Evaluating Forelimb and Hindlimb Joint Conformation of Morna Racehorses (Equus caballus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Study Design
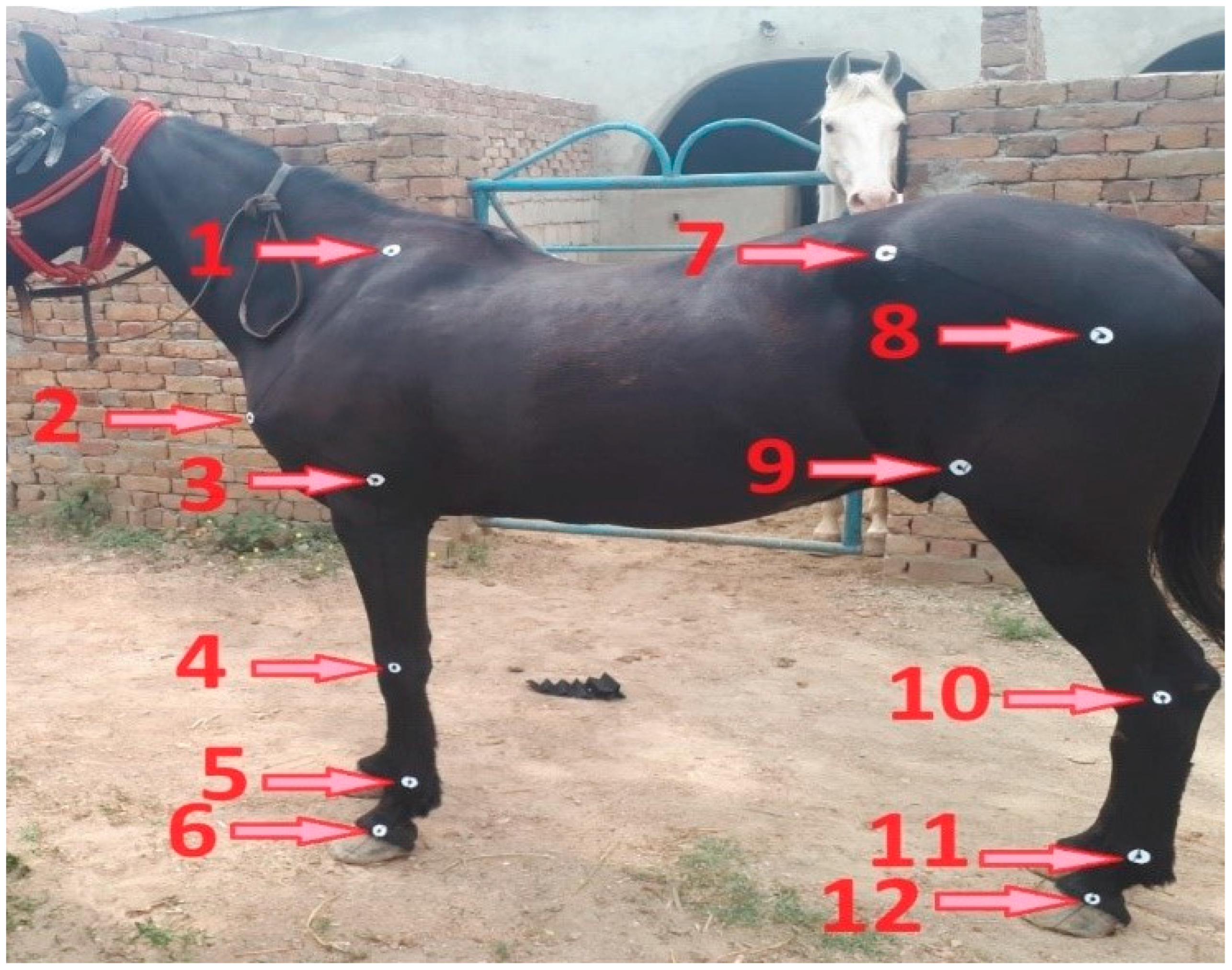
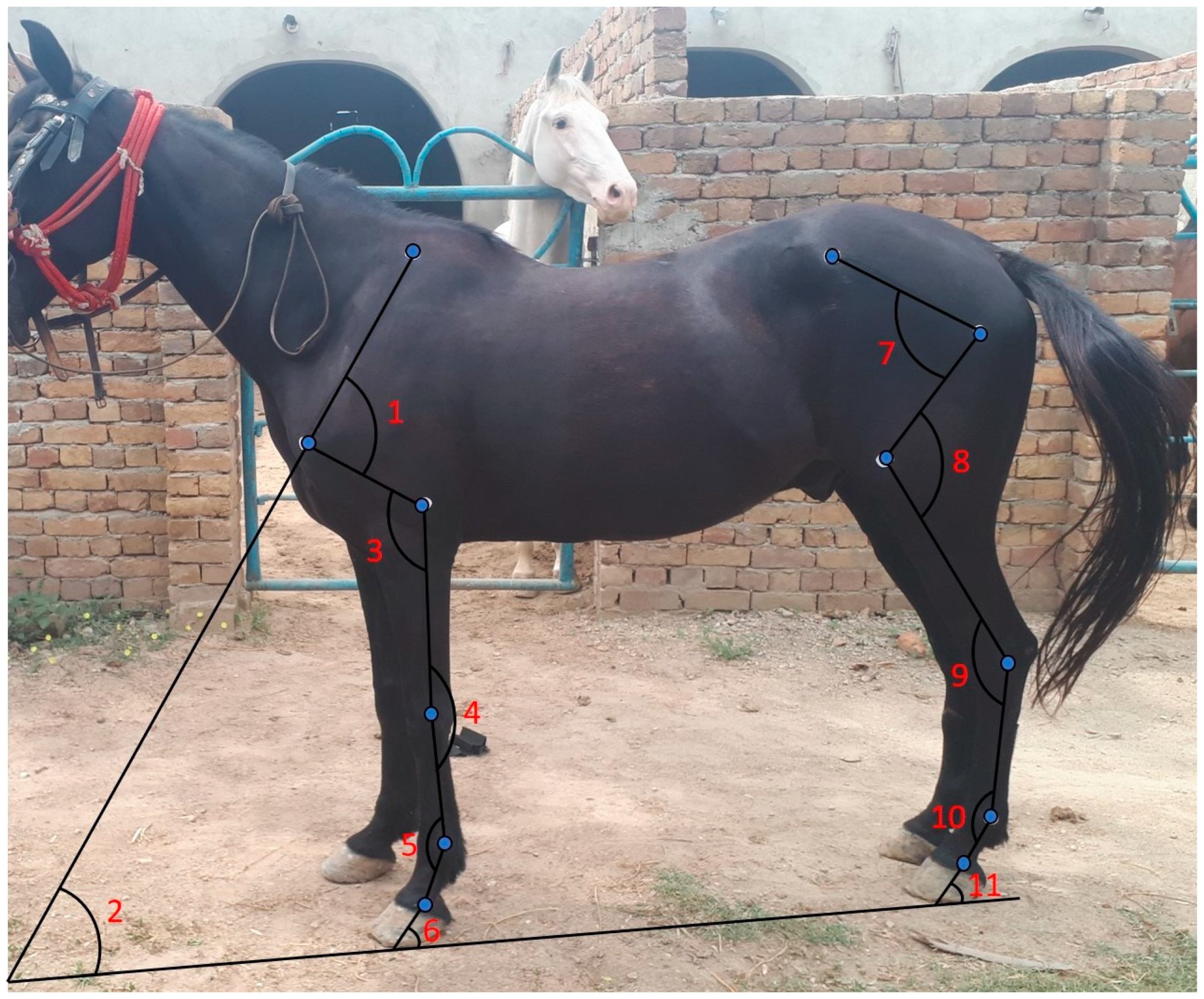
2.2. Measurements
2.3. Data Collection
2.3.1. Shoulder Versus Ground Angle
2.3.2. Shoulder Joint Angle
2.3.3. Elbow Joint Angle
2.3.4. Knee Joint Angle
2.3.5. Fore Fetlock Joint Angle
2.3.6. Fore Pastern Versus Ground Angle
2.3.7. Hip Joint Angle
2.3.8. Stifle Joint Angle
2.3.9. Hock Joint Angle
2.3.10. Hind Fetlock Joint Angle
2.3.11. Hind Pastern Versus Ground Angle
2.3.12. Girth Circumference and Body Length
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Body Measurements
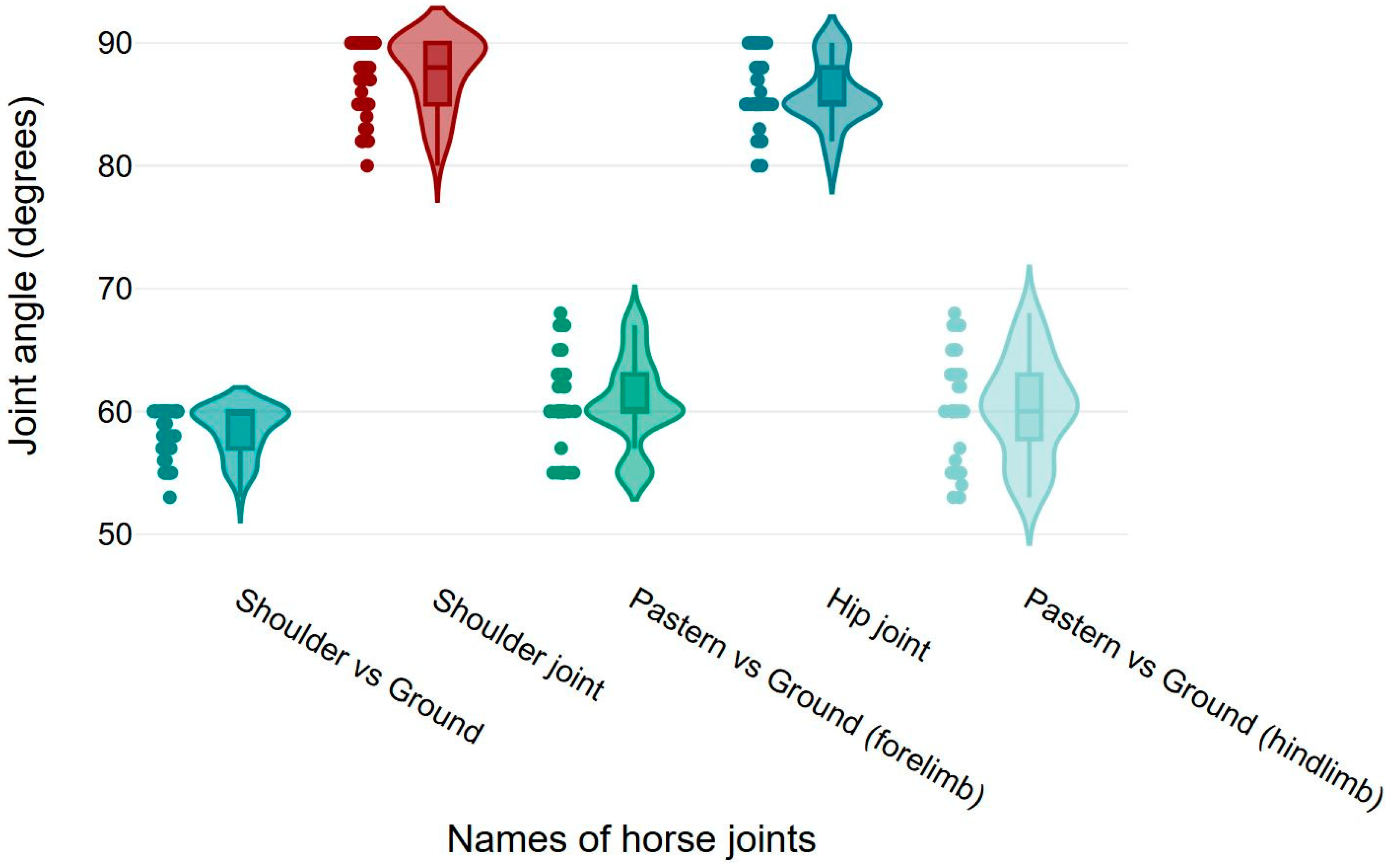
3.2. Elbow, Knee, Fetlock, Stifle, and Hock Joint Angles
3.3. Shoulder, Pastern, and Hip Joint Angles
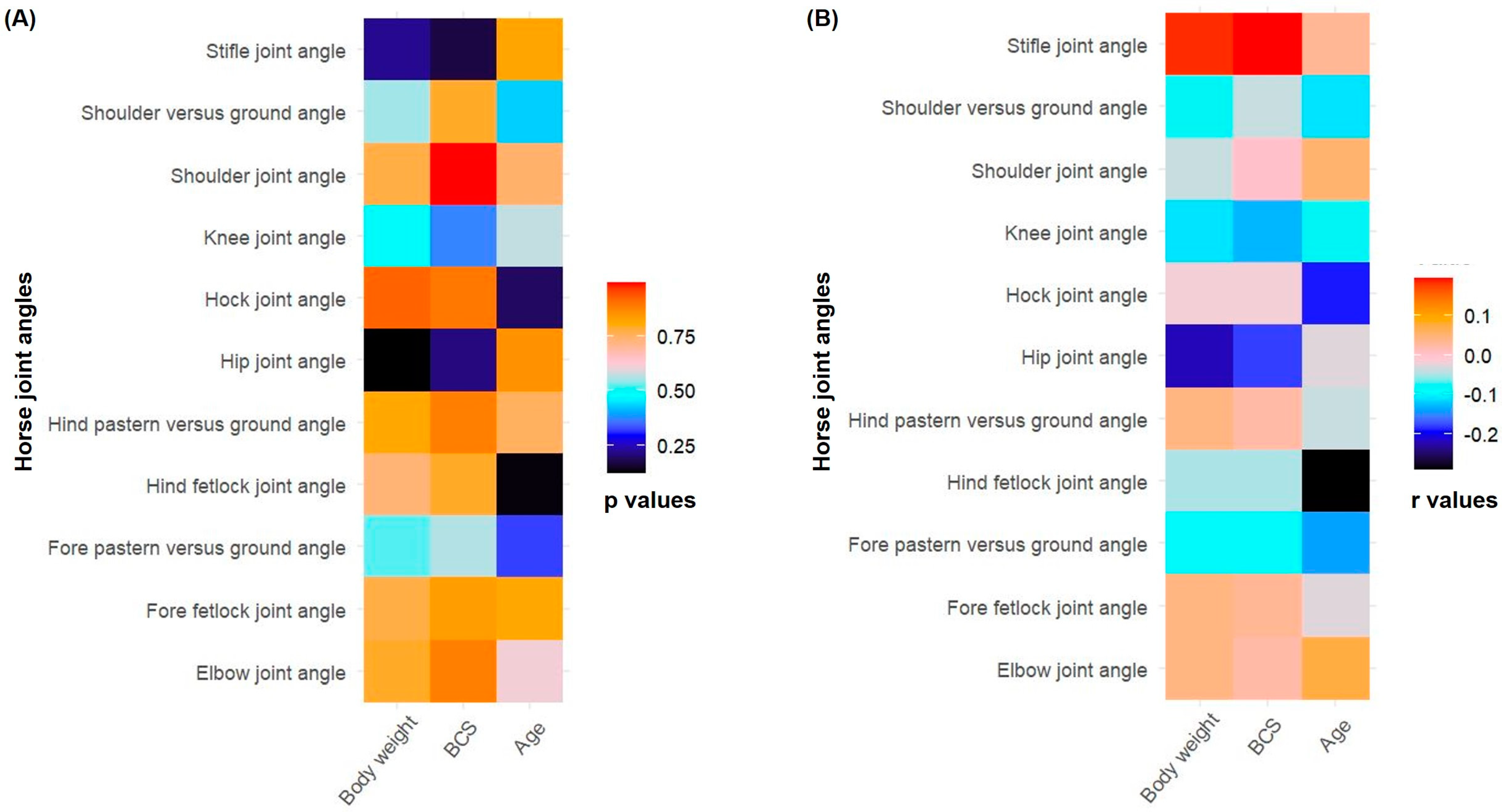
3.4. Correlation Between Horse Joint Angles, Age, Body Weight, and Body Condition Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klecel, W.; Martyniuk, E. From the eurasian steppes to the roman circuses: A review of early development of horse breeding and management. Animals 2021, 11, 1859. [Google Scholar] [CrossRef]
- Steel, C.; Morrice-West, A. Veterinary Aspects of Training, Conditioning, and Racing Thoroughbred Racehorses. In Equine Sports Medicine and Surgery; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1131–1168. [Google Scholar]
- McIlwraith, C.W.; Anderson, T.M.; Douay, P. The role of conformation in musculoskeletal problems in the racing Thoroughbred. Equine Vet. J. 2004, 36, 571–575. [Google Scholar] [CrossRef]
- Bukhari, S.S.U.H.; McElligott, A.G.; Parkes, R.S.V. Quantifying the Impact of Mounted Load Carrying on Equids: A Review. Animals 2021, 11, 1333. [Google Scholar] [CrossRef]
- Bukhari, S.S.U.H.; Parkes, R.S.V. Assessing the impact of draught load pulling on welfare in equids. Front. Vet. Sci. 2023, 10, 1214015. [Google Scholar] [CrossRef] [PubMed]
- Ripollés-Lobo, M.; Perdomo-González, D.I.; Valera, M.; Gómez, M.D. Conformational Defects in the Limbs of Menorca Purebred Horses and Their Relationship to Functionality. Animals 2024, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Domańska-Kruppa, N.; Wierzbicka, M.; Stefanik, E. Advances in the Clinical Diagnostics to Equine Back Pain: A Review of Imaging and Functional Modalities. Animals 2024, 14, 698. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Valette, J.-P.; Denoix, J.-M. Longitudinal development of equine forelimb conformation from birth to weaning in three different horse breeds. Vet. J. 2013, 198, e75–e80. [Google Scholar] [CrossRef] [PubMed]
- Van Weeren, P.R.; Crevier-Denoix, N. Equine conformation: Clues to performance and soundness? Equine Vet. J. 2006, 38, 591–596. [Google Scholar] [CrossRef]
- Crevier-Denoix, N.; Camus, M.; Falala, S.; Martino, J.; Pauchard, M.; Ravary-Plumioen, B.; Desquilbet, L.; Denoix, J.-M.; Chateau, H.; Pourcelot, P. Comparison of the loading of leading and trailing forelimbs in horses at landing. Equine Vet. J. 2014, 46, 38. [Google Scholar] [CrossRef]
- Boswell, R.P.; Mitchell, R.D.; Dyson, S.J. Lameness in the show hunter and show jumper. In Diagnosis and Management of Lameness in the Horse; Elsevier: Amsterdam, The Netherlands, 2011; pp. 965–975. [Google Scholar]
- Schlueter, A.E.; Orth, M.W. Equine osteoarthritis: A brief review of the disease and its causes. Equine Comp. Exerc. Physiol. 2004, 1, 221–231. [Google Scholar] [CrossRef]
- Yildirim, I.I.G.; Erden, H. Conformational characteristics in Arabian and Thoroughbred horses. Anim. Heal. Prod. Hyg. 2023, 12, 27–35. [Google Scholar] [CrossRef]
- Senna, N.A.; Mostafa, M.B.; Abu-Seida, A.M.; Elemmawy, Y.M. Evaluation of limb conformation in jumping thoroughbred horses. Asian J. Anim. Sci. 2015, 9, 208–216. [Google Scholar] [CrossRef]
- Agass, R.F.; Wilson, A.M.; Weller, R.; Pfau, T. The Relationship Between Foot Conformation, Foot Placement and Motion Symmetry in the Equine Hindlimb. Equine Vet. J. 2014, 46, 19–20. [Google Scholar] [CrossRef]
- Górniak, W.; Walkowicz, E.; Soroko, M.; Korczyński, M. Impact of the individual characteristics of French trotters on their racing performance. Turkish J. Vet. Anim. Sci. 2020, 44, 110–117. [Google Scholar] [CrossRef]
- Labuschagne, W.; Rogers, C.W.; Gee, E.K.; Bolwell, C.F. A Cross-Sectional Survey of Forelimb Hoof Conformation and the Prevalence of Flat Feet in a Cohort of Thoroughbred Racehorses in New Zealand. J. Equine Vet. Sci. 2017, 51, 1–7. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Anderson, T.M.; Sanschi, E.M. Conformation and musculoskeletal problems in the racehorse. Clin. Tech. Equine Pract. 2003, 2, 339–347. [Google Scholar] [CrossRef]
- Holmes, T.Q.; Brown, A.F. Champing at the bit for improvements: A review of equine welfare in equestrian sports in the United Kingdom. Animals 2022, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.B.; Senna, N.A.; Abu-Seida, A.M.; Elemmawy, Y.M. Evaluation of abnormal limb conformation in jumping Thoroughbred horses. J. Hell. Vet. Med. Soc. 2019, 70, 1533–1540. [Google Scholar] [CrossRef]
- Gmel, A.I.; Druml, T.; Portele, K.; von Niederhäusern, R.; Neuditschko, M. Repeatability, reproducibility and consistency of horse shape data and its association with linearly described conformation traits in Franches-Montagnes stallions. PLoS ONE 2018, 13, e0202931. [Google Scholar] [CrossRef] [PubMed]
- Hancock, G.E.; Hepworth, T.; Wembridge, K. Accuracy and reliability of knee goniometry methods. J. Exp. Orthop. 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Jacklin, B.D.; Hanousek, K.; Gillespie, S.; Liedtke, A.; Tucker, R.; Fiske-Jackson, A.; Smith, R.K. Validation of a novel clinical tool for monitoring distal limb stiffness. Front. Vet. Sci. 2023, 10, 127103. [Google Scholar] [CrossRef] [PubMed]
- Tuan, C.C.; Wu, Y.C.; Yeh, W.L.; Wang, C.C.; Lu, C.H.; Wang, S.W.; Yang, J.; Lee, T.F.; Kao, H.K. Development of Joint Activity Angle Measurement and Cloud Data Storage System. Sensors 2022, 22, 4684. [Google Scholar] [CrossRef]
- Jensen, R.B.; Rockhold, L.L.; Tauson, A.H. Weight estimation and hormone concentrations related to body condition in Icelandic and Warmblood horses: A field study. Acta Vet. Scand. 2019, 61, 63. [Google Scholar] [CrossRef]
- RStudio. Available online: https://www.rstudio.com/ (accessed on 11 November 2024).
- Addis, P.R.; Lawson, S.E.M. The role of tendon stiffness in development of equine locomotion with age. Equine Vet. J. 2010, 42, 556–560. [Google Scholar] [CrossRef]
- Ramos, S.; Pinto, A.; Cardoso, M.; Alexandre, N.; Bettencourt, E.; Monteiro, S.; Gama, L.T. Prevalence of Radiographic Signs of Osteoarthritis in Lusitano Purebred Horses. J. Equine Vet. Sci. 2020, 94, 103196. [Google Scholar] [CrossRef] [PubMed]
- Banwarth, M.R.; DeAtley, K.; Doyle, S. PSI-38 Relationship between body condition score, claw set, and foot angle in Angus and Red Angus beef cows grazing native annual-grass rangeland in northern California. J. Anim. Sci. 2020, 98, 474. [Google Scholar] [CrossRef]
- Mostafa, M.B.; Elemmawy, Y.M. Relationships between morphometric measurements and musculoskeletal disorders in jumping thoroughbred horses. J. Equine Sci. 2020, 31, 23–27. [Google Scholar] [CrossRef]
- Holmström, M.; Magnusson, L.E.; Philipsson, J. Variation in conformation of Swedish warmblood horses and conformational characteristics of élite sport horses. Equine Vet. J. 1990, 22, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Van Weeren, P.R.; Van Den Bogert, A.J.; Barneveld, A. A quantitative analysis of skin displacement in the trotting horse. Equine Vet. J. 1990, 22, 101–109. [Google Scholar] [CrossRef]
- Harrison, S.M.; Whitton, R.C.; King, M.; Haussler, K.K.; Kawcak, C.E.; Stover, S.M.; Pandy, M.G. Forelimb muscle activity during equine locomotion. J. Exp. Biol. 2012, 215, 2980–2991. [Google Scholar] [CrossRef]

| Measurements | Mean | SD |
|---|---|---|
| Age (years) | 4.81 | 0.67 |
| Girth Circumference (inch) | 65.06 | 0.91 |
| Body Length (inch) | 54.66 | 1.02 |
| Body Weight (kg) | 350.42 | 15.56 |
| BCS (/9) | 4.90 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.; Ijaz, S.; Usman, M.M.; Safdar, A.; Khan, I.U.; Zeeshan, M.; Bukhari, S.S.U.H. Evaluating Forelimb and Hindlimb Joint Conformation of Morna Racehorses (Equus caballus). Vet. Sci. 2025, 12, 20. https://doi.org/10.3390/vetsci12010020
Ahmad I, Ijaz S, Usman MM, Safdar A, Khan IU, Zeeshan M, Bukhari SSUH. Evaluating Forelimb and Hindlimb Joint Conformation of Morna Racehorses (Equus caballus). Veterinary Sciences. 2025; 12(1):20. https://doi.org/10.3390/vetsci12010020
Chicago/Turabian StyleAhmad, Israr, Sahar Ijaz, Mirza M. Usman, Ayesha Safdar, Imdad U. Khan, Muhammad Zeeshan, and Syed S. U. H. Bukhari. 2025. "Evaluating Forelimb and Hindlimb Joint Conformation of Morna Racehorses (Equus caballus)" Veterinary Sciences 12, no. 1: 20. https://doi.org/10.3390/vetsci12010020
APA StyleAhmad, I., Ijaz, S., Usman, M. M., Safdar, A., Khan, I. U., Zeeshan, M., & Bukhari, S. S. U. H. (2025). Evaluating Forelimb and Hindlimb Joint Conformation of Morna Racehorses (Equus caballus). Veterinary Sciences, 12(1), 20. https://doi.org/10.3390/vetsci12010020








