as S. salivarius and L. sakei, their identification was validated by two different databases. Specifically, SpeciesFinder v.2.0., which identifies bacteria according to the complete sequence of the 16S rDNA, and KmerFinder v.3.0.2., which relies on the frequency of overlapping kmers (i.e., 16-mers) [27,28].
2.3.2. Assembly Evaluation, Bacteriocin Identification, and Safety Evaluation
The assembled genomic sequences of the two strains were analyzed individually to confirm their taxonomic identification at the species level and to (i) evaluate the quality of the assembly; (ii) identify the genes and operons related to the biosynthesis and production of bacteriocins; and (iii) to evaluate their safety by identifying genes encoding potential virulence factors and antibiotic resistance genes by means of several web server programs (Table 1). The RAST platform was used to obtain general data for the two genomes analyzed and to assess the quality of the assembly by evaluating the L50 and N50 values. The identification of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPRs) and CRISPR-associated proteins (cas) (CRISPR-Cas) systems, and mobile genetic elements, for instance active prophages, insertion sequences (ISs), and plasmids, was carried out using several bioinformatics pipelines (Table 1). Additionally, a phylogenetic WGS-based mapping and the genomic maps of the two strains were created by the Type (Strain) Genome Server (TYGS) and the Proksee web server, respectively (Table 1).

Table 1.
Bioinformatic tools used for genome analyses of S. salivarius MDI13 and L. sakei MEI5.
2.4. Evaluation of the Deconjugation of Bile Salts
The capacity of the two strains to deconjugate taurocholate and taurodeoxycholate was evaluated using a protocol described by Noriega et al. (2006) [40]. First, bacteria were inoculated in MRS broth (Oxoid) and incubated at 30 °C under aerobic conditions for 16 h. After incubation, 10 μL of the cultures was inoculated onto MRS agar (1.5%, w/v) plates supplemented with L-cysteine (0.05%, w/v, Merck, Darmstadt, Germany) and the respective bile salts (taurocholate or taurodeoxycholate, 0.5%, w/v, Sigma-Aldrich, Darmstadt, Germany), and incubated at 37 °C in anaerobiosis (AnaeroGen, Oxoid) for 72 h. A fresh fecal slurry from a healthy adult cow was employed as a positive control.
2.5. Evaluation of the Degradation of Mucin
The capacity of the two strains to degrade gastric mucin was evaluated following the method described by Zhou et al. (2001) [41]. Mucin from porcine stomach type III (Sigma-Aldrich) and agar were added into medium B without glucose at concentrations of 0.5 and 1.5% (w/v), respectively. In brief, 10 μL of cultures grown in MRS broth was spotted onto the medium B containing mucin. After the incubation at 37 °C for 72 h in anaerobiosis (AnaeroGen, Oxoid), the plates were stained with a 0.1% (w/v) solution of amido black (Merck KGaA) in 3.5 mol/L acetic acid for 30 min and then washed with 1.2 mol/L acetic acid (Merck KGaA). The appearance of a discolored zone around the bacterial spots indicated a positive result. A fresh fecal slurry from a healthy adult cow served as a positive control.
2.6. Evaluation of the Production of Biogenic Amine
The presence of hdc, tdc, odc, and ldc, encoding histidine decarboxylase (HDC), tyrosine decarboxylase (TDC), ornithine decarboxylase (ODC), and lysine decarboxylase (LDC), respectively, was evaluated by PCR. PCR amplifications were performed using previously reported primers [42,43,44,45] and the PCR products were visualized in an agarose (1,5% w/v) (Pronadisa, Madrid, Spain) gel with GelRed Nucleic Acid Gel Stain (Biotium, Inc., Fremont, CA, USA) using a ChemiDoc Imaging System (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
2.7. Synthesis In Vitro of Bacteriocins and Evaluation of Their Antimicrobial Activity
Total genomic DNA from S. salivarius MDI13 was used to amplify the nucleotide sequences of the mature bacteriocins of interest (slvV, blpK, and blpE). Oligonucleotides (Table 2) were acquired from Thermo Fisher Scientific (Waltham, MA, USA). The nucleotide sequence of the T7 promoter was included in the forward primers, which was followed by the first 29, 28, and 27 bases corresponding to the genes of interest (slvV, blpK, and blpE, respectively) and starting with ATG. In the case of reverse primers, these contained the nucleotide sequence of the T7 terminator followed by the last 22, 24, and 24 bases of the gene of interest (slvV, blpK, and blpE, respectively). PCR amplifications were performed using the Phusion Hot Start II High-Fidelity DNA Polymerase (Thermo Fisher Scientific) and genomic DNA (5–100 ng). After visualization of PCR-amplified gene fragments as described above, amplicons were purified using the NucleoSpin® Gel and PCR Clean-up kit (Macherey-Nagel™, Allentown, PA, USA), and quantified with a Qubit fluorometer system (Invitrogen, Waltham, MA, USA). To conduct in vitro cell-free protein synthesis (IV-CFPS) reactions, purified PCR amplicons were used as templates using the PURExpress In vitro Protein Synthesis Kit (New England Biolabs, Ipswich, MA, USA) and a final DNA concentration of 10 ng/µL, and then incubated at 37 °C for 2 h [46,47,48].

Table 2.
Oligonucleotides used in this work.
The antimicrobial activity of the in vitro-synthesized peptides (bacteriocins) was assessed using the Spot-On-Agar Test (SOAT) [49]. In brief, 5 μL of samples was placed onto the surface of MRS, TSB, or BHI (Oxoid) agar (1.5%, w/v) plates, overlaid with a soft agar (0.8%, w/v) containing fresh overnight cultures (ca. 105 CFU/mL) of the indicators (Lc. garvieae CF00021, Yersinia ruckeri LMG3279, Aeromonas hydrophila CECT839, A. salmonicida CLFP-23, A. salmonicida CECT4237, Ls. anguillarum CECT4344, and S. parauberis LMG22252). The antimicrobial activity of the synthesized bacteriocins SlvV, BlpK, and BlpE was assessed in two ways, individually and in combination. When each bacteriocin was evaluated individually, 5 µL was added. When tested together, the bacteriocins (BlpK-SlvV, BlpK-BlpE, and BlpE-SlvV) were mixed at an equal ratio (1:1).
3. Results
3.1. WGS, Assembly, and Mapping of S. salivarius MDI13 and L. sakei MEI5
The WGS of S. salivarius MDI13 and L. sakei MEI5, two interesting potential probiotic candidates previously isolated from the gut of European hakes caught in the Northeast Atlantic Ocean [50], was determined and analyzed. In this respect, both genomes were found to meet the criteria established by EFSA [22], with contig counts below 500 (29 and 15 contigs in S. salivarius MDI13 and L. sakei MEI5, respectively) (Table 3).

Table 3.
General characteristics of the assembled genome of the two sequenced LAB strains.
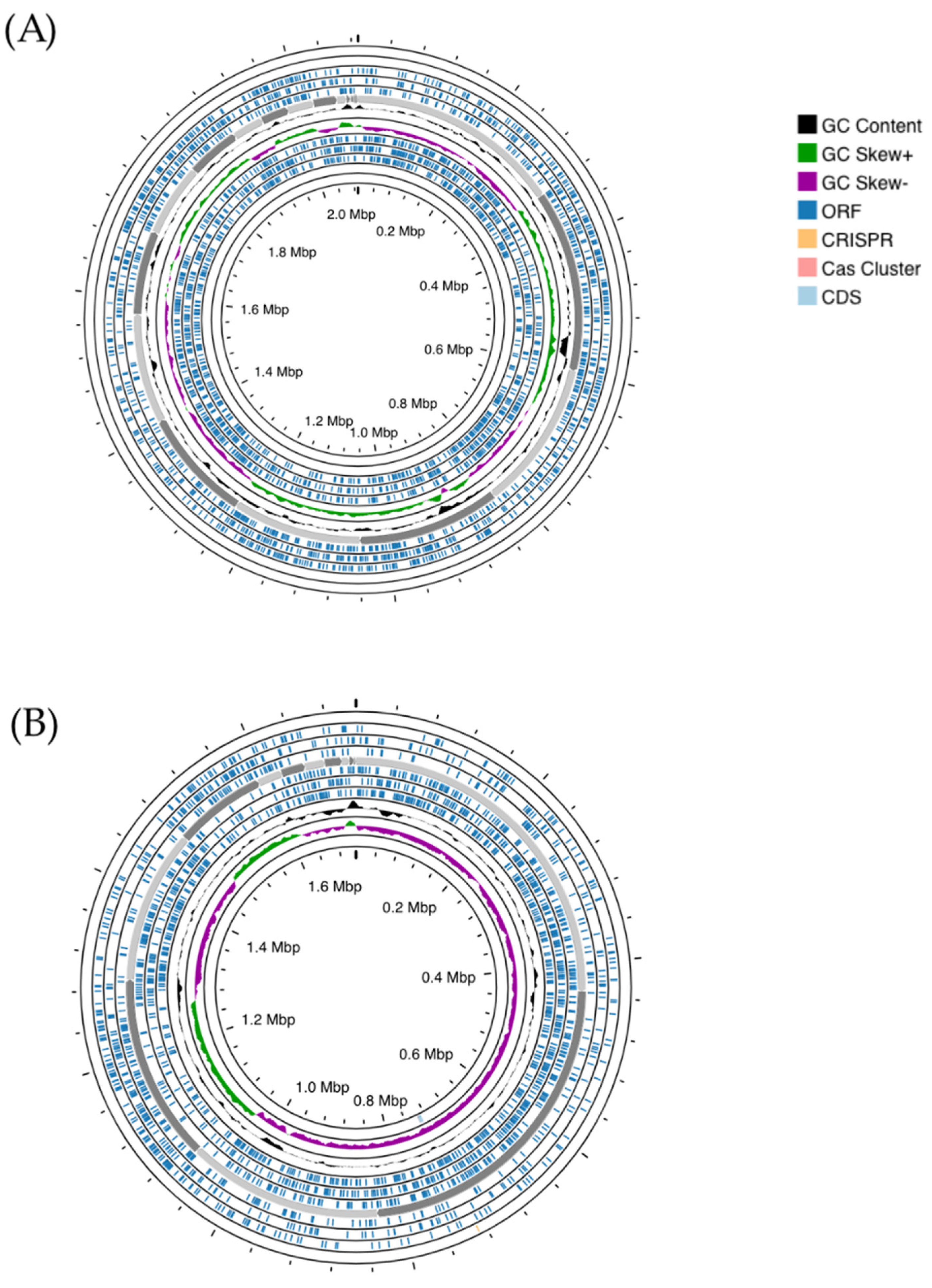
The genome of S. salivarius MDI13 comprises 29 contigs (2,088,084 bp), with 40.0% of G + C content, and N50 and L50 values of 195,997 and 5, respectively. In addition, this genome contains 219 gene subsystems (set of genes that synthesize proteins with similar or associated functions). The total numbers of coding DNA sequences (CDSs) and RNAs were 1,952 and 39, respectively. On the other hand, the genome of L. sakei MEI15 consists of 37 contigs (1,712,091 bp), with 37.0% of G + C content, and N50 and L50 values of 237,072 and 3, respectively. Moreover, this genome contains 197 gene subsystems. The total numbers of CDSs and RNAs were 1,711 and 48, respectively (Table 3). In addition, a genomic map was made in order to visualize features of interest in the bacterial genomes (Figure 1).
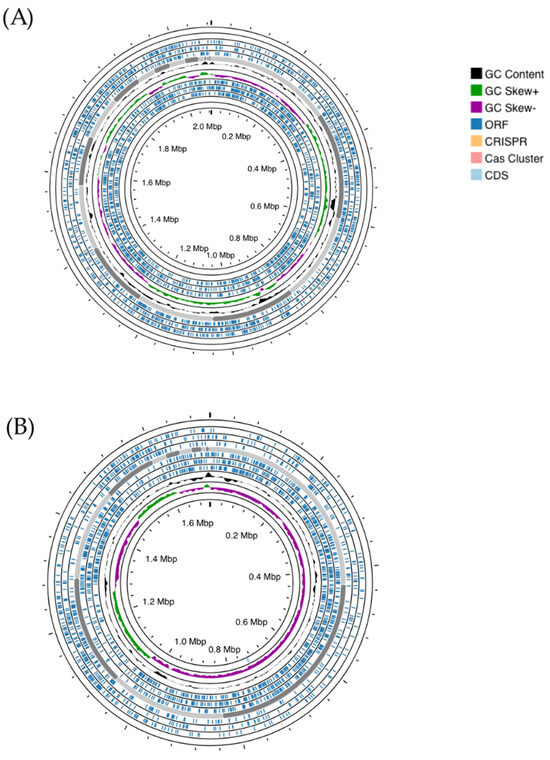
Figure 1.
Genome mapping of S. salivarius MDI13 (A) and L. sakei MEI5 (B), generated using the Proksee webserver. ORFs (Open Reading Frames) and CG skew + and − are indicated in dark blue, green, and violet, respectively. CRISPR arrays and cas cluster systems are shown in orange and light pink, respectively.
3.2. Bioinformatic and Functional Analyses of the Genome of S. salivarius MDI13 and L. sakei MEI5
3.2.1. Species Identification
In this work, we proceeded to sequence the genome of S. salivarius MDI13 and L. sakei MEI5. The SpeciesFinder and KmerFinder software programs confirmed their identification as S. salivarius MDI13 and L. sakei MEI5.
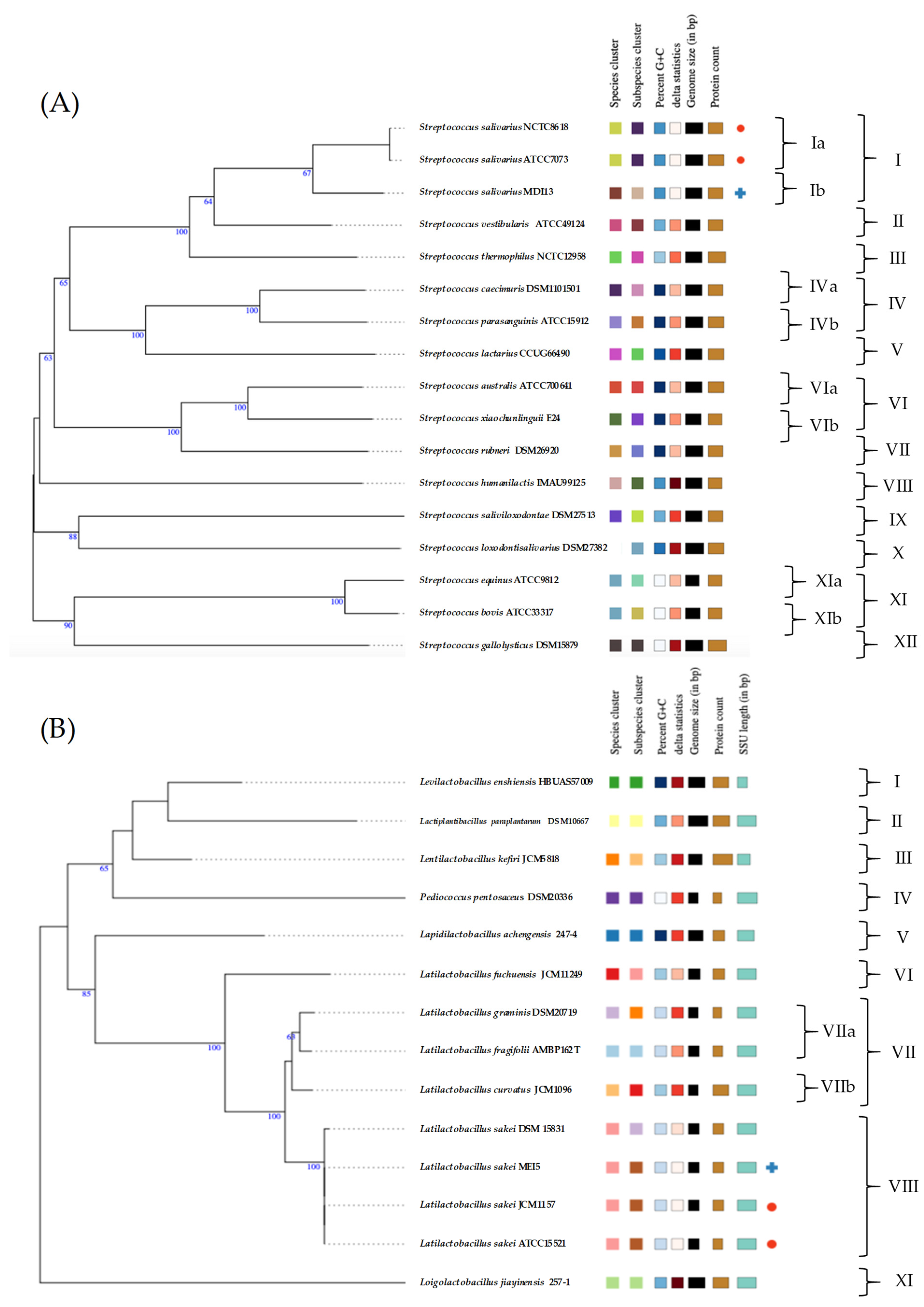
The TYGS server was used to perform species and subspecies similarity estimations from the closest defined type genome sequences. TYGS phylogenetic tree for the two probiotic candidates showed that S. salivarius MDI13 constitutes a single subcluster (Ib) with respect to subcluster Ia of the species type strains (S. salivarius NCTC8618 and S. salivarius ATCC7073; 67% identity). On the other hand, L. sakei MEI5 is part of a single cluster (VIII) including the species type strains (L. sakei JCM1157 and L. sakei ATCC15521; 100% identity) (Figure 2).
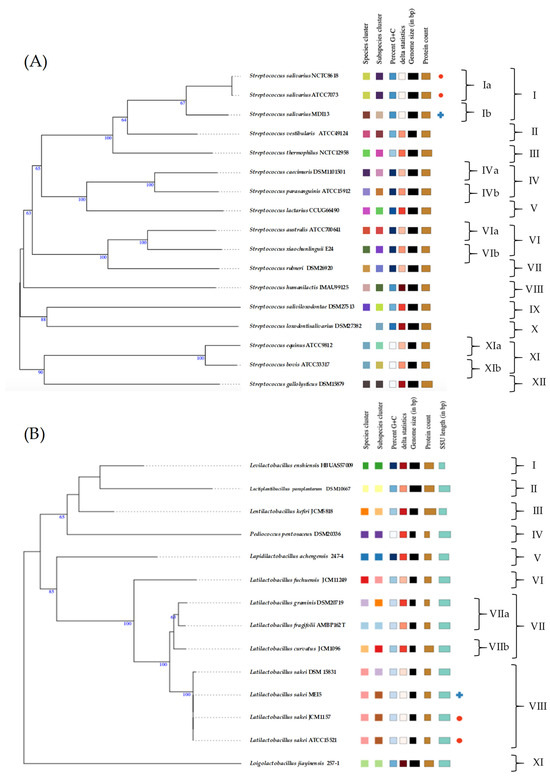
Figure 2.
Genome-based phylogenetic tree of S. salivarius MDI13 (A) and L. sakei MEI5 (B) created by the TYGS webserver. Blue crosses indicate the probiotic candidates evaluated in this study and red dots point out the species type strains.
3.2.2. Distribution of Functional Genetic Subsystems
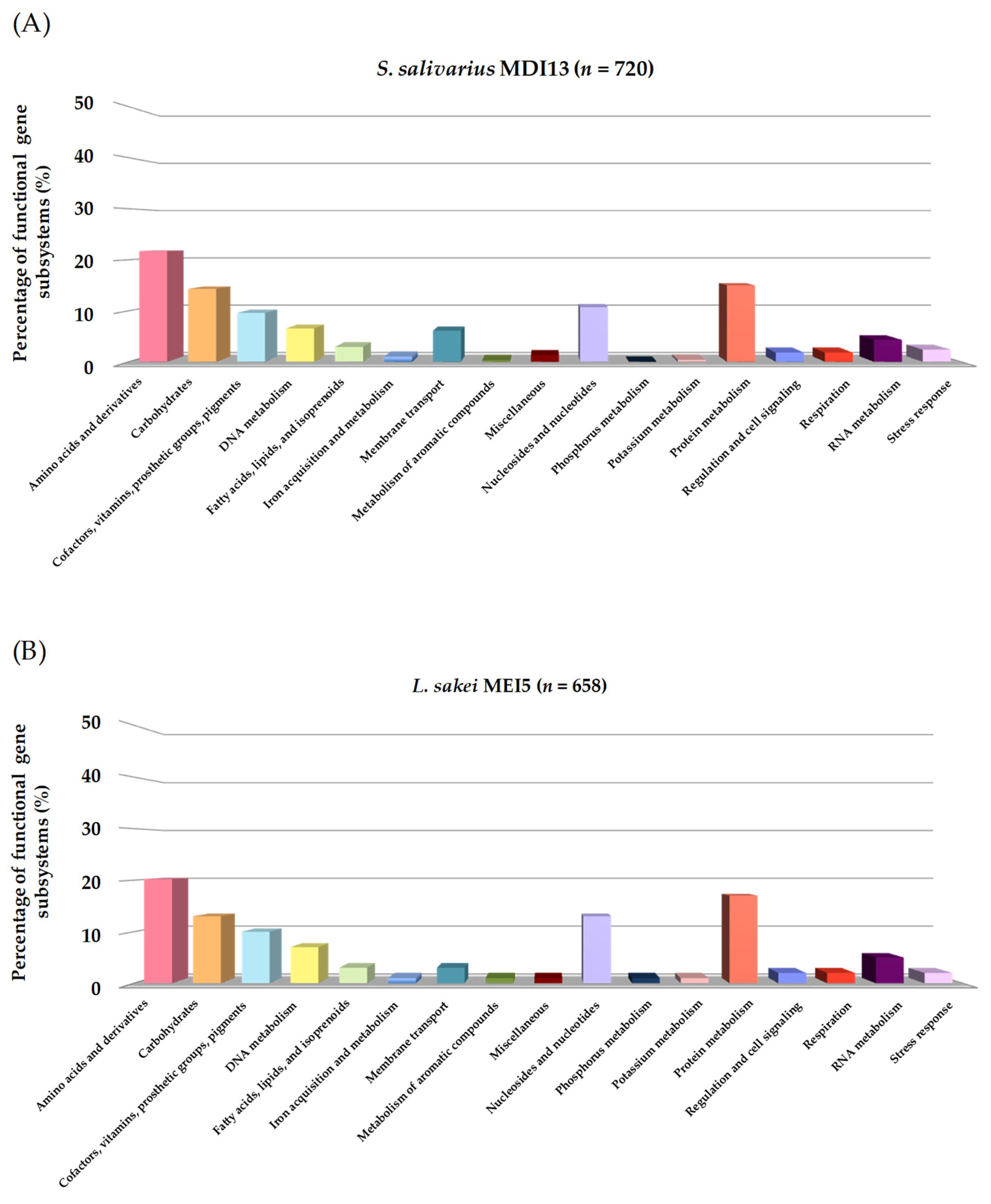
Using the RAST platform, the sequences of S. salivarius MDI13 and L. sakei MEI5 were analyzed, and 17 functional gene subsystems were identified (Figure 3). S. salivarius MDI13 showed a high number of genes associated with the biosynthesis of amino acids and derivatives (156; 21.7%), followed by protein metabolism (108; 15.0%), carbohydrate metabolism (103; 14.3%), and nucleosides and nucleotides (77; 10.7%). In the L. sakei MEI5 genome, genes related to amino acids and derivatives (133; 20.2%), protein metabolism (111; 16.9%), nucleosides and nucleotides (88; 13.4%), and carbohydrate metabolism (83, 12.6%) were also identified. In both genomes, genes associated with motility and chemotaxis, nitrogen metabolism, and photosynthesis were not found.
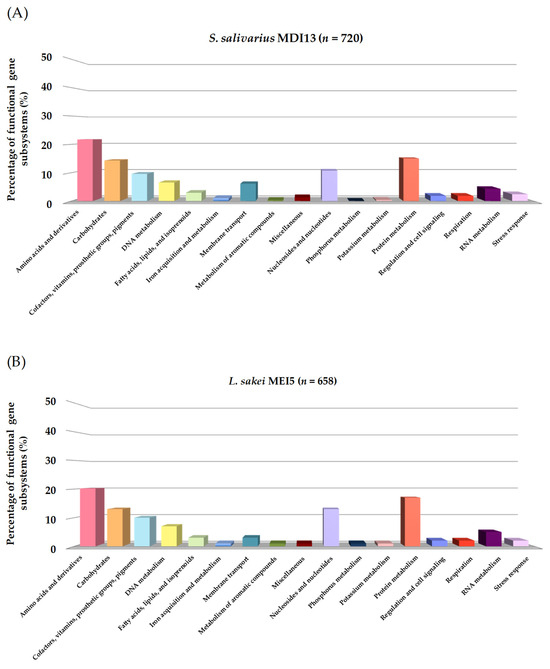
Figure 3.
Functional gene subsystems identified in the genome of S. salivarius MDI13 (A) and L. sakei MEI5 (B), obtained using the RAST program.
3.2.3. Identification of Genetic Determinants Related to Various Probiotic Traits
For the determination of potential probiotic characteristics, RAST annotation of the assembled genome of S. salivarius MDI13 and L. sakei MEI5 was used to identify genes involved in probiotic properties. In the case of the genome of S. salivarius MDI13, genes associated with vitamin biosynthesis, adhesion and aggregation, and amino acid metabolism were identified (Table S1). On the other hand, in the genome of L. sakei MEI5, not only were genes involved in adhesion and aggregation, vitamin biosynthesis, amino acid metabolism detected, but also genes responsible for antimicrobial activity, active metabolism, and stress adaptation/host gastrointestinal tract adaptation (tolerance to temperature, acid, pH, bile salts, osmotic stress, and oxidative stress) (Table S1).
The genome analyses of each strain evaluated in this work identified the existence of genes related to adhesion and aggregation. In this regard, S. salivarius MDI13 and L. sakei MEI5 had genes encoding proteins for the capacity to adhere to the host cells through the gastrointestinal tract, including those encoding the fibronectin-binding protein, exopolysaccharide (EPS), triosephosphate isomerase, and sortase A. Moreover, L. sakei MEI5 also had the enolase gene.
Regarding vitamin biosynthesis, the RAST software predicted the existence of genes linked to the biosynthesis of vitamins, such as thiamine (B1), riboflavin (B2), pyridoxin (B6), biotin (B7), and folate (B9), in the genome of S. salivarius MDI13 and L. sakei MEI5 (Table S1). The genome analyses also allowed the detection of genes associated with amino acid biosynthesis. Specifically, L. sakei MEI5 encodes genes related to the biosynthesis of tryptophan, leucine, arginine, taurine, methionine, threonine, and lysine, while S. salivarius MDI13 only presented genes related to the biosynthesis of threonine and tryptophan (Table S1). Genes related to the production of lactic acid, such as D-lactate and L-lactate dehydrogenase, were only predicted in the genome of L. sakei MEI5 (Table S1). In addition, genes related to stress adaptation (e.g., tolerance to acid, pH, temperature, bile salts, and osmotic and oxidative stress) were detected in the genome of L. sakei MEI5 but not in S. salivarius MDI13 (Table S1).
3.2.4. Identification of Antibiotic Resistance Genes and Other Virulence Factors
Regarding the safety evaluation of the two probiotic candidate LAB strains characterized in this work, no genes encoding virulence factors were detected using the VirulenceFactor 2.0 database and the PathogenFinder platform. In addition, no resistance genes were detected in any of the two probiotic candidates using the ResFinder 4.1 database.
3.2.5. Identification of Mobile Genetic Elements (MGEs) and CRISPR-Cas Systems
In the identification of MGEs, the possible existence of ISs was analyzed through the ISfinder program. ISs are DNA sequences that can move or integrate into the bacterial genome. The results revealed the presence of 7 and 11 true ISs in S. salivarius MDI13 and L. sakei MEI5 genomes, respectively (Table 4), which means that each ORF was identified as a transposase and the detected ISs displayed an e-value of 0.0 [51].

Table 4.
Mobile genetic elements (ISs, active prophages, and plasmids) and CRISPR-Cas systems identified in the genome of S. salivarius MDI13 and L. sakei MEI5.
No prophage was predicted by the Prophage Hunter web service in S. salivarius MDI13 or in L. sakei MEI5 (Table 4). After analysis with the PlasmidFinder server, no plasmids were found in the genome of S. salivarius MDI13 and L. sakei MEI5 (Table 4). The CRISPRCas-Finder program predicted the existence of CRISPR assemblies with an evidence level of 1 and 2 in the genome of L. sakei MEI5, whereas none were detected in S. salivarius MDI13 (Table 4).
3.2.6. Identification of Operons Responsible for the Production of Bacteriocins
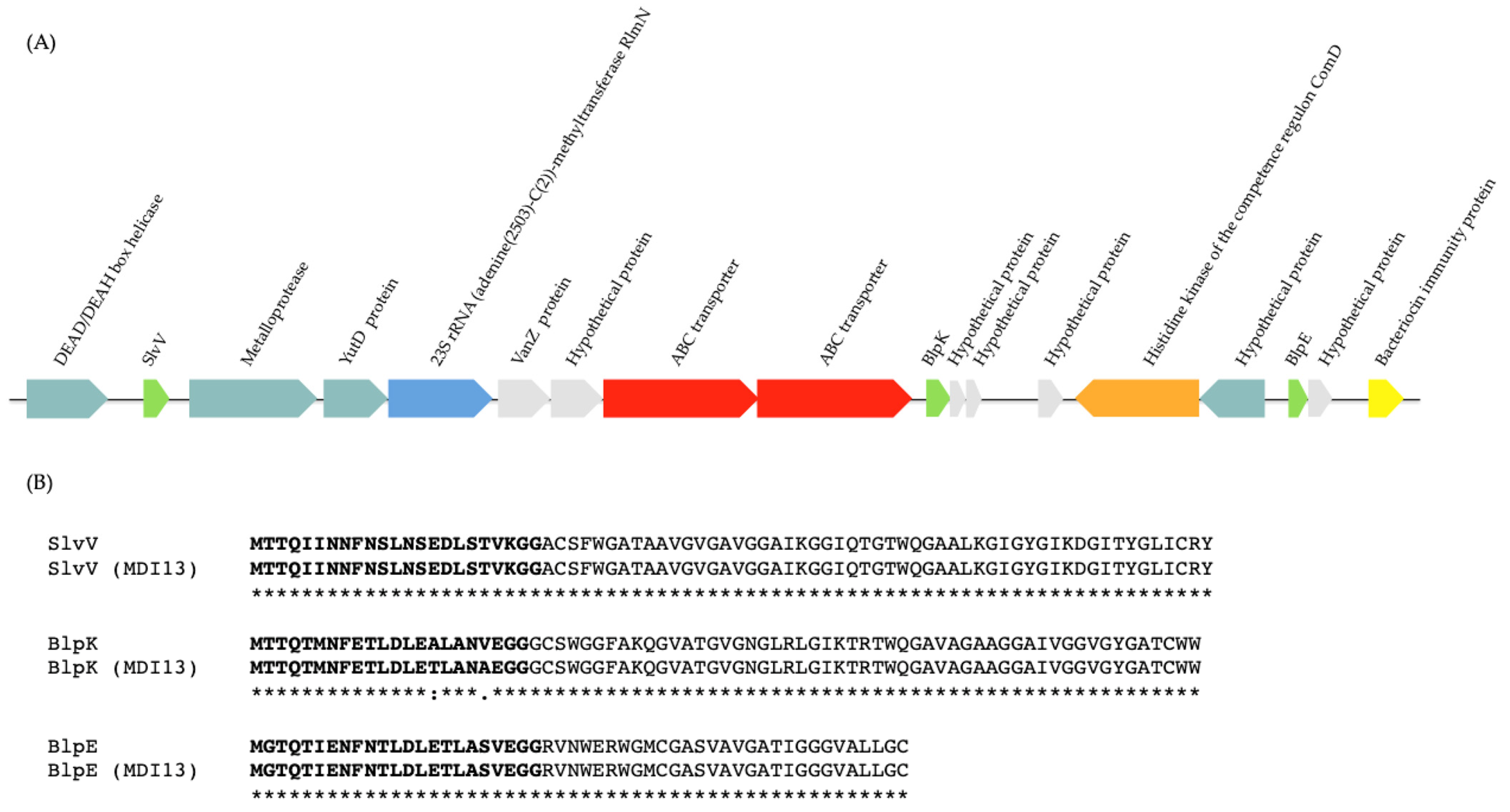
In our study, the BAGEL4 platform and BLASTp (NCBI) database were used to identify operons associated with the production of bacteriocins and to predict their functions, respectively. The bioinformatic analyses with both servers allowed us to detect in the genome of S. salivarius MDI13 a cluster with the structural genes of three putative bacteriocins (SlvV, BlpK, and BlpE) belonging to the class IId, taking into account the classification proposed by Álvarez-Sieiro et al. (2016) [52] (Figure 4A). In contrast, no bacteriocin genes were identified in the genome of L. sakei MEI5.
The multi-bacteriocinogenic gene cluster found in the genome of S. salivarius MDI13 (Figure 4A) showed a genetic structure similar to other blp operons characterized in S. salivarius, Streptococcus pneumoniae, and Streptococcus thermophilus, composed of genes encoding the following: (i) putative bacteriocins with the common double glycine (2-Gly) cleavage site in the leader peptides, (ii) an ATP-Binding Cassette (ABC) transporter system, (iii) a bacteriocin immunity protein, and (iv) a protein-histidine-kinase [53,54,55,56,57,58,59]. The identity of the predicted sequences of the three bacteriocins encoded in the genome of S. salivarius MDI13 was compared to those of the bacteriocins previously described in this species [59], using the Clustal Omega server (Figure 4B). The amino acid sequences of the bacteriocins encoded by S. salivarius MDI13 were 100% identical to those previously described [59]. However, two amino acids of the leader peptide of BlpK in S. salivarius MDI13 differed from those found in other strains (Figure 4B).
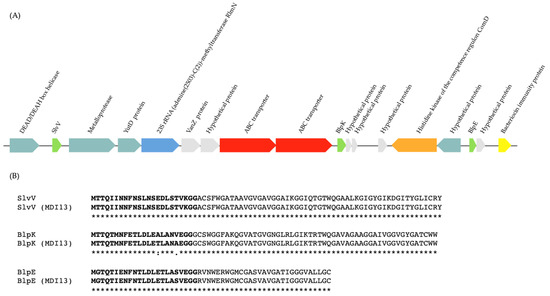
Figure 4.
(A) Gene cluster encoding the bacteriocins SlvV, BlpK, and BlpE in the genome of S. salivarius MDI13 using BAGEL v.4.0. ORFs are indicated by arrows and their putative functions are indicated. (B) Sequence alignment of the amino acid sequence of bacteriocins (SlvV, BlpK, and BlpE) detected in the genome of S. salivarius MDI13, with the amino acid sequence of bacteriocins previously described in this species [59]. The leader sequences with the double glycine are shown in bold. A single fully conserved residue is designated by an asterisk (*), conserved groups of residues with robustly similar properties are illustrated with a colon (:), and groups of residues that are feebly similar are shown by a period (.).
Figure 4.
(A) Gene cluster encoding the bacteriocins SlvV, BlpK, and BlpE in the genome of S. salivarius MDI13 using BAGEL v.4.0. ORFs are indicated by arrows and their putative functions are indicated. (B) Sequence alignment of the amino acid sequence of bacteriocins (SlvV, BlpK, and BlpE) detected in the genome of S. salivarius MDI13, with the amino acid sequence of bacteriocins previously described in this species [59]. The leader sequences with the double glycine are shown in bold. A single fully conserved residue is designated by an asterisk (*), conserved groups of residues with robustly similar properties are illustrated with a colon (:), and groups of residues that are feebly similar are shown by a period (.).
3.3. Evaluation of Mucin Degradation, Bile Salt Deconjugation, and Biogenic Amine Production
Neither of the two candidate probiotic LAB strains evaluated in this work showed mucinolytic activity, the capability to deconjugate bile salts, or the ability to produce biogenic amines.
3.4. In Vitro Cell-Free Protein Synthesis (IV-CFPS) of Bacteriocins and Evaluation of Their Antimicrobial Activity
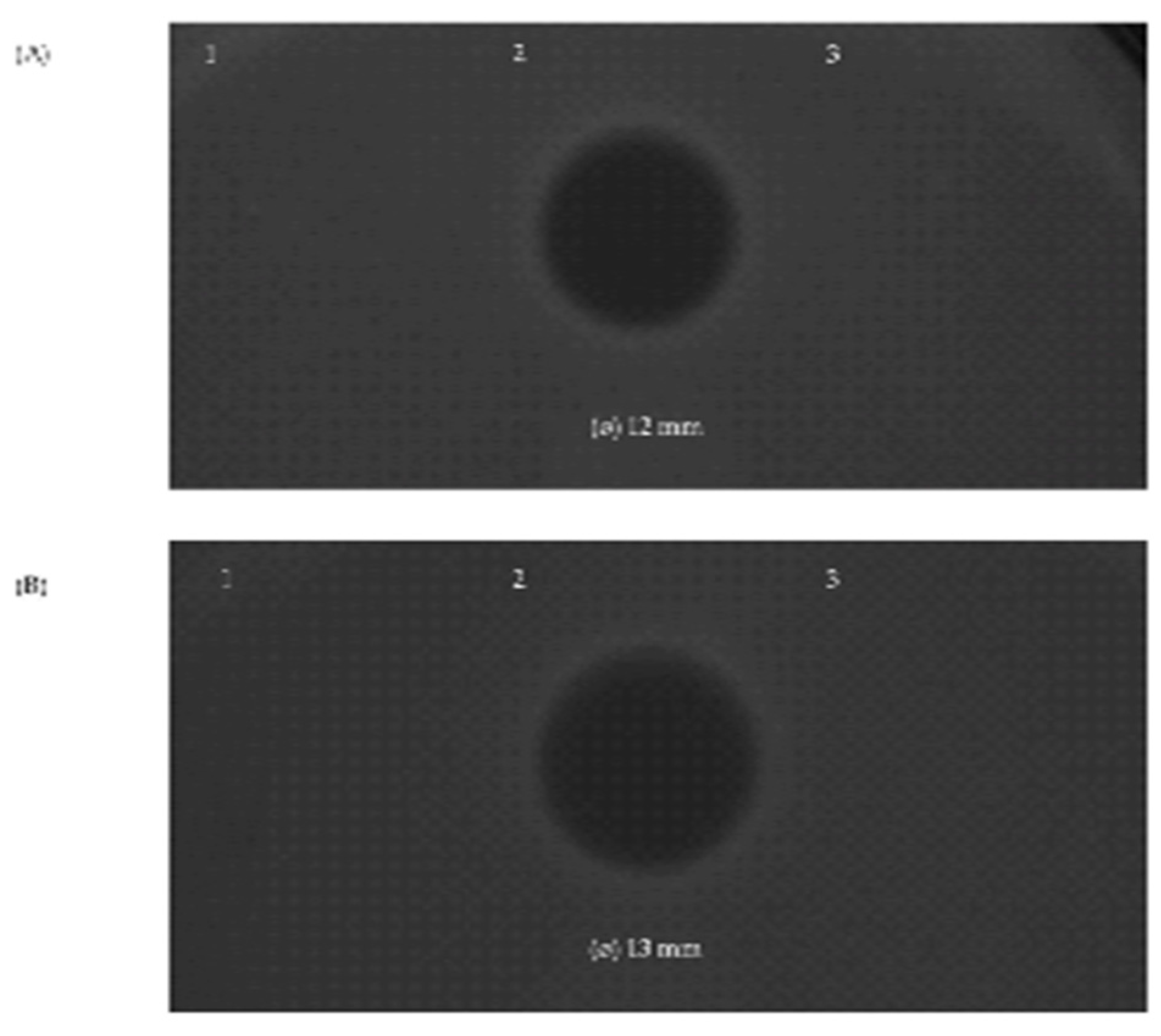
The three bacteriocins found to be encoded by the genome of S. salivarius MDI13 were synthesized by an in vitro cell-free protein synthesis (IV-CFPS) procedure and tested by a SOAT in order to evaluate their antimicrobial activity (Figure 5). In this sense, the in vitro-synthesized BlpK showed antimicrobial activity against Lc. garvieae CF00021 and S. parauberis LMG22252 (Figure 5).
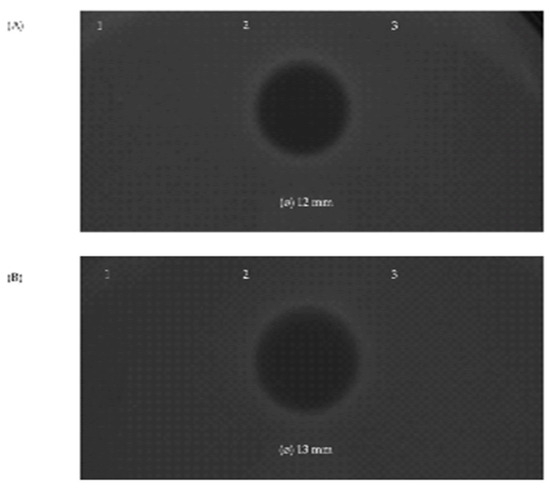
Figure 5.
Antimicrobial activity of the in vitro-synthesized bacteriocins SlvV (1), BlpK (2), and BlpE (3) against the ictyophathogens Lc. garvieae CF00021 (A) and S. parauberis LMG22252 (B) by using a SOAT.
In contrast, the in vitro-synthesized SlvV and BlpE did not show antimicrobial activity against any of the evaluated pathogens. Furthermore, the combination of two peptides (BlpK-SlvV, BlpK-BlpE, and BlpE-SlvV) did not show synergistic activity; nonetheless, the inhibition zone produced by BlpK was observed in all cases.
4. Discussion
The development of massive sequencing platforms together with the reduction in the cost of this technique have facilitated the sequencing of a large number of LAB genomes from different origins, which has allowed obtaining additional information on the potential biotechnological applications of these bacteria in the food industry, human medicine, veterinary field, and animal production [60,61].
The genome analysis of the strains studied, S. salivarius MDI13 and L. sakei MEI5, showed comparable annotation characteristics, with 1952 and 1711 CDSs organized in 219 and 197 subsystems, respectively. Similarly, a large proportion of the subsystems have also been predicted in other genera of LAB and assigned to amino acids and derivatives, protein metabolism, and carbohydrate metabolism [62,63].
Regarding the genetic determinants related to probiotic traits, S. salivarius MDI13 and L. sakei MEI5 had genes encoding proteins for the ability to adhere to host cells through the gastrointestinal tract, including those encoding fibronectin-binding protein, exopolysaccharide (EPS), triosephosphate isomerase, and sortase A. In addition, L. sakei MEI5 also had the enolase gene, which encodes a surface protein that mediates bacterial adhesion to laminin, which is a suitable attribute to be a probiotic candidate [64]. Regarding fibronectin, this is a frequent substrate for bacterial adhesins in the host gut and fibronectin-binding proteins have been recognized in many different bacteria. Because of their adhesive property, fibronectin-binding proteins from probiotic bacteria could be used for the exclusion of pathogens from the host gut and serve as a substrate for adhesion to the host epithelium and intestinal tract, which is key for the colonization and persistence of bacteria in the host intestine [65]. Other studies also detected the presence of genes encoding adhesins and sortase A in S. salivarius [66,67]. Moreover, several articles state that sortase A plays an important function in bacterial adhesion, biofilm formation and immunity [68,69]. Similarly, bacterial exopolysaccharides have been associated with adhesion to surfaces and protection against host immune systems [70]. Regarding vitamin biosynthesis, the RAST software predicted the presence of genes involved. Other studies have reported the ability of strains of L. sakei and Streptococcus spp. to produce folate, riboflavin, and thiamine [71,72,73,74]. In this regard, other studies confirm that LAB can synthesize essential biomolecules (e.g., vitamins or bioactive peptides) that can influence the composition, processing, and organoleptic properties, as well as the overall quality of, food and feed. In addition, they can enhance synergistic effects on digestion and alleviate symptoms of intestinal malabsorption [75,76]. It has been suggested that dietary micronutrients, including B vitamins, may impact fillet texture and increase the number of muscle cells in post-smolts [77]. In addition, the synthesis of B group vitamins, specifically thiamine (vitamin B1), could reduce the numbers of dead and deformed fry in the probiotic-diet-fed fish [51,78].
The genome analyses also allowed the identification of genes related to amino acid biosynthesis (tryptophan, leucine, arginine, taurine, methionine, threonine, and lysine). In this sense, tryptophan is a metabolic precursor of serotonin, melatonin, and niacin (vitamin B3) which is involved in numerous physiological functions, including the modulation of behavior, antioxidant, and immune and stress responses. Aquaculture has spent years optimizing the amount of tryptophan in feed, as this is an essential aromatic amino acid that must be supplied through the fish feed [79]. In the case of leucine, this is a branched-chain amino acid, nutritionally essential in animals. This is one of the most abundant amino acids in high-quality protein feeds and is involved in energy metabolism (i.e., glucose uptake and mitochondrial biogenesis), thus providing energy for protein synthesis [80]. In this regard, approximately 80% of leucine is used for protein synthesis, [80]. Zhao et al. (2023) [81] suggested that fish feed with a diet supplemented with leucine could recover the function of the intestinal barrier by increasing antioxidant capacities, humoral immune response, and the level of tight junction protein. On the other hand, among the essential amino acids, arginine is one of the functional amino acids in animals. Specifically, arginine is an indispensable amino acid for fish, but its endogenous biosynthesis is limited. In particular, arginine specifically modulates the adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) pathway to regulate energy balance and also activates the target of the rapamycin (TOR) signaling pathway to regulate protein synthesis [82,83]. Cysteine is a precursor of taurine, and has physiological roles, such as antioxidant stress and immune enhancement. Studies have shown that cysteine supplementation greatly increases the weight and growth rate of juvenile golden pompano (Trachinotus ovatus) [84]. Furthermore, the possibility of the reduction in mercury contamination in fish with dietary cysteine supplementation has been proposed [85]. Methionine is also an essential amino acid that stimulates protein synthesis, reduces green liver syndrome, increases cell survival, and improves digestibility and growth in some fish species, for instance rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar) [86,87].
Genes involved in the production of lactic acid and related to stress adaptation were predicted in the genome of L. sakei MEI5. In this sense, one of the beneficial properties of lactic acid production is to hinder the survival of pathogenic bacteria [51,88,89,90]. The bacterial tolerance to acid and bile salts represents an adaptive advantage for probiotics, as it allows them to survive during their transit through the stomach and small intestine and, therefore, to reach the large intestine in greater numbers and with greater viability [91,92].
Regarding the safety evaluation of the two probiotic candidate LAB strains characterized in this work, no genes encoding virulence factors were detected. Although some S. salivarius strains have been used as oral probiotics [93], a recent EFSA report has notified that this species is not recommended for the QPS list because of its potential pathogenic characteristics [15]. However, as mentioned above, bioinformatic analyses of the S. salivarius MDI13 genome did not identify any virulence factor. Regarding L. sakei, this species is considered potentially as safe as many other LAB species with the QPS status [94]. Independently of this, all strains proposed as probiotics for animals and humans should be evaluated for susceptibility to antibiotics of relevance in therapeutic practice in humans and animals [94]. In this regard, the identification of acquired transmissible antibiotic resistance(s) in a strain would automatically invalidate its use, due to the risk of spreading the resistance genes to commensal and pathogenic bacteria, which would increase the current major public health problem generated by the emergence and dissemination of antibiotic (multi)resistant bacteria [16]. In our study, no resistance genes were detected in any of the two probiotic candidates using the ResFinder 4.1 database. These results are in agreement with our previous in vitro assays in which both strains were found to be sensitive to the most commonly used antibiotics in human medicine and animal health (unpublished data).
Concerning the identification of MGEs, no prophage was detected in S. salivarius MDI13 or L. sakei MEI5. This result can be considered as a positive probiotic characteristic, since some prophages may be implicated in antibiotic resistance and/or in the development of virulent characteristics [36,95]. Also, no plasmids were found in the genome of S. salivarius MDI13 and L. sakei MEI5. On the other hand, CRISPR assemblies (evidence level of 1 and 2) were found in the genome of L. sakei MEI5. In this regard, sequences with an evidence level lower than 3 were discarded as they indicate potentially invalid CRISPR assemblies [38,96]. Furthermore, the absence of associated cas genes in the predicted CRISPR arrays in the L. sakei MEI5 genome suggests the presence of orphan loci. This finding is not a surprising feature, as they have been previously described in other bacteria as remnants of previous functional CRISPR-Cas systems [97,98].
On the other hand, the strains studied do not have mucinolytic capacity. The gastrointestinal epithelium is covered by a mucus layer that prevents the penetration of microorganisms, so bacteria with mucinolytic capacity are considered pathogenic for fish by facilitating their extraintestinal migration [11,99]. According to the FAO, the capacity to deconjugate bile salts is an advantageous characteristic in probiotics for use in humans [6]. This beneficial effect was attributed to the correlation observed amongst the capability of bacteria to deconjugate bile salts with the capability to survive in the gastrointestinal tract [100]. Although this finding was supported by later studies [101,102], there are some studies that found no relationship between survival in the presence of bile and the ability to deconjugate bile salts [103,104]. In any case, the deconjugation of bile salts makes them less effective at emulsifying ingested lipids, thereby reducing fatty acid absorption and the reabsorption of bile acids themselves [105]. Although this may be a desirable feature in human nutrition, it is not desirable in production animals, as their feed becomes less efficient. Furthermore, according to some authors, the deconjugation of bile salts can lead to the formation of toxic compounds that alter the intestinal microbiota, resulting in diarrhea, enteritis, or the activation of carcinogens in the intestinal contents [11,51,106,107,108]. Moreover, none of the strains evaluated in this work showed the capacity to produce biogenic amines, which are organic bases of low molecular size found in various foods that can exert adverse effects with symptoms similar to allergy [43]. Histamine and tyramine have been extensively studied because of their toxic effects due to their vasoactive and psychoactive properties. In this regard, there is legislation in the European Union that regulates the presence of histamine in fish [109]. Tyramine is related to migraines and hypertensive crises in patients treated with the enzyme monoamine oxidase inhibitor. Regarding putrescine and cadaverine, the problem lies in the fact that they potentiate the effects of other biogenic amines and/or hinder their detoxification in the body [51,110,111,112,113].
The bioinformatic analyses allowed the detection of a cluster with the structural genes of three putative bacteriocins (SlvV, BlpK, and BlpE) in the genome of S. salivarius MDI13. Previous studies focused on genome mining from marine bacteria have also predicted bacteriocin gene clusters [114], including blp operons [115]. Interestingly, previous studies reported that approximately 50% of the salivaricins or related peptides are unique to S. salivarius, whereas others (e.g., BlpK) are mostly identified in other streptococcal species (e.g., S. thermophilus, S. pneumoniae, and S. pyogenes) [59]. In recent decades, a huge number of bacteriocins produced by LAB have been described [48,51,61,116]. In this regard, bacteriocin production by LAB is considered an important probiotic antimicrobial strategy to inhibit the growth of other sensitive strains co-existing in the same ecosystem, colonizing their microbial community and displacing pathogens [115], and is also linked to immunostimulatory effects [117]. In our work, BlpK synthesized in vitro showed antimicrobial activity against Lc. garvieae CF00021 and S. parauberis LMG22252. In another study, BlpK produced in vitro by a cell-free system was shown to be active against S. salivarius, S. thermophilus, and Lactococcus lactis [59]. In addition, a recombinant strain encoding BlpK exerted antimicrobial activity against phylogenetically closely related streptococcal species, and non-closely related pathogenic species, such as Listeria monocytogenes, Enterococcus faecium, Enterococcus faecalis, and Staphylococcus aureus [59]. To our knowledge, our study reports for the first time the antimicrobial activity of this broad-antimicrobial-spectrum bacteriocin against the fish pathogens Lc. garvieae and S. parauberis. Nevertheless, taking into account that the production of most bacteriocins in Streptococcus spp. is induced by transcriptional regulatory systems that require in most cases that the producer microorganism grows in presence of the indicator microorganism, future works will be needed to demonstrate that this bacteriocin is produced by S. salivarius MDI13 in liquid coculture with the microorganism of interest, followed by bacteriocin purification and detection by mass spectrometry. On the other hand, SlvV and BlpE did not exert antimicrobial activity against any of the evaluated pathogens. Furthermore, the combination of two peptides (BlpK-SlvV, BlpK-BlpE, and BlpE-SlvV) did not show synergistic activity. In this respect, it has been reported that the activity of fusion constructions between the expression–secretion signal of the blpK gene and the mature part of slvV and blpE in S. salivarius showed a narrow spectrum, targeting some streptococcal species, such as S. thermophilus and S. salivarius in the case of the expression of SlvV, and also Streptococcus vestibularis and Streptococcus pyogenes when expressing BlpE [59]. Therefore, the absence of the antimicrobial activity of SlvV and BlpE against the indicators tested in our study suggests that these microorganisms are not sensitive to both peptides.
The results obtained in this study suggest the safety and possible probiotic potential of both strains. Nevertheless, further in vivo rearing trials and challenge tests carried out in fish are needed to validate these findings.
5. Conclusions
This work highlights the importance of subjecting probiotic candidates for the food chain to WGS and bioinformatic and functional analyses. The bioinformatic analyses of the genomes of S. salivarius MDI13 and L. sakei MEI5, both strains isolated from European hakes, allowed confirming their safety and identifying genes related to their potential probiotic characteristics, including genes associated with vitamin biosynthesis adhesion and aggregation and amino acid metabolism. Specifically, in the case of L. sakei MEI5, other genes potentially related to probiotic traits were identified, such as genes related to lactic acid production, active metabolism, and adaptation to stress and adverse conditions in the host gastrointestinal tract. Moreover, in vitro-synthesized BlpK encoded by the genome of S. salivarius MDI13 displayed antimicrobial activity against Lc. garvieae and S. parauberis, two ichthyopathogens of utmost relevance in aquaculture. The broad antimicrobial spectrum against ichthyopathogens, the interesting probiotic properties, and the safety profile of S. salivarius MDI13 and L. sakei MEI5 reveals their potential as probiotics to prevent fish bacterial diseases in aquaculture as a complementary/alternative strategy to the use of antibiotics. However, before proposing their use as probiotics, a thorough in vivo evaluation of their safety and probiotic efficacy should be carried out. These findings are very promising, since aquaculture needs to have effective, safe, sustainable, and economically profitable preventive and therapeutic strategies for ichthyopathologies of relevance that will improve the health and productivity of aquaculture species and the safety of marketed products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci11080365/s1. Table S1. Probiotic characteristics based on genome analysis.
Author Contributions
Conceptualization, L.M.C. and E.M.-A.; methodology, L.D.-F., D.C., E.M.-A. and J.F.; software, L.D.-F. and D.C.; validation, J.F. and J.B.; formal analysis, L.D.-F., J.B. and J.F.; investigation, L.D.-F., E.M.-A., J.B. and D.C.; resources, J.B., P.E.H. and L.M.C.; data curation, L.D.-F.; writing—original draft preparation, L.D.-F.; writing—review and editing, E.M.-A. and L.M.C.; visualization, L.D.-F. and E.M.-A.; supervision, L.M.C. and E.M.-A.; project administration, L.M.C., P.E.H., E.M.-A. and J.B.; funding acquisition, L.M.C., J.B. and P.E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerio de Ciencia, Innovación y Universidades (MCIU, Spain) (Projects RTI2018-094907-B-I00 and PID2019-104808RA-I00). L.D.F. received support by the Programa Investigo (Ministerio de Trabajo y Economía Social, MITES, Spain), funded by the EU (NextGenerationEU). D.C. had a research contract from the project RTI2018-094907-B-I00 (MCIU, Madrid, Spain). J.F. was supported by a FEI16/54 contract from the Universidad Complutense de Madrid (UCM, Spain) and held a predoctoral contract from the UCM. J.B. was supported by the Programa Atracción de Talento (2018-T1/BIO-10158) from the Comunidad de Madrid (Spain).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The whole-genome sequences of S. salivarius MDI13 and L. sakei MEI5 are deposited in GenBank under the accession numbers JAYKZL000000000 and JBEJGS000000000, respectively.
Acknowledgments
We acknowledge I. Ruiz Zarzuela (Facultad de Veterinaria, Universidad de Zaragoza, Spain) and C. Michel (INRA, Jouy-en-Josas, France) for providing some of the fish pathogens.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Natnan, M.E.; Mayalvanan, Y.; Jazamuddin, F.M.; Aizat, W.M.; Low, C.-F.; Goh, H.-H.; Azizan, K.A.; Bunawan, H.; Baharum, S.N. Omics strategies in current advancements of infectious fish disease management. Biology 2021, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Hegde, A.; Kabra, S.; Basawa, R.M.; Khile, D.A.; Abbu, R.U.F.; Thomas, N.A.; Manickam, N.B.; Raval, R. Bacterial diseases in marine fish species: Current trends and future prospects in disease management. World J. Microbiol. Biotechnol. 2023, 39, 317. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Buschmann, A.H.; Dölz, H.J. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect. Dis. 2016, 16, e127–e133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Q.E.; Zhou, X.; Wang, F.; Muurinen, J.; Virta, M.P.; Brandt, K.K.; Zhu, Y. Antibiotic resistome in the livestock and aquaculture industries: Status and solutions. Crit. Rev. Environ. Sci. 2021, 51, 2159–2196. [Google Scholar] [CrossRef]
- FAO; WHO. Probióticos en los alimentos. Propiedades saludables y nutricionales y directrices para la evaluación. Estudios FAO. Alimentación y Nutrición. 2006, 85, 52. [Google Scholar]
- Soliman, W.S.; Shaapan, R.M.; Mohamed, L.A.; Gayed, S.S.R. Recent biocontrol measures for fish bacterial diseases, in particular to probiotics, bio-encapsulated vaccines, and phage therapy. Open Vet. J. 2019, 9, 190–195. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. The nature and consequences of co-infections in tilapia: A review. J. Fish. Dis. 2020, 43, 651–664. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish. Shellfish. Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2020/151 of 4 February 2020 concerning the authorisation of Pediococcus acidilactici CNCM I-4622 as a feed additive for all porcine species for fattening and for breeding other than sows, all avian species, all fish species and all crustaceans and repealing Regulations (EC) No 911/2009, (EU) No 1120/2010 and (EU) No 212/2011 and Implementing Regulations (EU) No 95/2013, (EU) No 413/2013 and (EU) 2017/2299 (holder of authorisation Danstar Ferment AG represented in the Union by Lallemand SAS). Off. J. Eur. Union 2020, 33, 12–15. Available online: https://eur-lex.europa.eu/eli/reg_impl/2020/151/oj (accessed on 6 August 2024).
- Muñoz-Atienza, E.; Gómez-Sala, B.; Araújo, C.; Campanero, C.; del Campo, R.; Hernández, P.E.; Herranz, C.; Cintas, L.M. Antimicrobial activity, antibiotic susceptibility and virulence factors of Lactic Acid Bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol. 2013, 13, 15. [Google Scholar] [CrossRef]
- Stiles, M.E.; Holzapfel, W.H. Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 1997, 36, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish. Shellfish. Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Ray, A.K. Impact of microbial proteases on biotechnological industries. Biotechnol. Genet. Eng. Rev. 2017, 33, 119–143. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Statement on the update of the list of QPS-recommended microbiological agents intentionally added to food or feed as notified to EFSA 16: Suitability of taxonomic units notified to EFSA until March 2022. EFSA J. 2022, 20, 7408. [Google Scholar] [CrossRef]
- EFSA. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 15: Suitability of taxonomic units notified to EFSA until September 2021. EFSA J. 2022, 20, 7045. [Google Scholar] [CrossRef]
- Ringø, E. Probiotics in shellfish aquaculture. Aquac. Fish. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Ringø, E.; Li, X.; Doan, H.; Ghosh, K. Interesting probiotic bacteria other than the more widely used lactic acid bacteria and bacilli in finfish. Front. Mar. Sci. 2022, 9, 848037. [Google Scholar] [CrossRef]
- Simón, R.; Docando, F.; Nuñez-Ortiz, N.; Tafalla, C.; Díaz-Rosales, P. Mechanisms used by probiotics to confer pathogen resistance to teleost fish. Front. Immunol. 2021, 12, 653025. [Google Scholar] [CrossRef]
- Sumon, M.A.A.; Molla, M.H.R.; Hakeem, I.J.; Ahammad, F.; Amran, R.H.; Jamal, M.T.; Gabr, M.H.; Islam, M.S.; Alam, M.T.; Brown, C.L.; et al. Epigenetics and probiotics application toward the modulation of fish reproductive performance. Fishes 2022, 7, 189. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef]
- EFSA. Statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain. EFSA J. 2021, 19, 434–438. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: A mini-review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.V.; Cosentino, S.; Lukjancenko, O.; Saputra, D.; Rasmussen, S.; Hasman, H.; Sicheritz-Pontén, T.; Aarestrup, F.M.; Ussery, D.W.; Lund, O. Benchmarking of methods for genomic taxonomy. J. Clin. Microbiol. 2014, 52, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Hasman, H.; Saputra, D.; Sicheritz-Ponten, T.; Lund, O.; Svendsen, C.A.; Frimodt-Møller, N.; Aarestrup, F.M. Rapid whole-genome sequencing for detection and characterization of microorganisms directly from clinical samples. J. Clin. Microbiol. 2014, 52, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 2018, 46, 278–281. [Google Scholar] [CrossRef]
- Singh, H.; Raghava, G.P. BLAST-based structural annotation of protein residues using Protein Data Bank. Biol. Direct. 2016, 11, 4. [Google Scholar] [CrossRef]
- Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; Thompson, J.D.; Higgins, D.G. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.F.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Larsen, M.V.; Aarestrup, F.M.; Lund, O. PathogenFinder—Distinguishing friend from foe using bacterial Whole Genome Sequence data. PLoS ONE 2013, 8, e77302. [Google Scholar] [CrossRef] [PubMed]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic. Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Sun, H.X.; Zhang, C.; Cheng, L.; Peng, Y.; Deng, Z.; Wang, D.; Wang, Y.; Hu, M.; Liu, W.; et al. Prophage Hunter: An integrative hunting tool for active prophages. Nucleic Acids Res. 2019, 47, 74–80. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents. Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic. Acids Res. 2018, 46, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Noriega, L.; Cuevas, I.; Margolles, A.; de los Reyes-Gavilán, C.G. Deconjugation and bile salts hydrolase activity by Bifidobacterium strains with acquired resistance to bile. Int. Dairy. J. 2006, 16, 850–855. [Google Scholar] [CrossRef]
- Zhou, J.S.; Gopal, P.K.; Gill, H.S. Potential probiotic lactic acid bacteria Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) do not degrade gastric mucin in vitro. Int. J. Food Microbiol. 2001, 63, 81–90. [Google Scholar] [CrossRef]
- Le Jeune, C.; Lonvaud-Funel, A.; ten Brink, B.; Hofstra, H.; van der Vossen, J.M. Development of a detection system for histidine decarboxylating lactic acid bacteria based on DNA probes, PCR and activity test. J. Appl. Bacteriol. 1995, 78, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Coton, M.; Coton, E.; Lucas, P.; Lonvaud, A. Identification of the gene encoding a putative tyrosine decarboxylase of Carnobacterium divergens 508. Development of molecular tools for the detection of tyramine-producing bacteria. Food Microbiol. 2004, 21, 125–130. [Google Scholar] [CrossRef]
- Marcobal, A.; de las Rivas, B.; Moreno-Arribas, M.V.; Muñoz, R. Multiplex PCR method for the simultaneous detection of histamine-, tyramine-, and putrescine-producing lactic acid bacteria in foods. J. Food Prot. 2005, 68, 874–878. [Google Scholar] [CrossRef]
- De las Rivas, B.; Marcobal, A.; Carrascosa, A.V.; Muñoz, R. PCR detection of foodborne bacteria producing the biogenic amines histamine, tyramine, putrescine, and cadaverine. J. Food Prot. 2006, 69, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Gabant, P.; Borrero, J. PARAGEN 1.0: A standardized synthetic gene library for fast cell-free bacteriocin synthesis. Front. Bioeng. Biotechnol. 2019, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Montalbán-López, M.; Scott, T.A.; Ramesh, S.; Rahman, I.R.; van Heel, A.J.; Viel, J.H.; Bandarian, V.; Dittmann, E.; Genilloud, O.; Goto, Y.; et al. New developments in RiPP discovery, enzymology and engineering. Nat. Prod. Rep. 2021, 38, 130–239. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, E.; Peña, N.; Lafuente, I.; Cintas, L.M.; Muñoz-Atienza, E.; Hernández, P.E.; Borrero, J. Nisin S, a novel Nisin variant produced by Ligilactobacillus salivarius P1CEA3. Int. J. Mol. Sci. 2023, 24, 6813. [Google Scholar] [CrossRef] [PubMed]
- Cintas, L.M.; Casaus, P.; Holo, H.; Hernández, P.E.; Nes, I.F.; Håvarstein, L.S. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J. Bacteriol. 1998, 180, 1988–1994. [Google Scholar] [CrossRef]
- Díaz-Formoso, L.; Silva, V.; Contente, D.; Feito, J.; Hernández, P.E.; Borrero, J.; Igrejas, G.; Del Campo, R.; Muñoz-Atienza, E.; Poeta, P.; et al. Antibiotic resistance genes, virulence factors, and biofilm formation in coagulase-negative Staphylococcus spp. isolates from European hakes (Merluccius merluccius, L.) caught in the Northeast Atlantic Ocean. Pathogens 2023, 12, 1447. [Google Scholar] [CrossRef]
- Feito, J.; Contente, D.; Ponce-Alonso, M.; Díaz-Formoso, L.; Araújo, C.; Peña, N.; Borrero, J.; Gómez-Sala, B.; del Campo, R.; Muñoz-Atienza, E.; et al. Draft genome sequence of Lactococcus lactis subsp. cremoris WA2-67: A promising Nisin-producing probiotic strain isolated from the rearing environment of a Spanish rainbow trout (Oncorhynchus mykiss, Walbaum) farm. Microorganisms 2022, 10, 521. [Google Scholar] [CrossRef]
- Álvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Hols, P.; Hancy, F.; Fontaine, L.; Grossiord, B.; Prozzi, D.; Leblond-Bourget, N.; Decaris, B.; Bolotin, A.; Delorme, C.; Dusko Ehrlich, S.; et al. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol. Rev. 2005, 29, 435–463. [Google Scholar] [CrossRef] [PubMed]
- Son, M.R.; Shchepetov, M.; Adrian, P.V.; Madhi, S.A.; de Gouveia, L.; von Gottberg, A.; Klugman, K.P.; Weiser, J.N.; Dawid, S. Conserved mutations in the pneumococcal bacteriocin transporter gene, blpA, result in a complex population consisting of producers and cheaters. mBio 2011, 2, e00179-11. [Google Scholar] [CrossRef] [PubMed]
- Renye, J.A.; Somkuti, G.A. BlpC-regulated bacteriocin production in Streptococcus thermophilus. Biotechnol. Lett. 2013, 35, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Valente, C.; Dawid, S.; Pinto, F.R.; Hinds, J.; Simões, A.S.; Gould, K.A.; Mendes, L.A.; de Lencastre, H.; Sá-Leão, R. The blp locus of Streptococcus pneumoniae plays a limited role in the selection of strains that can cocolonize the human nasopharynx. Appl. Environ. Microbiol. 2016, 82, 5206–5215. [Google Scholar] [CrossRef] [PubMed]
- Vertillo Aluisio, G.; Spitale, A.; Bonifacio, L.; Privitera, G.F.; Stivala, A.; Stefani, S.; Santagati, M. Streptococcus salivarius 24SMBc genome analysis reveals new biosynthetic gene clusters involved in antimicrobial effects on Streptococcus pneumoniae and Streptococcus pyogenes. Microorganisms 2022, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Rozman, V.; Mohar Lorbeg, P.; Chanishvili, N.; Accetto, T.; Kakabadze, E.; Grdzelishvili, N.; Rupnik, M.; Matijasic, B. Genomic insights into the safety and bacteriocinogenic potential of isolates from artisanal fermented milk Matsoni. LWT Food Sci. Technol. 2023, 185, 115183. [Google Scholar] [CrossRef]
- Damoczi, J.; Knoops, A.; Martou, M.S.; Jamaux, F.; Gabant, P.; Mahillon, J.; Mignolet, J.; Hols, P. Uncovering the class II-bacteriocin predatiome in salivarius streptococci. bioRxiv 2024. [Google Scholar] [CrossRef]
- Mileriene, J.; Aksomaitiene, J.; Kondrotiene, K.; Asledottir, T.; Vegarud, G.E.; Serniene, L.; Malakauskas, M. Whole-Genome Sequence of Lactococcus lactis subsp. lactis LL16 confirms safety, probiotic potential, and reveals functional traits. Microorganisms 2023, 11, 1034. [Google Scholar] [CrossRef]
- Contente, D.; Díaz-Formoso, L.; Feito, J.; Hernández, P.E.; Muñoz-Atienza, E.; Borrero, J.; Poeta, P.; Cintas, L.M. Genomic and functional evaluation of two Lacticaseibacillus paracasei and two Lactiplantibacillus plantarum strains, isolated from a rearing tank of rotifers (Brachionus plicatilis), as probiotics for aquaculture. Genes 2024, 15, 64. [Google Scholar] [CrossRef]
- Pakroo, S.; Tarrah, A.; Takur, R.; Wu, M.; Corich, V.; Giacomini, A. Limosilactobacillus fermentum ING8, a potential multifunctional non-starter strain with relevant technological properties and antimicrobial activity. Foods 2022, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Kim, D.-H. Genome-wide comparison reveals a probiotic strain Lactococcus lactis WFLU12 isolated from the gastrointestinal tract of olive flounder (Paralichthys olivaceus) harboring genes supporting probiotic action. Mar. Drugs 2018, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Bergonzelli, G.E.; Pridmore, R.D.; Marvin, L.; Rouvet, M.; Corthésy-Theulaz, I.E. Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect. Immun. 2004, 72, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Singh, K.S.; Choudhary, R.; Kumar, S.; Grover, S.; Mohanty, A.K.; Pande, V.; Kaushik, J.K. Expression of fibronectin-binding protein of L. acidophilus NCFM and in vitro refolding to adhesion capable native-like protein from inclusion bodies. Protein Expr. Purif. 2018, 145, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Couvigny, B.; Kulakauskas, S.; Pons, N.; Quinquis, B.; Abraham, A.-L.; Meylheuc, T.; Delorme, C.; Renault, P.; Briandet, B.; Lapaque, N.; et al. Identification of new factors modulating adhesion abilities of the pioneer commensal bacterium Streptococcus salivarius. Front. Microbiol. 2018, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Chaffanel, F.; Charron-Bourgoin, F.; Soligot, C.; Kebouchi, M.; Bertin, S.; Payot, S.; Le Roux, Y.; Leblond-Bourget, N. Surface proteins involved in the adhesion of Streptococcus salivarius to human intestinal epithelial cells. Appl. Microbiol. Biotechnol. 2018, 102, 2851–2865. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.; Roos, S.; Jonsson, H.; Rud, I.; Grimmer, S.; van Pijkeren, J.P.; Britton, R.A.; Axelsson, L. Role of Lactobacillus reuteri cell and mucus-binding protein A (CmbA) in adhesion to intestinal epithelial cells and mucus in vitro. Microbiology 2014, 160, 671–681. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.; Qu, H.; Wang, K.; Jing, S.; Guan, S.; Su, L.; Li, Q.; Wang, D. Taxifolin, an inhibitor of Sortase A, interferes with the adhesion of methicillin-resistant Staphylococcal aureus. Front. Microbiol. 2021, 12, 686864. [Google Scholar] [CrossRef] [PubMed]
- Low, K.E.; Howell, P.L. Gram-negative synthase-dependent exopolysaccharide biosynthetic machines. Curr. Opin. Struct. Biol. 2018, 53, 32–44. [Google Scholar] [CrossRef]
- Burghout, P.; Zomer, A.; van der Gaast-de Jongh, C.E.; Janssen-Megens, E.M.; Françoijs, K.J.; Stunnenberg, H.G.; Hermans, P.W. Streptococcus pneumoniae folate biosynthesis responds to environmental CO2 levels. J. Bacteriol. 2013, 195, 1573–1582. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Liu, Y.; Hu, F.; Xu, L.; Zheng, Q.; Wang, Q.; Zeng, G.; Zhang, K. Genomic and phenotypic characterization of Streptococcus mutans isolates suggests key gene clusters in regulating its interaction with Streptococcus gordonii. Front. Microbiol. 2022, 13, 945108. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Sun, Y.; Zeng, L.; Wu, H.; Gu, Q.; Li, P. Probiotic potential of a folate-producing strain Latilactobacillus sakei LZ217 and its modulation effects on human gut microbiota. Foods. 2022, 11, 234. [Google Scholar] [CrossRef]
- Vidal Amaral, J.R.; Jucá Ramos, R.T.; Almeida Araújo, F.; Bentes Kato, R.; Figueira Aburjaile, F.; de Castro Soares, S.; Góes-Neto, A.; Matiuzzi da Costa, M.; Azevedo, V.; Brenig, B.; et al. Bacteriocin producing Streptococcus agalactiae strains isolated from bovine mastitis in Brazil. Microorganisms. 2022, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Shah, N.; Prajapati, J.B. Biosynthesis of vitamins and enzymes in fermented foods by lactic acid bacteria and related genera—A promising approach. Croat. J. Food Sci. Technol. 2013, 5, 85–91. Available online: https://hrcak.srce.hr/113693 (accessed on 6 August 2024).
- Chen, S.; Zhang, F.; Ananta, E.; Muller, J.A.; Liang, Y.; Lee, Y.K.; Liu, S. Inoculation of Latilactobacillus sakei with Pichia kluyveri or Saccharomyces boulardii improves flavor compound profiles of salt-free fermented wheat-gluten: Effects from single strain inoculation. Curr. Res. Food. Sci. 2023, 6, 100492. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.C.; Saito, T.; Espe, M.; Whatmore, P.; Fernandes, J.M.O.; Vikeså, V.; Skjærven, K.H. Metabolic and molecular signatures of improved growth in Atlantic salmon (Salmo salar) fed surplus levels of methionine, folic acid, vitamin B6 and B12 throughout smoltification. Br. J. Nutr. 2022, 127, 1289–1302. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, A.; Sahu, C. Effect of probiotic on reproductive performance in female livebearing ornamental fish. Aquac. Res. 2007, 38, 518–526. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Pérez-Jiménez, A.; Costas, B.; Azeredo, R.; Gesto, M. Physiological roles of tryptophan in teleosts: Current knowledge and perspectives for future studies. Rev. Aquac. 2019, 11, 3–24. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, W.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids. 2016, 48, 41–51. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Liu, H.; Cao, Q.; Feng, L.; Zhang, Z.; Jiang, W.; Wu, P.; Liu, J.; Luo, W.; et al. Dietary leucine improves fish intestinal barrier function by increasing humoral immunity, antioxidant capacity, and tight junction. Int. J. Mol. Sci. 2023, 24, 4716. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Khan, M.A.; Yousefi, M.; Costas, B. Roles of arginine in fish nutrition and health: Insights for future researches. Rev. Aquac. 2020, 12, 2091–2108. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Z.; Ai, Q. Arginine metabolism and its functions in growth, nutrient utilization, and immunonutrition of fish. Anim. Nutr. 2021, 7, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Zhu, K.C.; Guo, H.Y.; Liu, B.S.; Zhang, N.; Zhang, D.C. Effects of cysteine addition to low-fishmeal diets on the growth, anti-oxidative stress, intestine immunity, and Streptococcus agalactiae resistance in juvenile golden pompano (Trachinotus ovatus). Front. Immunol. 2022, 13, 1066936. [Google Scholar] [CrossRef] [PubMed]
- Mok, W.J.; Hatanaka, Y.; Seoka, M.; Itoh, T.; Tsukamasa, Y.; Ando, M. Effects of additional cysteine in fish diet on mercury concentration. Food Chem. 2014, 147, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Belghit, I.; Skiba-Cassy, S.; Geurden, I.; Dias, K.; Surget, A.; Kaushik, S.; Panserat, S.; Seiliez, I. Dietary methionine availability affects the main factors involved in muscle protein turnover in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2014, 112, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.M.; Waagbø, R.; Espe, M. Functional amino acids in fish nutrition, health and welfare. Front. Biosci. (Elite Ed.). 2016, 8, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.E.; Jung, H.C.; Rhee, J.S.; Pan, J.G. Homofermentative production of D- or L-lactate in metabolically engineered Escherichia coli RR1. Appl. Environ. Microbiol. 1999, 65, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; HuangFu, H.; Wang, X.; Zhao, S.; Liu, Y.; Lv, H.; Qin, G.; Tan, Z. Antibacterial activity of lactic acid producing Leuconostoc mesenteroides QZ1178 against pathogenic Gallibacterium anatis. Front. Vet. Sci. 2021, 8, 630294. [Google Scholar] [CrossRef]
- Alayande, K.A.; Aiyegoro, O.A.; Nengwekhulu, T.M.; Katata-Seru, L.; Ateba, C.N. Integrated genome-based probiotic relevance and safety evaluation of Lactobacillus reuteri PNW1. PLoS ONE 2020, 15, e0235873. [Google Scholar] [CrossRef]
- Tuomola, E.; Crittenden, R.; Playne, M.; Isolauri, E.; Salminen, S. Quality assurance criteria for probiotic bacteria. Am. J. Clin. Nutr. 2001, 73, 393S–398S. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Saraiva, T.D.; Silva, W.M.; Pereira, U.P.; Campos, B.C.; Benevides, L.J.; Rocha, F.S.; Figueiredo, H.C.; Azevedo, V.; Soares, S.C. Analyses of the probiotic property and stress resistance-related genes of Lactococcus lactis subsp. lactis NCDO 2118 through comparative genomics and in vitro assays. PLoS ONE 2017, 12, e0175116. [Google Scholar] [CrossRef] [PubMed]
- Heng, N.C.; Haji-Ishak, N.S.; Kalyan, A.; Wong, A.Y.; Lovric, M.; Bridson, J.M.; Artamonova, J.; Stanton, J.A.; Wescombe, P.A.; Burton, J.P.; et al. Genome sequence of the bacteriocin-producing oral probiotic Streptococcus salivarius strain M18. J. Bacteriol. 2011, 193, 6402–6403. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, 5206. [Google Scholar] [CrossRef]
- Argov, T.; Azulay, G.; Pasechnek, A.; Stadnyuk, O.; Ran-Sapir, S.; Borovok, I.; Sigal, N.; Herskovits, A.A. Temperate bacteriophages as regulators of host behavior. Curr. Opin. Microbiol. 2017, 38, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Katyal, I.; Chaban, B.; Ng, B.; Hill, J.E. CRISPRs of Enterococcus faecalis and E. hirae isolates from pig feces have species-specific repeats but share some common spacer sequences. Microb. Ecol. 2013, 66, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Butiuc-Keul, A.; Farkas, A.; Carpa, R.; Iordache, D. CRISPR-Cas System: The powerful modulator of accessory genomes in prokaryotes. Microb. Physiol. 2022, 32, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Ruseler-van Embden, J.; van Lieshout, L.; Gosselink, M.J.; Marteau, P. Inability of Lactobacillus casei strain GG, L. acidophilus, and Bifidobacterium bifidum to degrade intestinal mucus glycoproteins. Scand. J. Gastroenterol. 1995, 30, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Conway, P.L.; Gorbach, S.L.; Goldin, B.R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 1987, 70, 1–12. [Google Scholar] [CrossRef]
- De Smet, I.; Van Hoorde, L.; Vande Woestyne, M.; Christiaens, H.; Verstraete, W. Significance of bile salt hydrolytic activities of lactobacilli. J. Appl. Bacteriol. 1995, 79, 292–301. [Google Scholar] [CrossRef]
- Grill, J.P.; Cayuela, C.; Antoine, J.M.; Schneider, F. Isolation and characterization of a Lactobacillus amylovorus mutant depleted in conjugated bile salt hydrolase activity: Relation between activity and bile salt resistance. J. Appl. Microbiol. 2000, 89, 553–563. [Google Scholar] [CrossRef]
- Usman Hosono, A. Bile tolerance, taurocholate deconjugation, and binding of cholesterol by Lactobacillus gasseri strains. J. Dairy Sci. 1999, 82, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.A.; Savage, D.C. Bile salt hydrolase activity and resistance to toxicity of conjugated bile salts are unrelated properties in lactobacilli. Appl. Environ. Microbiol. 2001, 67, 3476–3480. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef]
- Marteau, P.; Gerhardt, M.; Myara, A.; Bouvier, E.; Trivin, F.C.; Rambaud, J. Metabolism of bile salts by alimentary bacteria during transit in the human small intestine. Microb. Ecol. Health Dis. 1995, 8, 151–157. [Google Scholar] [CrossRef][Green Version]
- Nagengast, F.M.; Grubben, M.; van Munster, I. Role of bile acids in colorectal carcinogenesis. Eur. J. Cancer. 1995, 31, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Isolauri, E.; Salminen, E. Clinical uses of probiotics for stabilizing the gut mucosal barrier: Successful strains and future challenges. Antonie van Leeuwenhoek. 1996, 70, 347–358. [Google Scholar] [CrossRef] [PubMed]
- European Commission. “Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs”. Off. J. Eur. Union 2005, L322, 1–19. [Google Scholar]
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Vidal-Carou, M.C.; Izquierdo-Pulido, M.L.; Martín-Morro, M.C.; Mariné-Font, A. Histamine and tyramine in meat products: Relationship with meat spoilage. Food Chem. 1990, 37, 239–249. [Google Scholar] [CrossRef]
- Landete, J.M.; de Las Rivas, B.; Marcobal, A.; Muñoz, R. Molecular methods for the detection of biogenic amine-producing bacteria on foods. Int. J. Food Microbiol. 2007, 117, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.O.; Gopal, T.K.S. Biogenic amines in seafood: A review. J. Food Sci. Technol. 2016, 53, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Uniacke-Lowe, S.; Collins, F.W.J.; Hill, C.; Ross, R.P. Bioactivity screening and genomic analysis reveals deep-sea fish microbiome isolates as sources of novel antimicrobials. Mar Drugs. 2023, 21, 444. [Google Scholar] [CrossRef] [PubMed]
- Teber, R.; Asakawa, S. In silico screening of bacteriocin gene clusters within a set of marine Bacillota genomes. Int. J. Mol. Sci. 2024, 25, 2566. [Google Scholar] [CrossRef]
- Araújo, C.; Muñoz-Atienza, E.; Ramírez, M.; Poeta, P.; Igrejas, G.; Hernández, P.E.; Herranz, C.; Cintas, L.M. Safety assessment, genetic relatedness and bacteriocin activity of potential probiotic Lactococcus lactis strains from rainbow trout (Oncorhynchus mykiss, Walbaum) and rearing environment. Eur. Food Res. Technol. 2015, 241, 647–662. [Google Scholar] [CrossRef]
- Contente, D.; Díaz-Rosales, P.; Feito, J.; Díaz-Formoso, L.; Docando, F.; Simón, R.; Borrero, J.; Hernández, P.E.; Poeta, P.; Muñoz-Atienza, E.; et al. Immunomodulatory effects of bacteriocinogenic and non-bacteriocinogenic Lactococcus cremoris of aquatic origin on rainbow trout (Oncorhynchus mykiss, Walbaum). Front. Immunol. 2023, 14, 1178462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).