TLR Agonist Immunoadjuvants Provide Effective Protection Against PCV2 and PRV Infections in a Bivalent Subunit Vaccine for PCV2 and PRV
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. PRV (ZJM-1 Strain) Virus and ST Cells
2.2. Preparation of the PCV2 and PRV Bivalent Subunit Vaccine
2.3. Schedule of Vaccination and Challenge
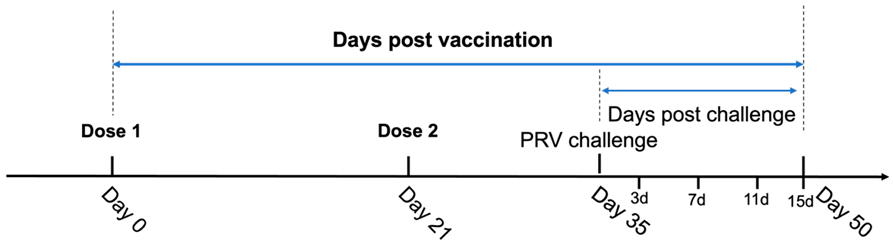
| Groups | Vaccine Groups | Vaccine Dosage (IM) | Serum Sample | Whole Blood, Throat Swabs, and Anal Swabs |
|---|---|---|---|---|
| Days Post-Vaccination | Days Post-Challenge | |||
| A | 1 | 1 mL | 0d/21d/35d/50d | 3d/7d/11d/15d |
| B | 2 | 1 mL | 0d/21d/35d/50d | 3d/7d/11d/15d |
| C | none | None | 0d/21d/35d/50d | 3d/7d/11d/15d |
| D | none | None | 0d/21d/35d/50d | 3d/7d/11d/15d |
2.4. Detection of Antibodies in Serum Samples
2.5. PRV Neutralization Assay
2.6. Clinical Observations Post-PRV Challenge
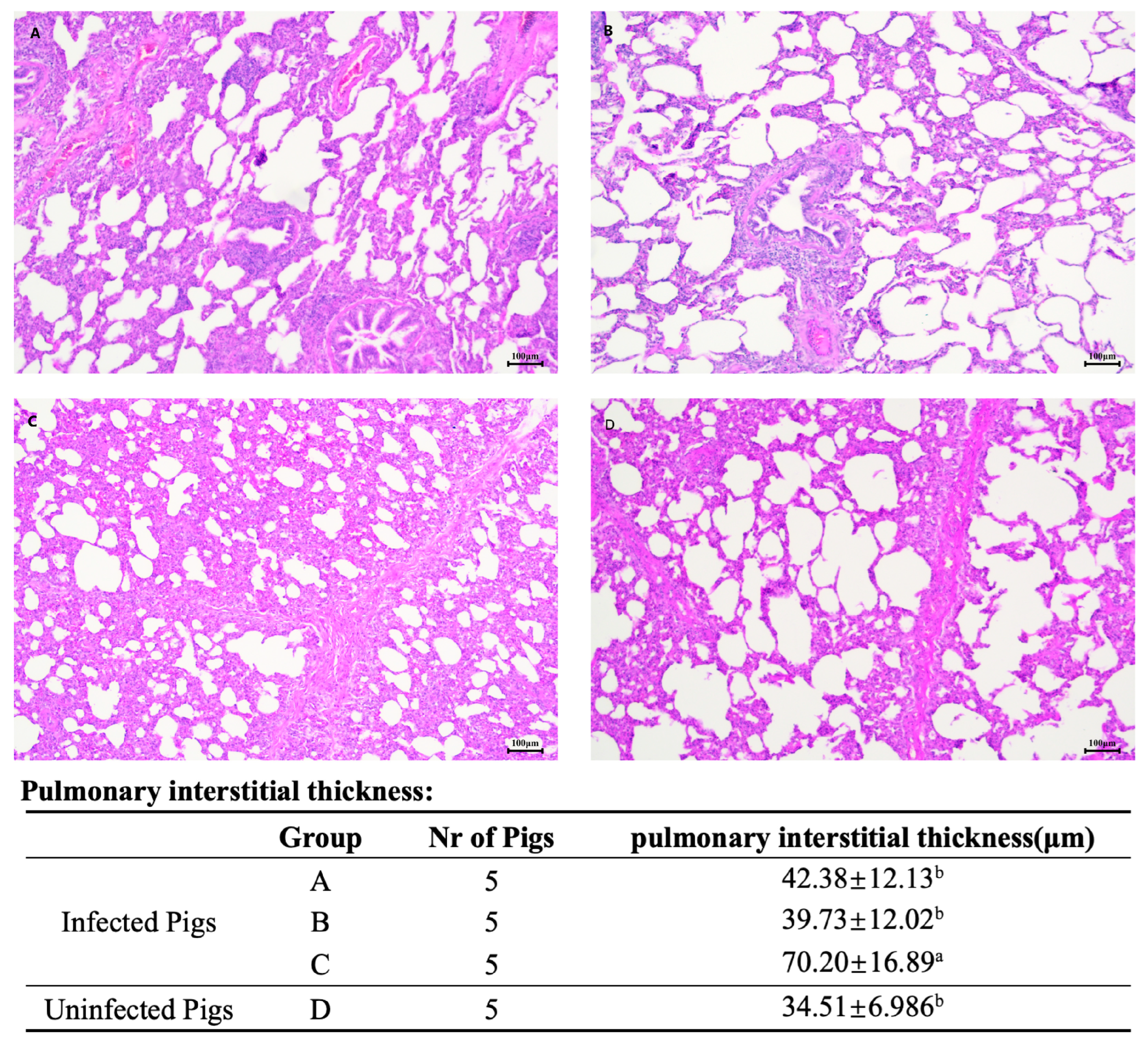
2.7. Pathological Evaluation
2.8. Quantification of PRV Load
2.9. Statistical Analysis
3. Results
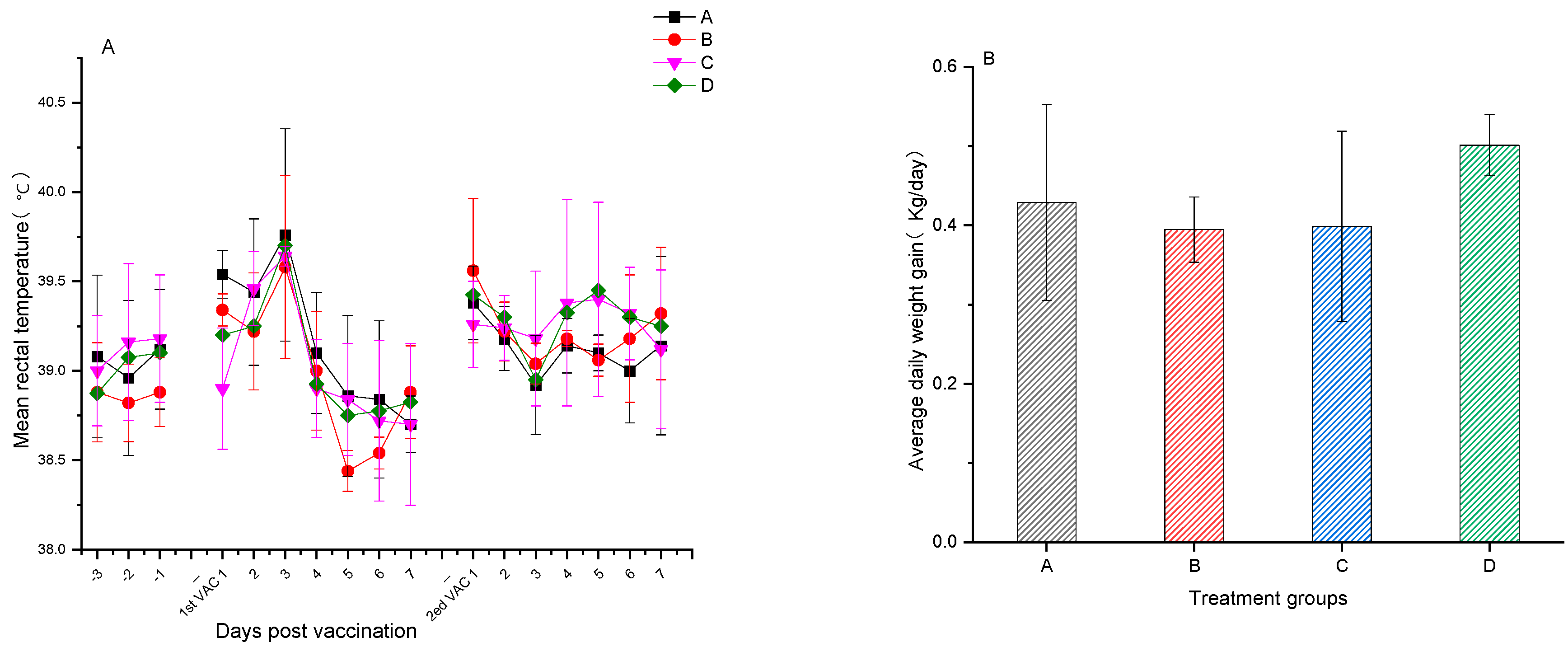
3.1. Safety Assessment of the PRV and PCV2 Bivalent Subunit Vaccine
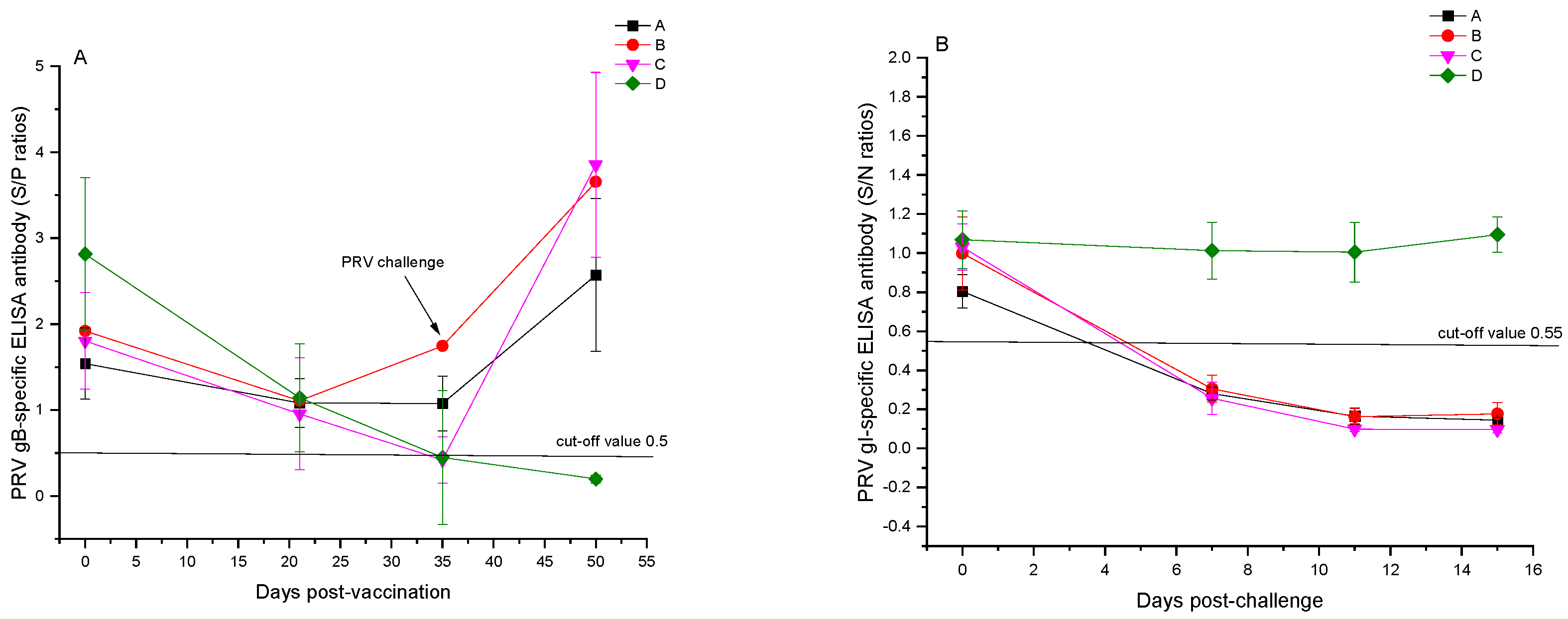
3.2. Results of ELISA Antibody Tests Post-Immunization with the PCV2 and PRV Bivalent Subunit Vaccine in Experimental Pigs
3.3. Results of PRV-Neutralizing Antibody Tests
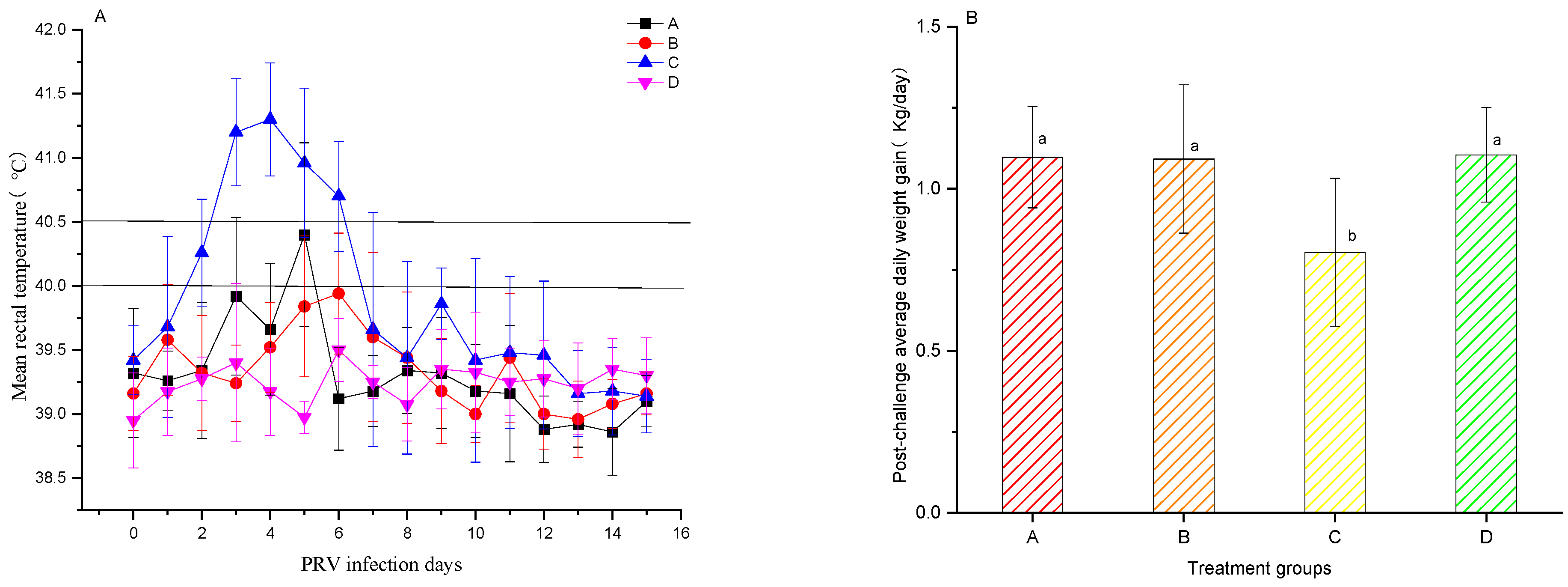
3.4. PRV Challenge of Pigs
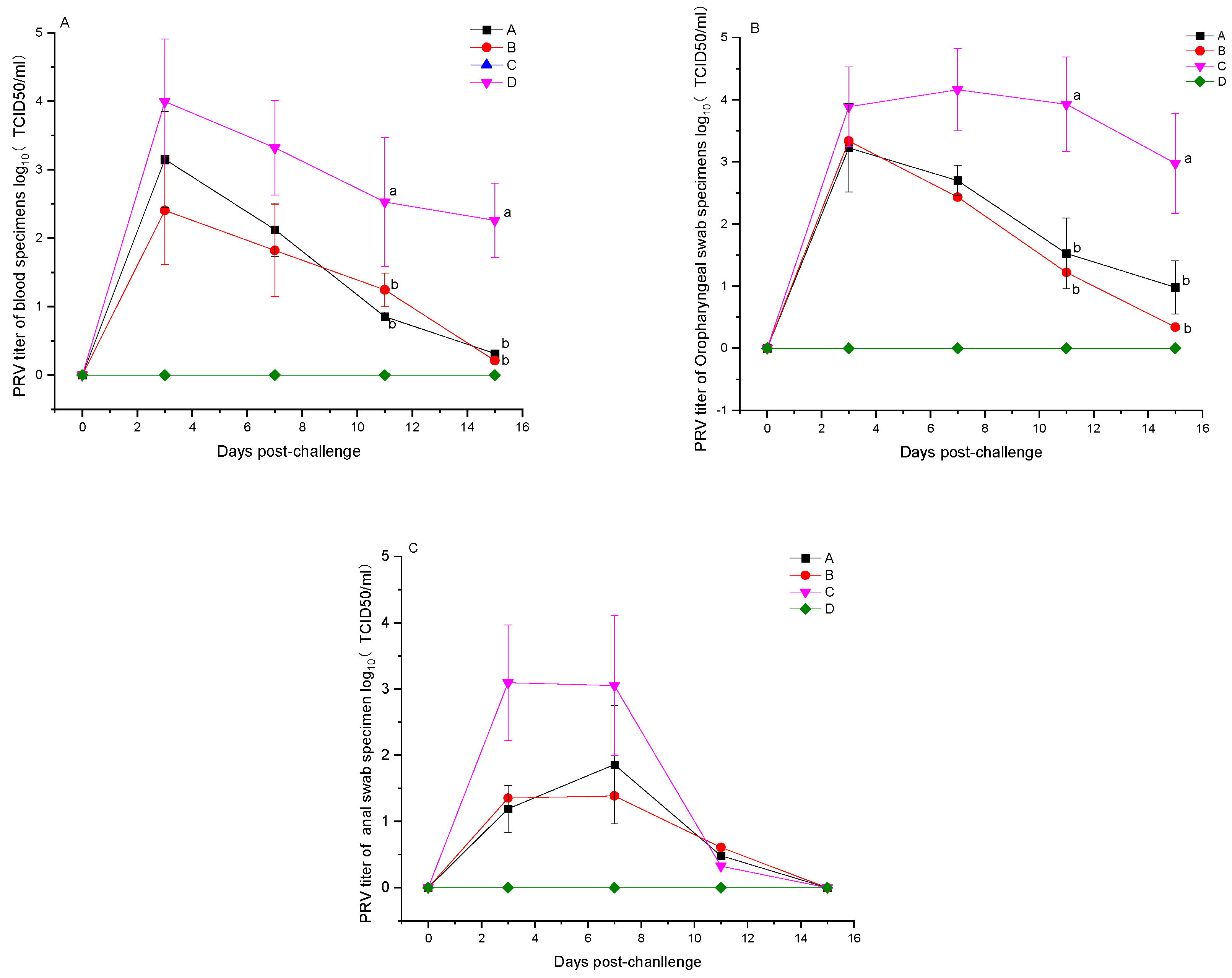
3.5. Detection of Viral Load Post-PRV Challenge
3.6. Post-PRV Challenge Clinical Signs and Pathological Examination
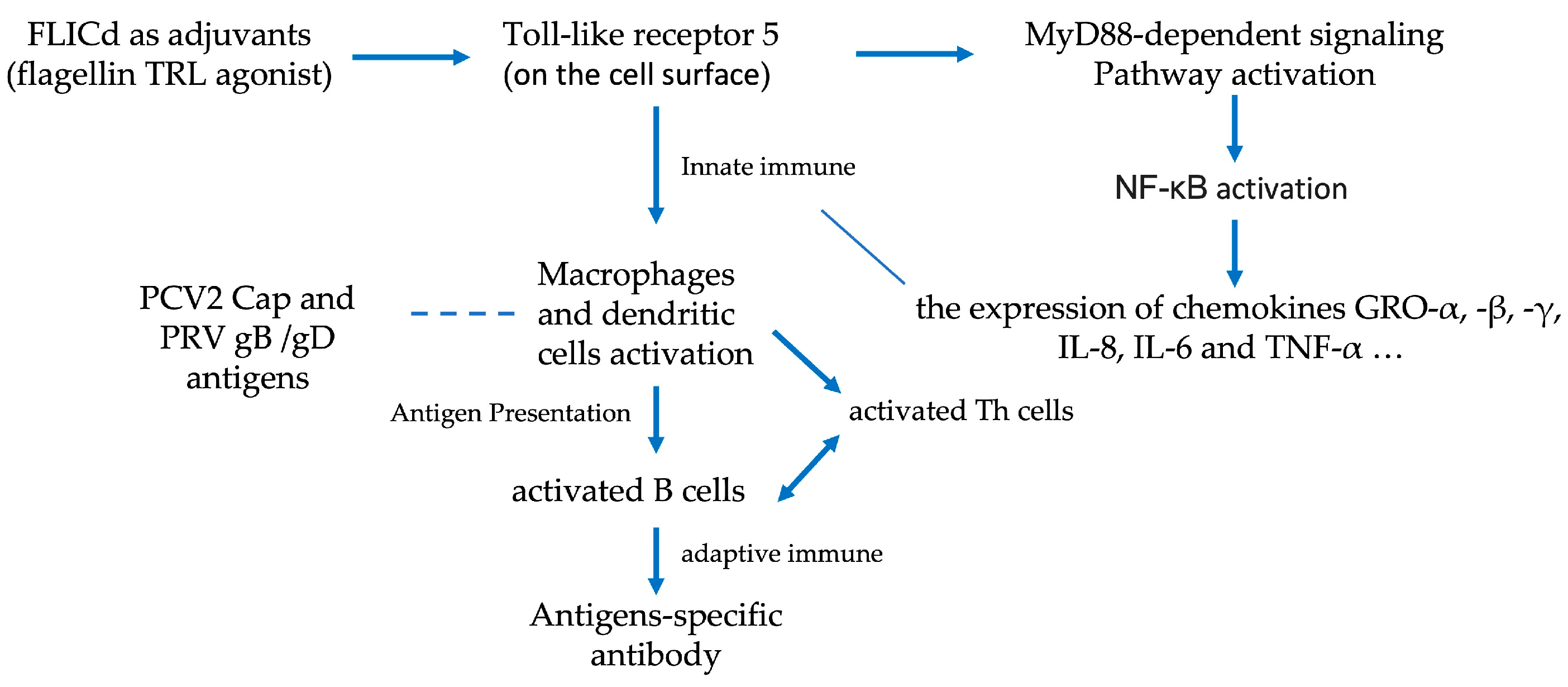
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alarcon, P.; Rushton, J.; Wieland, B. Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical infection in England—An economic disease model. Prev. Vet. Med. 2013, 110, 88–102. [Google Scholar] [CrossRef]
- Shi, R.; Hou, L.; Liu, J. Host immune response to infection with porcine circoviruses. Anim. Dis. 2021, 1, 23. [Google Scholar] [CrossRef]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies virus variant in Bartha-K61–Vaccinated pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Zhang, X.; Niu, G.; Yang, L.; Ji, W.; Zhang, L.; Ren, L. PCV2 and PRV coinfection induces endoplasmic reticulum stress via PERK-eIF2α-ATF4-CHOP and IRE1-XBP1-EDEM pathways. Int. J. Mol. Sci. 2022, 23, 4479. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Zhang, L.; Niu, G.; Zhang, X.; Yang, L.; Ji, W.; Ren, L. Coinfection of porcine circovirus 2 and pseudorabies virus enhances immunosuppression and inflammation through NF-κB, JAK/STAT, MAPK, and NLRP3 pathways. Int. J. Mol. Sci. 2022, 23, 4469. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, Q.; Ku, X.; He, D.; Li, Z.; Ghonaim, A.H.; Fan, S.; He, Q. The epidemiological investigation of co-infection of major respiratory bacteria with pseudorabies virus in intensive pig farms in China. Vet. Med. Sci. 2021, 7, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.; Rushton, J.; Nathues, H.; Wieland, B. Economic efficiency analysis of different strategies to control post-weaning multi-systemic wasting syndrome and porcine circovirus type 2 subclinical infection in 3-weekly batch system farms. Prev. Vet. Med. 2013, 110, 103–118. [Google Scholar] [CrossRef]
- Bowie, A.G.; Unterholzner, L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 2008, 8, 911–922. [Google Scholar] [CrossRef]
- Mizel, S.B.; Bates, J.T. Flagellin as an adjuvant: Cellular mechanisms and potential. J. Immunol. 2010, 185, 5677–5682. [Google Scholar] [CrossRef]
- Sokol, O.O.; Nikitin, N.A.; Evtushenko, E.A.; Karpova, O.V.; Matveeva, I.N.; Gryn, S.A.; Popova, V.M.; Ivanov, I.V.; Fedorov, Y.N.; Litenkova, I.Y. Protective activity of inactivated rabies vaccine using flagellin-based adjuvant. Biochemistry 2024, 89, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D.; Andersen-Nissen, E.; Hayashi, F.; Strobe, K.; Bergman, M.A.; Barrett, S.L.R.; Cookson, B.T.; Aderem, A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 2003, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.I.; Kurnasov, O.; Natarajan, V.; Hong, M.; Gudkov, A.V.; Osterman, A.L.; Wilson, I.A. Structural basis of TLR5-flagellin recognition and signaling. Science 2012, 335, 859–864. [Google Scholar] [CrossRef]
- Zahid, A.; Ismail, H.; Li, B.; Jin, T. Molecular and structural basis of DNA sensors in antiviral innate immunity. Front. Immunol. 2020, 11, 613039. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Liu, H.; Zhou, Q.; Liu, X.; Huang, L.; Weng, C. A tug of war: Pseudorabies virus and host antiviral innate immunity. Viruses 2022, 14, 547. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Segalés, J. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS ONE 2018, 13, e0208585. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Oh, T.; Suh, J.; Chae, C. Comparison of pathogenicity of 4 porcine circovirus type 2 (PCV2) genotypes (2a, 2b, 2d, and 2e) in experimentally infected pigs. J. Vet. Med. Sci. 2023, 85, 83–87. [Google Scholar] [CrossRef]
- Opriessnig, T.; Xiao, C.T.; Halbur, P.G.; Gerber, P.F.; Matzinger, S.R.; Meng, X.J. A commercial porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viremia and shedding and prevents PCV2d transmission to naïve pigs under experimental conditions. Vaccine 2017, 35, 248–254. [Google Scholar] [CrossRef]
- Yu, C.; Cao, M.; Wei, Y.; Liu, J.; Zhang, H.; Liu, C.; Feng, L.; Huang, L. Evaluation of cross-immunity among major porcine circovirus type 2 genotypes by infection with PCV2b and PCV2d circulating strains. Vet. Microbiol. 2023, 283, 109796. [Google Scholar] [CrossRef] [PubMed]
- Pileri, E.; Cortey, M.; Rodríguez, F.; Sibila, M.; Fraile, L.; Segalés, J. Comparison of the immunoperoxidase monolayer assay and three commercial ELISAs for detection of antibodies against porcine circovirus type 2. Vet. J. 2014, 201, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Evaluation of natural porcine circovirus type 2 (PCV2) subclinical infection and seroconversion dynamics in piglets vaccinated at different ages. Vet. Res. 2016, 47, 121. [Google Scholar] [CrossRef] [PubMed]
- Kiss, I.; Szigeti, K.; Homonnay, Z.G.; Tamás, V.; Smits, H.; Krejci, R. Maternally derived antibody levels influence on vaccine protection against PCV2d challenge. Animals 2021, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, K.; Grivas, I.; Chiotelli, M.; Theodoridis, A.; Panteris, E.; Papadopoulos, D.; Petridou, E.; Papaioannou, N.; Nauwynck, H.; Kritas, S.K. Age-dependent invasion of pseudorabies virus into porcine central nervous system via maxillary nerve. Pathogens 2022, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, N.; Cong, X.; Wang, C.H.; Du, M.; Li, L.; Zhao, B.; Yuan, J.; Liu, D.D.; Li, S.; et al. Pathogenicity and genomic characterization of a pseudorabies virus variant isolated from Bartha-K61-vaccinated swine population in China. Vet. Microbiol. 2014, 174, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Liu, F.; Zheng, H.; Liang, C.; Zhou, Y.J.; Jiang, Y.F.; Shan, T.L.; Gao, F.; Li, G.X.; Tong, G.Z. Emergence of a Pseudorabies virus variant with increased virulence to piglets. Vet. Microbiol. 2015, 181, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Miao, Y.; Xi, R.; Gao, X.; Miao, D.; Chen, H.; Jung, Y.S.; Qian, Y.; Dai, J. Emergence of a novel pathogenic recombinant virus from Bartha vaccine and variant pseudorabies virus in China. Transbound. Emerg. Dis. 2021, 68, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ma, Z.; Bai, J.; Sun, Y.; Cao, M.; Wang, X.; Jiang, P.; Liu, X. Comparison of the protective efficacy between the candidate vaccine ZJ01R carrying gE/gI/TK deletion and three commercial vaccines against an emerging pseudorabies virus variant. Vet. Microbiol. 2023, 276, 109623. [Google Scholar] [CrossRef]
- Zhou, J.; Li, S.; Wang, X.; Zou, M.; Gao, S. Bartha-k61 vaccine protects growing pigs against challenge with an emerging variant pseudorabies virus. Vaccine 2017, 35, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, L.; Fu, Z.F. Effective cross-protection of a lyophilized live gE/gI/TK-deleted pseudorabies virus (PRV) vaccine against classical and variant PRV challenges. Vet. Microbiol. 2022, 267, 109387. [Google Scholar] [CrossRef]


| Groups | Adjuvant (v/v) | Immunomodulating Adjuvant | Antigen | |
|---|---|---|---|---|
| PRV | PCV2 | |||
| A | 7749 1/9 | Non | 60 μg gB 60 μg gD | 70 μg |
| B | 7749 1/9 | 100 μg FLICd | 60 μg gB 60 μg gD | 70 μg |
| Group | Number | Virus Titer (TCID50/mL) | Challenge Dose (mL)/Challenge Route | Clinical Signs | Morbidity | Mortality | ||
|---|---|---|---|---|---|---|---|---|
| Recital Temperature (>40.5 °C) | Nervous Signs | Respiratory Signs | ||||||
| A | 5 | 106.0 | 1/IM + 1/IN | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| B | 5 | 106.0 | 1/IM + 1/IN | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| C | 5 | 106.0 | 1/IM + 1/IN | 5/5 | 2/5 | 5/5 | 5/5 | 0/5 |
| D | 5 | None | None | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Xiang, W.; Ou, W.; Li, Y.; Shu, X.; Li, X. TLR Agonist Immunoadjuvants Provide Effective Protection Against PCV2 and PRV Infections in a Bivalent Subunit Vaccine for PCV2 and PRV. Vet. Sci. 2025, 12, 25. https://doi.org/10.3390/vetsci12010025
Yu F, Xiang W, Ou W, Li Y, Shu X, Li X. TLR Agonist Immunoadjuvants Provide Effective Protection Against PCV2 and PRV Infections in a Bivalent Subunit Vaccine for PCV2 and PRV. Veterinary Sciences. 2025; 12(1):25. https://doi.org/10.3390/vetsci12010025
Chicago/Turabian StyleYu, Fulai, Wei Xiang, Weiye Ou, Yang Li, Xinbiao Shu, and Xiaoliang Li. 2025. "TLR Agonist Immunoadjuvants Provide Effective Protection Against PCV2 and PRV Infections in a Bivalent Subunit Vaccine for PCV2 and PRV" Veterinary Sciences 12, no. 1: 25. https://doi.org/10.3390/vetsci12010025
APA StyleYu, F., Xiang, W., Ou, W., Li, Y., Shu, X., & Li, X. (2025). TLR Agonist Immunoadjuvants Provide Effective Protection Against PCV2 and PRV Infections in a Bivalent Subunit Vaccine for PCV2 and PRV. Veterinary Sciences, 12(1), 25. https://doi.org/10.3390/vetsci12010025







