Oral Fecal Microbiota Transplantation in Dogs with Tylosin-Responsive Enteropathy—A Proof-of-Concept Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Ethics
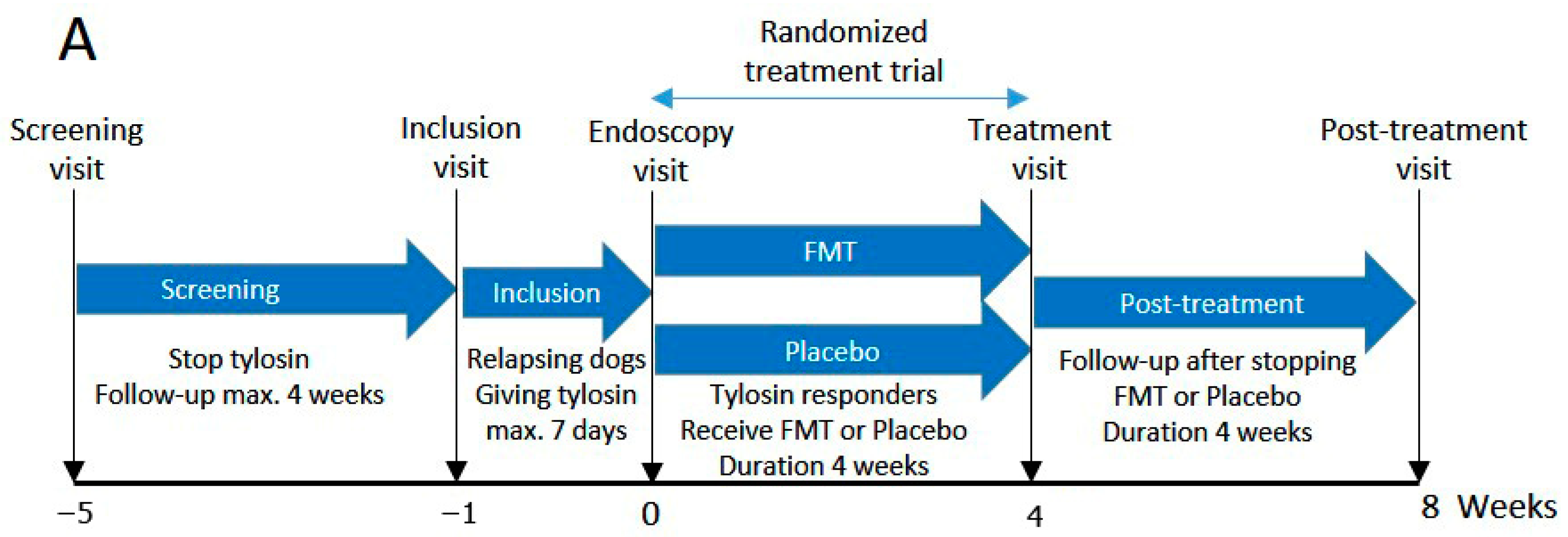
2.2. Trial Design
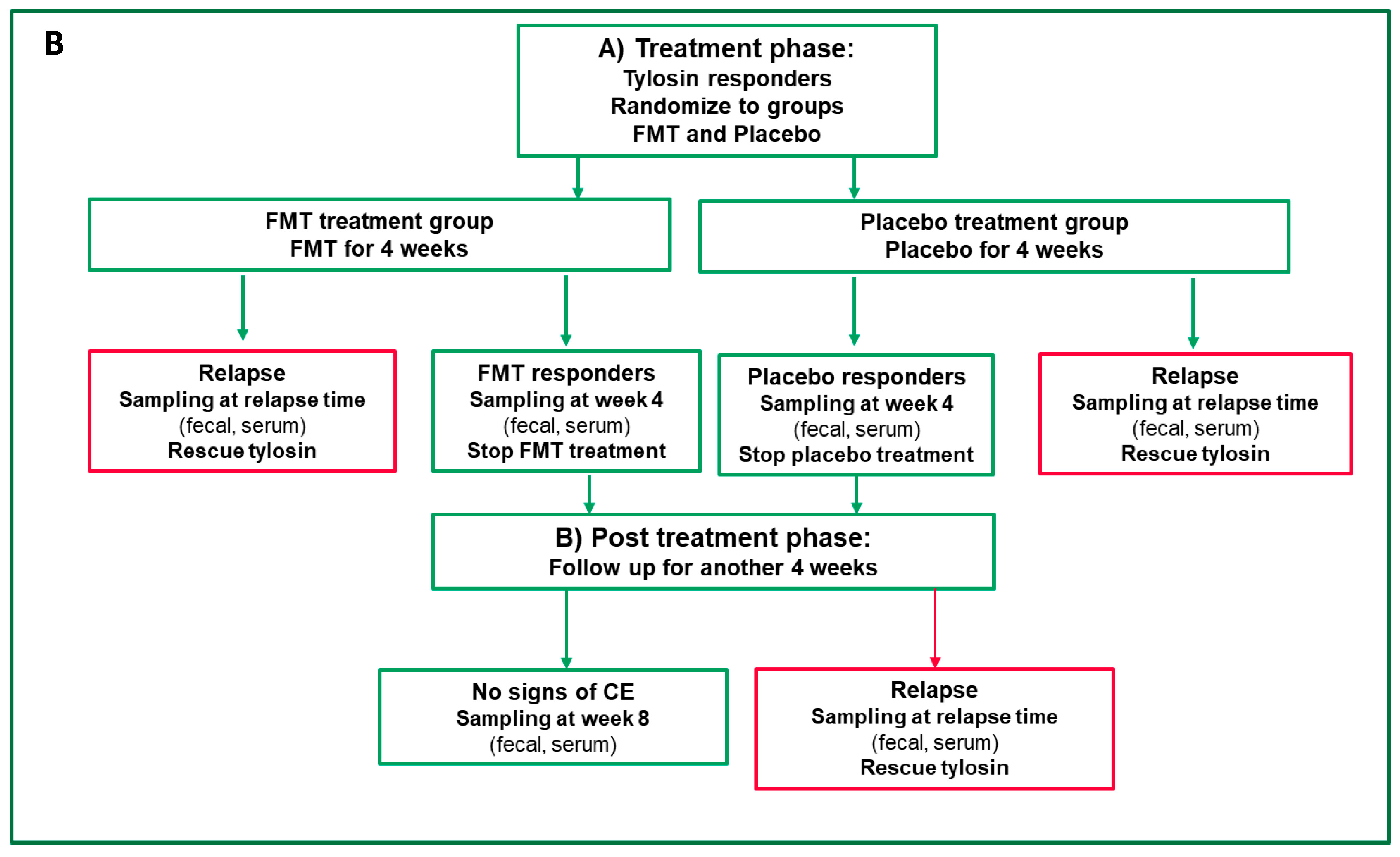
2.3. Definition of Clinical Remission and Relapse
2.4. Test Product and Mode of Patient Randomization
2.4.1. Fecal Microbiota Transplantation (FMT) or Placebo Products
2.4.2. Mode of Randomization
2.5. Endoscopy and Histopathology
2.6. Fecal Dry Matter
2.7. Healthy Dog Microbiomes
2.8. DNA Extraction and Full-Length 16S rRNA Amplicon Gene Sequencing of Fecal Microbiome
2.9. Bioinformatic Processing and Data Analysis of Fecal Microbiota Sequencing
2.10. Statistical Analysis
2.10.1. Analysis of CCECAI, FCS, and FDM Variables
2.10.2. Microbiome Statistical Analyses
3. Results
3.1. Study Population
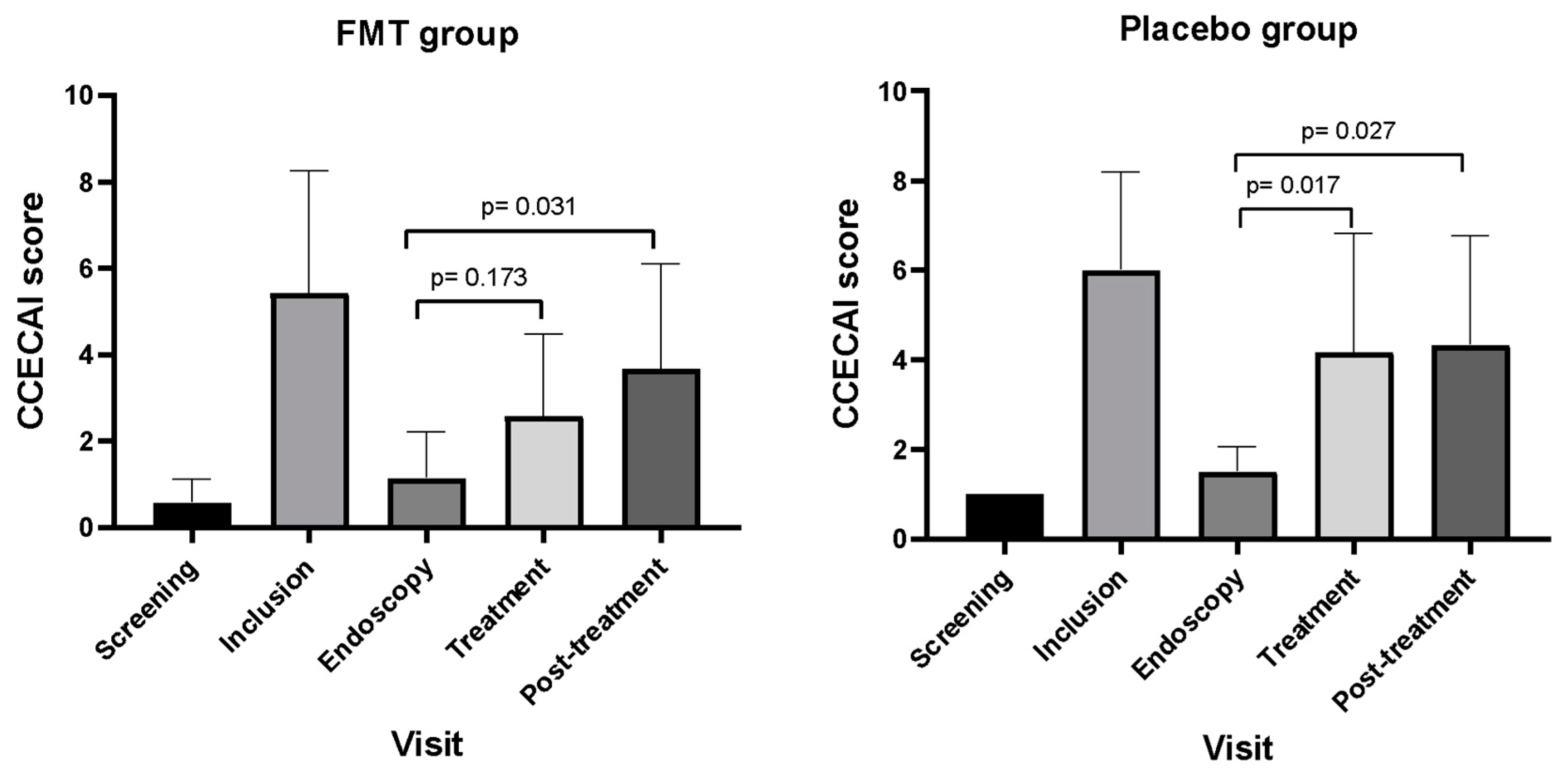
3.2. Outcomes Based on CCECAI Scores
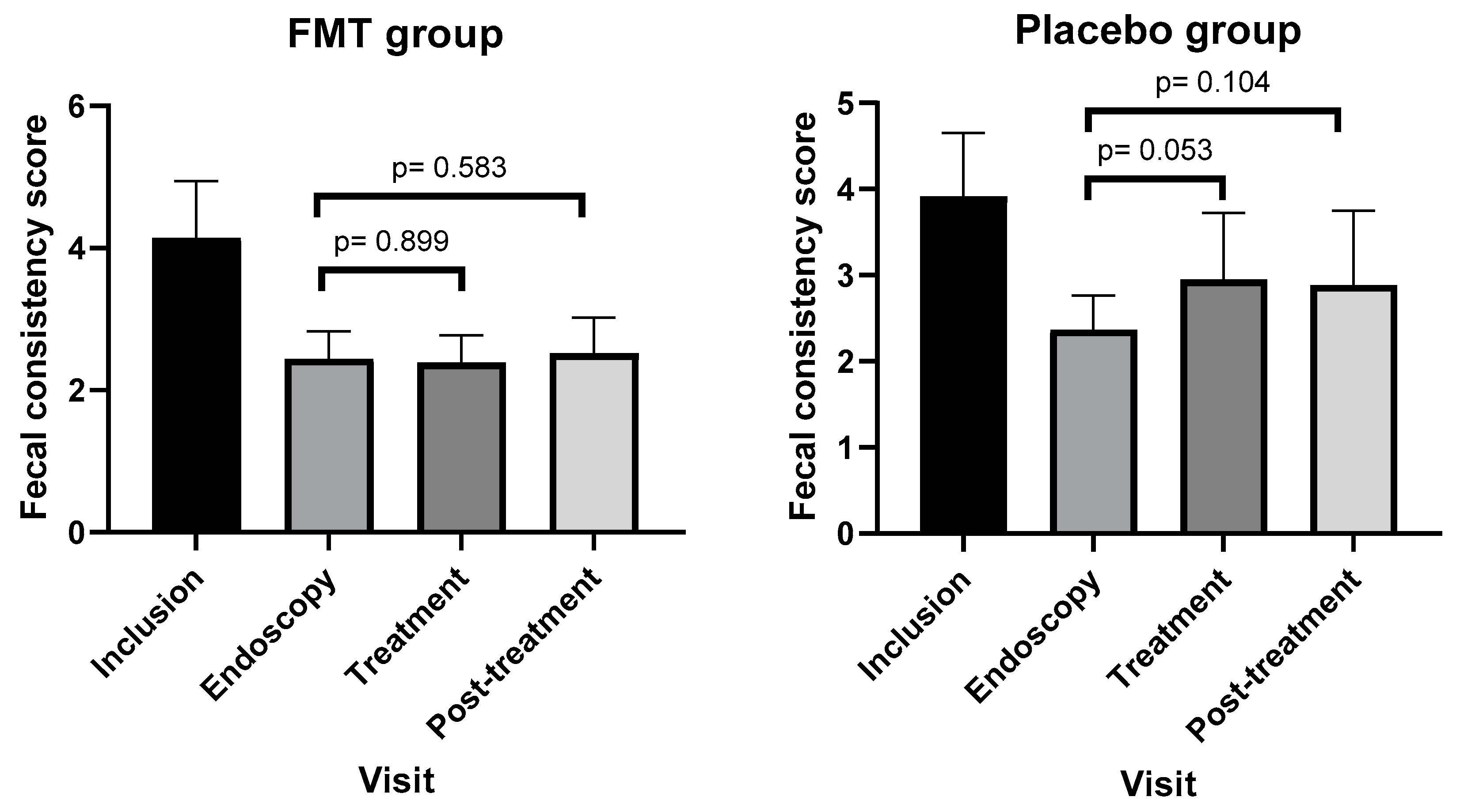
3.3. Outcomes Based on Fecal Consistency Scores
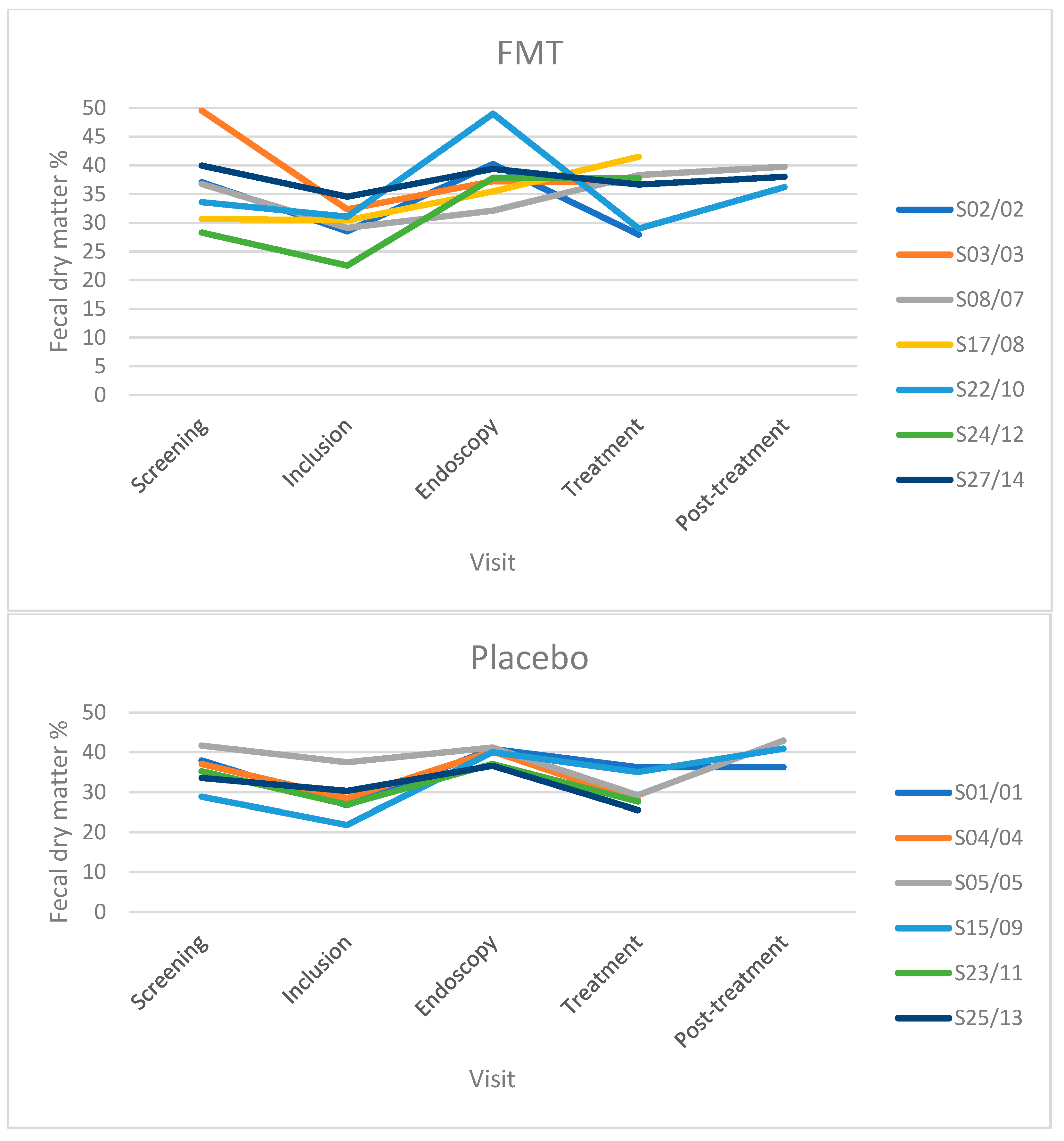
3.4. Fecal Dry Matter
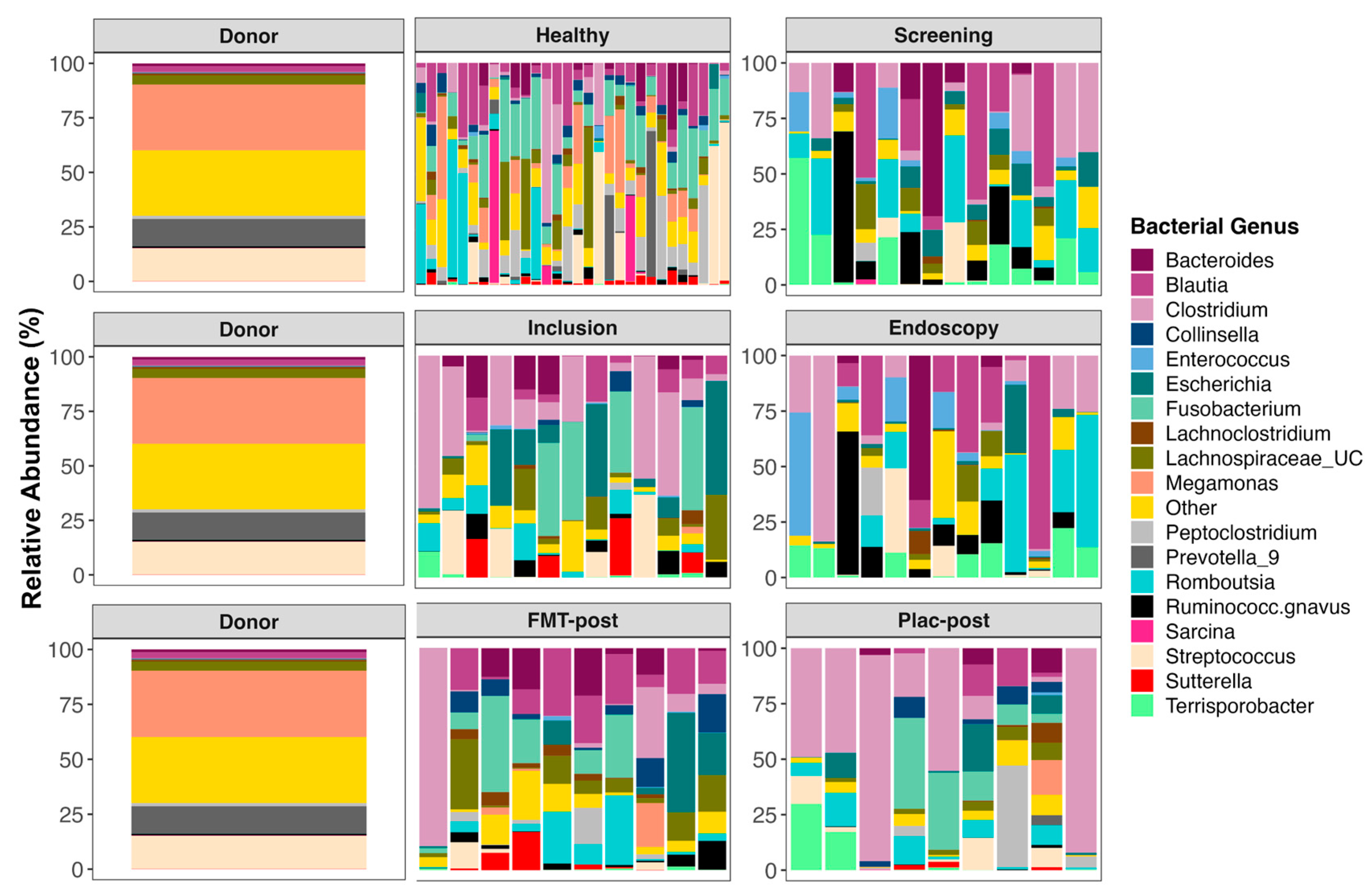
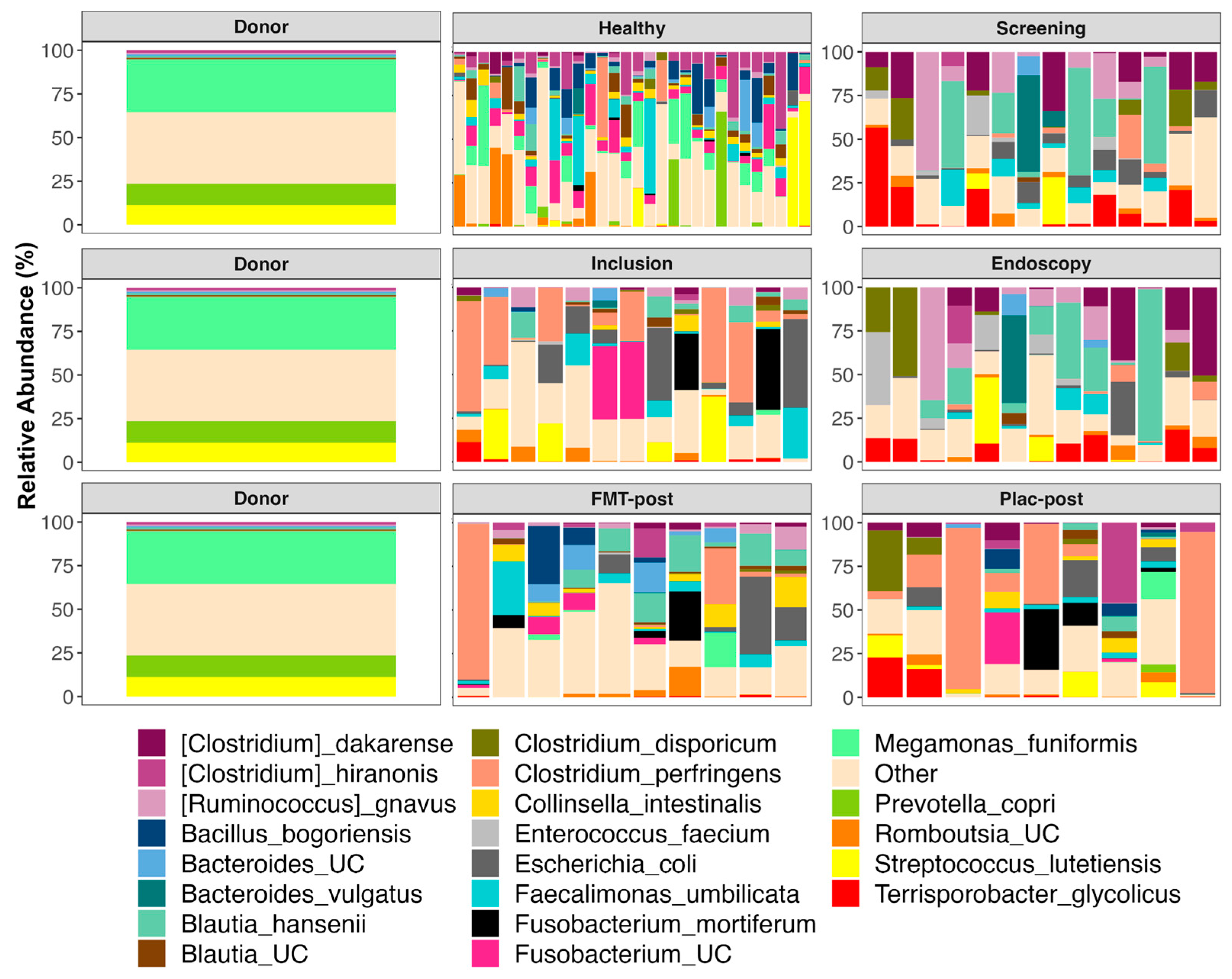
3.5. The Composition of Fecal Microbiomes before and after Treatment
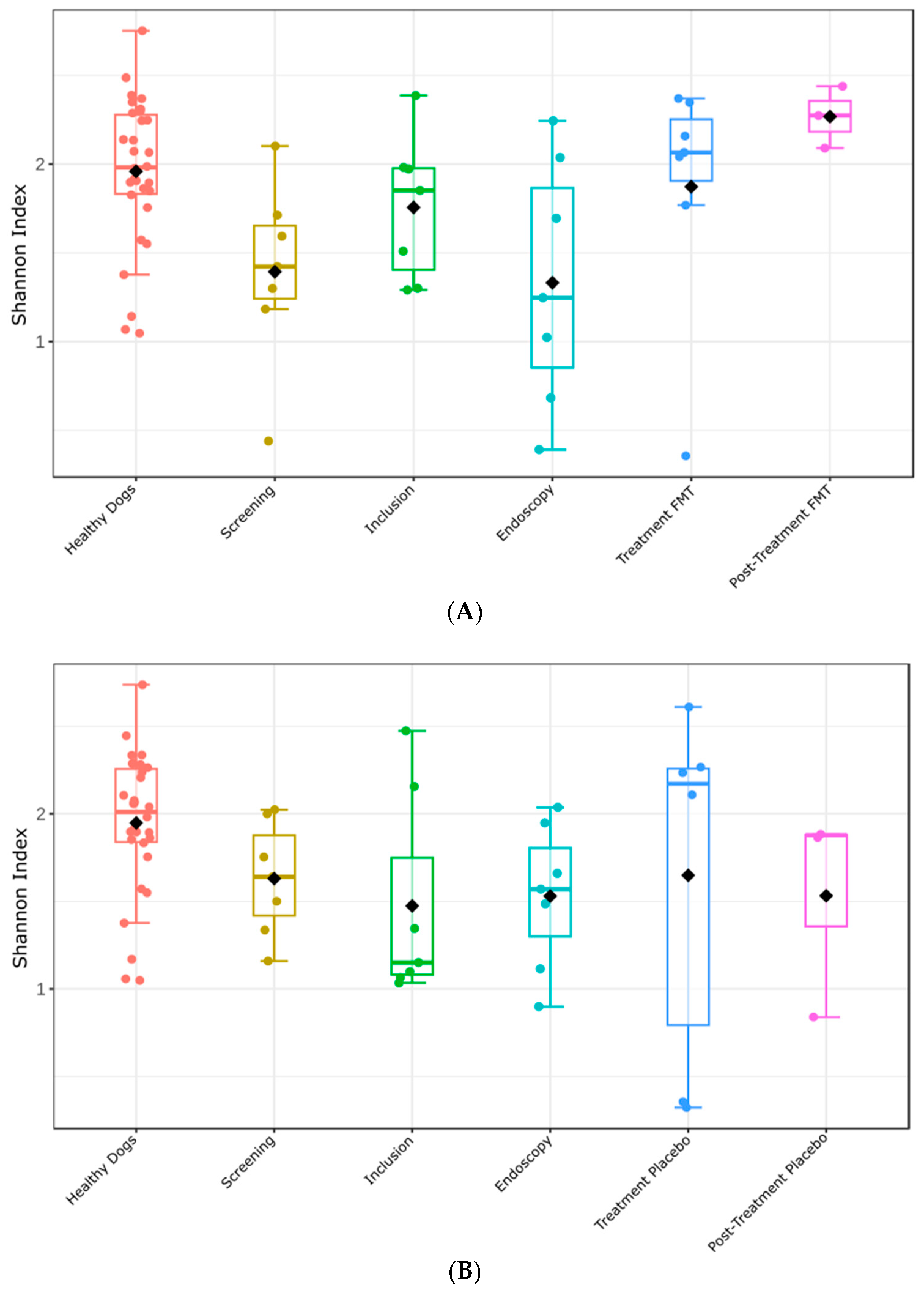
3.6. Fecal Microbiome Alpha- and Beta-Diversity before and after Treatment
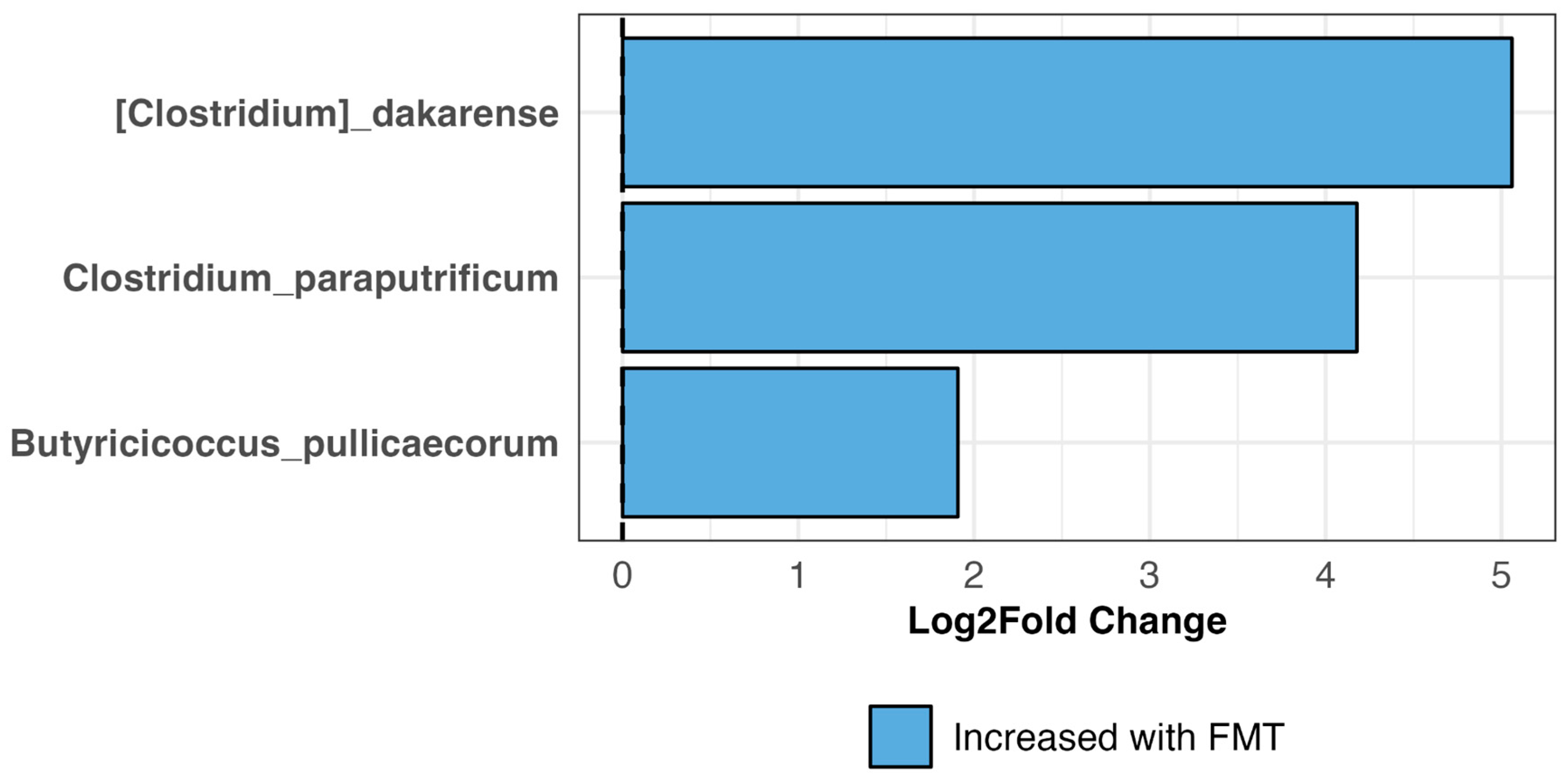
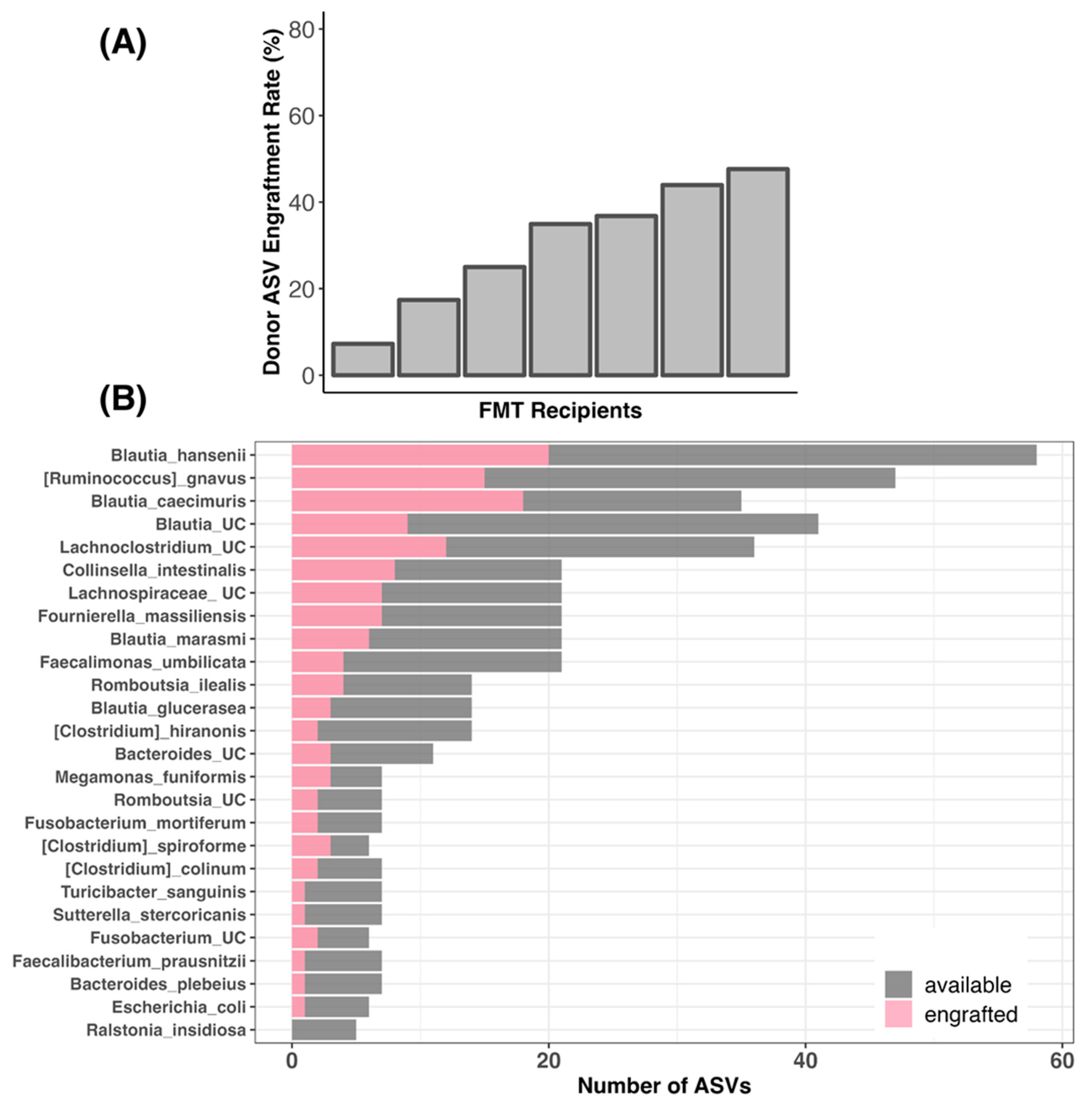
3.7. Bacterial Engraftment in TRE Dogs Receiving FMT Treatment
3.8. Adverse Events (AEs)
3.9. Histopathology
4. Discussion
4.1. Efficacy of FMT Based on CCECAI, FCS, and FDM
4.2. Impact of FMT on Microbiome Composition
4.3. Impact of FMT on Microbiome Alpha and Beta Diversity
4.4. Efficacy of FMT in Regards to Bacterial Engraftment
4.5. Adverse Effects and Study Implications
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dandrieux, J.R.S.; Mansfield, C.S. Chronic Enteropathy in Canines: Prevalence, Impact and Management Strategies. Vet. Med. 2019, 10, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, S.; Spillmann, T.; Syrja, P.; Skrzypczak, T.; Louhelainen, M.; Westermarck, E. Effect of tylosin on dogs with suspected tylosin-responsive diarrhea: A placebo-controlled, randomized, double-blinded, prospective clinical trial. Acta Vet. Scand. 2011, 53, 26. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, S.; Spillmann, T.; Westermarck, E. Efficacy of two low-dose oral tylosin regimens in controlling the relapse of diarrhea in dogs with tylosin-responsive diarrhea: A prospective, single-blinded, two-arm parallel, clinical field trial. Acta Vet. Scand. 2014, 56, 43. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Dowd, S.E.; Westermarck, E.; Steiner, J.M.; Wolcott, R.D.; Spillmann, T.; Harmoinen, J.A. The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rRNA gene sequencing. BMC Microbiol. 2009, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Ma, X.; Geng, S.; Jiang, X.; Li, Y.; Hu, L.; Li, J.; Wang, Y.; Han, X. Fecal Microbiota Transplantation Beneficially Regulates Intestinal Mucosal Autophagy and Alleviates Gut Barrier Injury. mSystems 2018, 3, e00137-18. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Vaughn, B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 2017, 8, 238–252. [Google Scholar] [CrossRef]
- El Hage, R.; Hernandez-Sanabria, E.; Van de Wiele, T. Emerging Trends in “Smart Probiotics”: Functional Consideration for the Development of Novel Health and Industrial Applications. Front. Microbiol. 2017, 8, 1889. [Google Scholar] [CrossRef]
- Burrello, C.; Giuffrè, M.R.; Macandog, A.D.; Diaz-Basabe, A.; Cribiù, F.M.; Lopez, G.; Borgo, F.; Nezi, L.; Caprioli, F.; Vecchi, M.; et al. Fecal Microbiota Transplantation Controls Murine Chronic Intestinal Inflammation by Modulating Immune Cell Functions and Gut Microbiota Composition. Cells 2019, 8, 517. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Li, Y.; Zhao, H.; He, C.; Wang, Z.; Zhao, H. Fecal microbiota transplantation reconstructs the gut microbiota of septic mice and protects the intestinal mucosal barrier. bioRxiv 2021, 11:736204. [Google Scholar] [CrossRef]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Fecal microbiota transplantation as a new treatment for canine inflammatory bowel disease. Biosci. Microbiota Food Health 2021, 40, 98–104. [Google Scholar] [CrossRef]
- Pereira, G.Q.; Gomes, L.A.; Santos, I.S.; Alfieri, A.F.; Weese, J.S.; Costa, M.C. Fecal microbiota transplantation in puppies with canine parvovirus infection. J. Vet. Intern. Med. 2018, 32, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Toresson, L.; Spillmann, T.; Pilla, R.; Ludvigsson, U.; Hellgren, J.; Olmedal, G.; Suchodolski, J.S. Clinical Effects of Faecal Microbiota Transplantation as Adjunctive Therapy in Dogs with Chronic Enteropathies—A Retrospective Case Series of 41 Dogs. Vet. Sci. 2023, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Tuniyazi, M.; Hu, X.; Fu, Y.; Zhang, N. Canine Fecal Microbiota Transplantation: Current Application and Possible Mechanisms. Vet. Sci. 2022, 9, 396. [Google Scholar] [CrossRef]
- Collier, A.J.; Gomez, D.E.; Monteith, G.; Plattner, B.L.; Verbrugghe, A.; Webb, J.; Weese, J.S.; Blois, S.L. Investigating fecal microbial transplant as a novel therapy in dogs with inflammatory bowel disease: A preliminary study. PLoS ONE 2022, 17, e0276295. [Google Scholar] [CrossRef] [PubMed]
- Innocente, G.; Patuzzi, I.; Furlanello, T.; Di Camillo, B.; Bargelloni, L.; Giron, M.C.; Facchin, S.; Savarino, E.; Azzolin, M.; Simionati, B. Machine Learning and Canine Chronic Enteropathies: A New Approach to Investigate FMT Effects. Vet. Sci. 2022, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef]
- Winston, J.A.; Suchodolski, J.S.; Gaschen, F.; Busch, K.; Marsilio, S.; Costa, M.C.; Chaitman, J.; Coffey, E.L.; Dandrieux, J.R.S.; Gal, A.; et al. Clinical Guidelines for Fecal Microbiota Transplantation in Companion Animals. Adv. Small Anim. Care, 2024; in press. [Google Scholar] [CrossRef]
- Rojas, C.A.; Park, B.; Jospin, G.; Scarsella, E.; Entrolezo, Z.; Jarett, J.K.; Martin, A.; Ganz, H.H. Species-level characterization of the core microbiome in healthy dogs using fulllength 16S rRNA gene sequencing. Front. Vet. Sci. 2024, 11, 1405470. [Google Scholar] [CrossRef]
- Ohno, H.; Murakami, H.; Tanisawa, K.; Konishi, K.; Miyachi, M. Validity of an observational assessment tool for multifaceted evaluation of faecal condition. Sci. Rep. 2019, 9, 3760. [Google Scholar] [CrossRef]
- Horng, K.R.; Ganz, H.H.; Eisen, J.A.; Marks, S.L. Effects of preservation method on canine (Canis lupus familiaris) fecal microbiota. PeerJ 2018, 6, e4827. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Zhou, H.; He, K.; Chen, J.; Zhang, X. LinDA: Linear models for differential abundance analysis of microbiome compositional data. Genome Biol. 2022, 23, 95. [Google Scholar] [CrossRef]
- Rojas, C.A.; Entrolezo, Z.; Jarett, J.K.; Jospin, G.; Martin, A.; Ganz, H.H. Microbiome Responses to Oral Fecal Microbiota Transplantation in a Cohort of Domestic Dogs. Vet. Sci. 2024, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; Hara, R.; Solymos, P.; Stevens, H.; Szöcs, E.; et al. Vegan Community Ecology Package, version 2.6-8; The Comprehensive R Archive Network: 2022. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 4 June 2024).
- Fritsch, D.A.; Jackson, M.I.; Wernimont, S.M.; Feld, G.K.; MacLeay, J.M.; Brejda, J.J.; Cochrane, C.Y.; Gross, K.L. Microbiome function underpins the efficacy of a fiber-supplemented dietary intervention in dogs with chronic large bowel diarrhea. BMC Vet. Res. 2022, 18, 245. [Google Scholar] [CrossRef]
- Glanemann, B.; Seo, Y.J.; Priestnall, S.L.; Garden, O.A.; Kilburn, L.; Rossoni-Serao, M.; Segarra, S.; Mochel, J.P.; Allenspach, K. Clinical efficacy of prebiotics and glycosaminoglycans versus placebo In dogs with food responsive enteropathy receiving a hydrolyzed diet: A pilot study. PLoS ONE 2021, 16, e0250681. [Google Scholar] [CrossRef]
- Ing, N.H.; Steiner, J.M. The Use of Diets in the Diagnosis and Treatment of Common Gastrointestinal Diseases in Dogs and Cats. Adv. Exp. Med. Biol. 2024, 1446, 39–53. [Google Scholar] [CrossRef]
- Manchester, A.C.; Dow, S.; Chow, L.; Gagne, J.; Lappin, M.R. Efficacy of an elemental diet in achieving clinical remission in dogs with chronic enteropathy. J. Vet. Intern. Med. 2023, 37, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.A.; Parker, V.J.; Winston, J.A.; Rudinsky, A.J. Dietary fiber aids in the management of canine and feline gastrointestinal disease. J. Am. Vet. Med. Assoc. 2022, 260, S33–S45. [Google Scholar] [CrossRef] [PubMed]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Improvement in Clinical Symptoms and Fecal Microbiome After Fecal Microbiota Transplantation in a Dog with Inflammatory Bowel Disease. Vet. Med. 2019, 10, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Yanuma, N.; Ohno, H.; Takahashi, K.; Kawano, K.; Morita, H.; Ohmori, K. Oral faecal microbiota transplantation for the treatment of Clostridium difficile-associated diarrhoea in a dog: A case report. BMC Vet. Res. 2019, 15, 11. [Google Scholar] [CrossRef]
- Ingle, D.J.; Valcanis, M.; Kuzevski, A.; Tauschek, M.; Inouye, M.; Stinear, T.; Levine, M.M.; Robins-Browne, R.M.; Holt, K.E. In silico serotyping of E. coli from short read data identifies limited novel O-loci but extensive diversity of O:H serotype combinations within and between pathogenic lineages. Microb. Genom. 2016, 2, e000064. [Google Scholar] [CrossRef]
- Sakamoto, M.; Ikeyama, N.; Yuki, M.; Ohkuma, M. Draft Genome Sequence of Faecalimonas umbilicata JCM 30896(T), an Acetate-Producing Bacterium Isolated from Human Feces. Microbiol. Resour. Announc. 2018, 7, e01091-18. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Maclean, P.; Thomas, D.G.; Cave, N.J.; Young, W. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. PeerJ 2017, 5, e3019. [Google Scholar] [CrossRef]
- Rojas, C.A.; Entrolezo, Z.; Jarett, J.K.; Jospin, G.; Kingsbury, D.D.; Martin, A.; Eisen, J.A.; Ganz, H.H. Microbiome Responses to Fecal Microbiota Transplantation in Cats with Chronic Digestive Issues. Vet. Sci. 2023, 10, 561. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.L. Species diversity of fecal microbial flora in Canis lupus familiaris infected with canine parvovirus. Vet. Microbiol. 2019, 237, 108390. [Google Scholar] [CrossRef]
- Leite, S.; Cotias, C.; Rainha, K.C.; Santos, M.G.; Penna, B.; F. Moraes, R.F.; Harmanus, C.; Smits, W.K.; Ferreira, E.d.O. Prevalence of Clostridioides difficile in dogs (Canis familiaris) with gastrointestinal disorders in Rio de Janeiro. Anaerobe 2023, 83, 102765. [Google Scholar] [CrossRef]
- Berlanda, M.; Innocente, G.; Simionati, B.; Di Camillo, B.; Facchin, S.; Giron, M.C.; Savarino, E.; Sebastiani, F.; Fiorio, F.; Patuzzi, I. Faecal Microbiome Transplantation as a Solution to Chronic Enteropathies in Dogs: A Case Study of Beneficial Microbial Evolution. Animals 2021, 11, 1433. [Google Scholar] [CrossRef]
- Manchester, A.C.; Webb, C.B.; Blake, A.B.; Sarwar, F.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. J. Vet. Intern. Med. 2019, 33, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Marclay, M.; Dwyer, E.; Suchodolski, J.S.; Lidbury, J.A.; Steiner, J.M.; Gaschen, F.P. Recovery of Fecal Microbiome and Bile Acids in Healthy Dogs after Tylosin Administration with and without Fecal Microbiota Transplantation. Vet. Sci. 2022, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, F.; Hou, Q.; Huang, W.; Liu, Y.; Zhang, H.; Sun, Z. Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhoea. Food Funct. 2019, 10, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Morelli, G.; Patuzzi, I.; Losasso, C.; Ricci, A.; Contiero, B.; Andrighetto, I.; Ricci, R. Characterization of intestinal microbiota in normal weight and overweight Border Collie and Labrador Retriever dogs. Sci. Rep. 2022, 12, 9199. [Google Scholar] [CrossRef]
- Togo, A.H.; Durand, G.; Khelaifia, S.; Armstrong, N.; Robert, C.; Cadoret, F.; Di Pinto, F.; Delerce, J.; Levasseur, A.; Raoult, D.; et al. Fournierella massiliensis gen. nov., sp. nov., a new human-associated member of the family Ruminococcaceae. Int. J. Syst. Evol. Microbiol. 2017, 67, 1393–1399. [Google Scholar] [CrossRef]
- Imdad, A.; Pandit, N.G.; Zaman, M.; Minkoff, N.Z.; Tanner-Smith, E.E.; Gomez-Duarte, O.G.; Acra, S.; Nicholson, M.R. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 2023, 4, CD012774. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef]
- Cerquetella, M.; Marchegiani, A.; Rossi, G.; Trabalza-Marinucci, M.; Passamonti, F.; Isidori, M.; Rueca, F. Case Report: Oral Fecal Microbiota Transplantation in a Dog Suffering From Relapsing Chronic Diarrhea—Clinical Outcome and Follow-Up. Front. Vet. Sci. 2022, 9, 893342. [Google Scholar] [CrossRef]
- Youngster, I.; Mahabamunuge, J.; Systrom, H.K.; Sauk, J.; Khalili, H.; Levin, J.; Kaplan, J.L.; Hohmann, E.L. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016, 14, 134. [Google Scholar] [CrossRef]
- Petrof, E.O.; Gloor, G.B.; Vanner, S.J.; Weese, S.J.; Carter, D.; Daigneault, M.C.; Brown, E.M.; Schroeter, K.; Allen-Vercoe, E. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome 2013, 1, 3. [Google Scholar] [CrossRef] [PubMed]


| FMT (N = 7) | Placebo (N = 7) | Total (N = 14) | p Values | ||
|---|---|---|---|---|---|
| Variable | n (%) | n (%) | n (%) | ||
| Age [years] | N | 7 | 7 | 14 | 0.452 |
| Min | 1 | 2 | 1 | ||
| Median | 2.0 | 4.0 | 2.5 | ||
| Max | 13 | 13 | 13 | ||
| Sex | Female | 4 (57.1) | 2 (28.6) | 6 (42.9) | 0.28 |
| Male | 3 (42.9) | 5 (71.4) | 8 (57.1) | ||
| Weight [kg] | N | 7 | 7 | 14 | 0.729 |
| Min | 6.4 | 9.8 | 6.4 | ||
| Median | 16.80 | 20.40 | 18.90 | ||
| Max | 49.4 | 34.7 | 49.4 | ||
| Neutered | No | 3 (42.9) | 4 (57.1) | 7 (50.0) | 0.592 |
| Yes | 4 (57.1) | 3 (42.9) | 7 (50.0) | ||
| Breed | Collie | 1 | 3 | 4 | |
| Cavalier King Charles Spaniel | 2 | 0 | 2 | ||
| Australian Kelpie | 1 | 0 | 1 | ||
| Coton de tulear | 0 | 1 | 1 | ||
| Giant Poodle | 1 | 0 | 1 | ||
| Golden Retriever | 1 | 0 | 1 | ||
| Lagotto Romagnolo | 0 | 1 | 1 | ||
| Mixed breed | 1 | 0 | 1 | ||
| Schapendoes | 0 | 1 | 1 | ||
| White Shepherd | 0 | 1 | 1 | ||
| Group | Visit | |||||||
|---|---|---|---|---|---|---|---|---|
| Screening | Inclusion | Endoscopy | Treatment | Post-Treatment | ||||
| Relapse | No Relapse | Relapse | No Relapse | |||||
| FMT | Number of dogs | 7 | 7 | 7 | 2 | 5 | 1 | 2 |
| Median (range) of FDM percentage | 36.75 (28.3–49.6) | 30.45 (22.6–34.6) | 37.85 (32.1–49) | 34.73 (27.95–41.5) | 36.95 (29–38.3) | 38 | 37.98 (36.2–39.75) | |
| Placebo | Number of dogs | 6 | 6 | 6 | 3 | 3 | 0 | 3 |
| Median (range) of FDM percentage | 36.6 (28.9–45) | 28.60 (21.9–37.5) | 38.58 (33.7–41.2) | 27.65 (25.55–28.75) | 35.05 (29.25–36.25) | 40.95 (36.25–42.95) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanifeh, M.; Scarsella, E.; Rojas, C.A.; Ganz, H.H.; Huhtinen, M.; Laine, T.; Spillmann, T. Oral Fecal Microbiota Transplantation in Dogs with Tylosin-Responsive Enteropathy—A Proof-of-Concept Study. Vet. Sci. 2024, 11, 439. https://doi.org/10.3390/vetsci11090439
Hanifeh M, Scarsella E, Rojas CA, Ganz HH, Huhtinen M, Laine T, Spillmann T. Oral Fecal Microbiota Transplantation in Dogs with Tylosin-Responsive Enteropathy—A Proof-of-Concept Study. Veterinary Sciences. 2024; 11(9):439. https://doi.org/10.3390/vetsci11090439
Chicago/Turabian StyleHanifeh, Mohsen, Elisa Scarsella, Connie A. Rojas, Holly H. Ganz, Mirja Huhtinen, Tarmo Laine, and Thomas Spillmann. 2024. "Oral Fecal Microbiota Transplantation in Dogs with Tylosin-Responsive Enteropathy—A Proof-of-Concept Study" Veterinary Sciences 11, no. 9: 439. https://doi.org/10.3390/vetsci11090439
APA StyleHanifeh, M., Scarsella, E., Rojas, C. A., Ganz, H. H., Huhtinen, M., Laine, T., & Spillmann, T. (2024). Oral Fecal Microbiota Transplantation in Dogs with Tylosin-Responsive Enteropathy—A Proof-of-Concept Study. Veterinary Sciences, 11(9), 439. https://doi.org/10.3390/vetsci11090439









