Efficacy of Fat Supplements with Different Unsaturated/Saturated FA Ratios Undergoing First Postpartum Ovulation in Lactating Anovulatory Goats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet Management
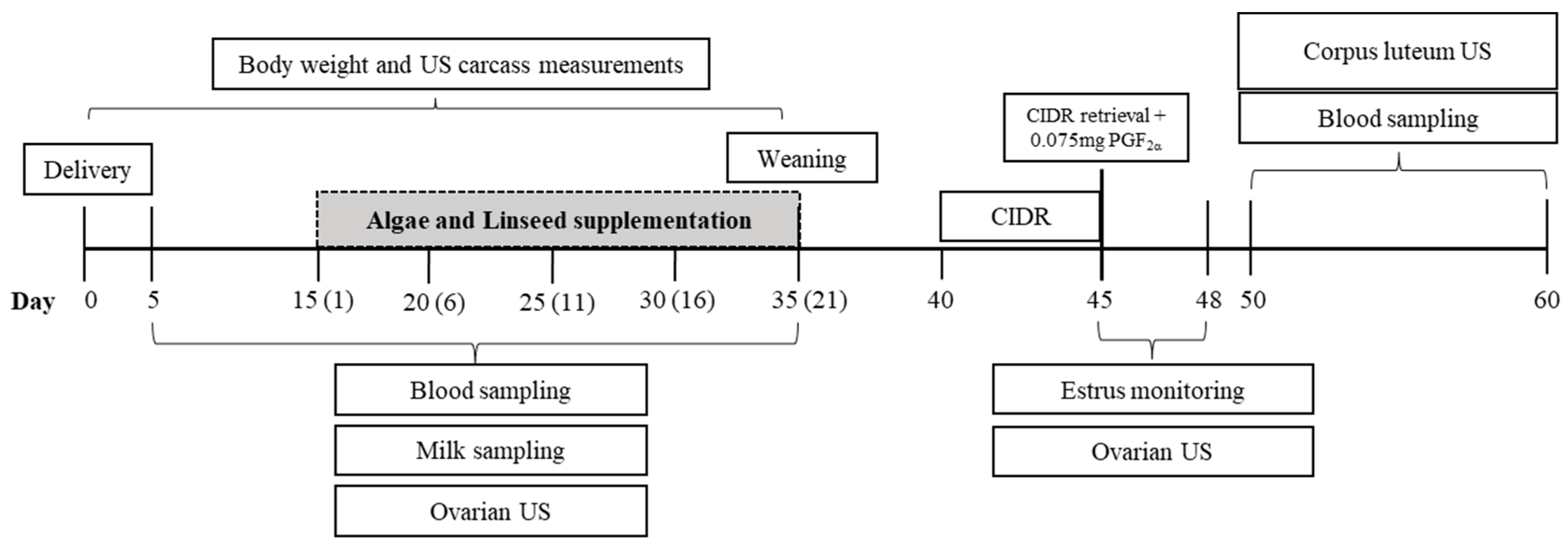
2.2. Nutritional Treatments and Experimental Design
2.3. Synchronization Program
2.4. In Vivo Performance and Adipose Carcass Marker Measurements
2.5. Ovarian Function
Follicular Dynamics, Ultrasonography Analysis, and Ovulatory Rate
2.6. Luteal Function
2.6.1. Progesterone Assay
2.6.2. Postpartum Luteal Activity
2.6.3. Corpus Luteum Ultrasonography
2.6.4. Metabolites, β-Hydroxybutyrate (BHB), Glutathione Peroxidase (GPx) Assays, and Fat–Protein Milk Ratio
2.7. Statistical Analysis
3. Results
3.1. Response During Supplementation
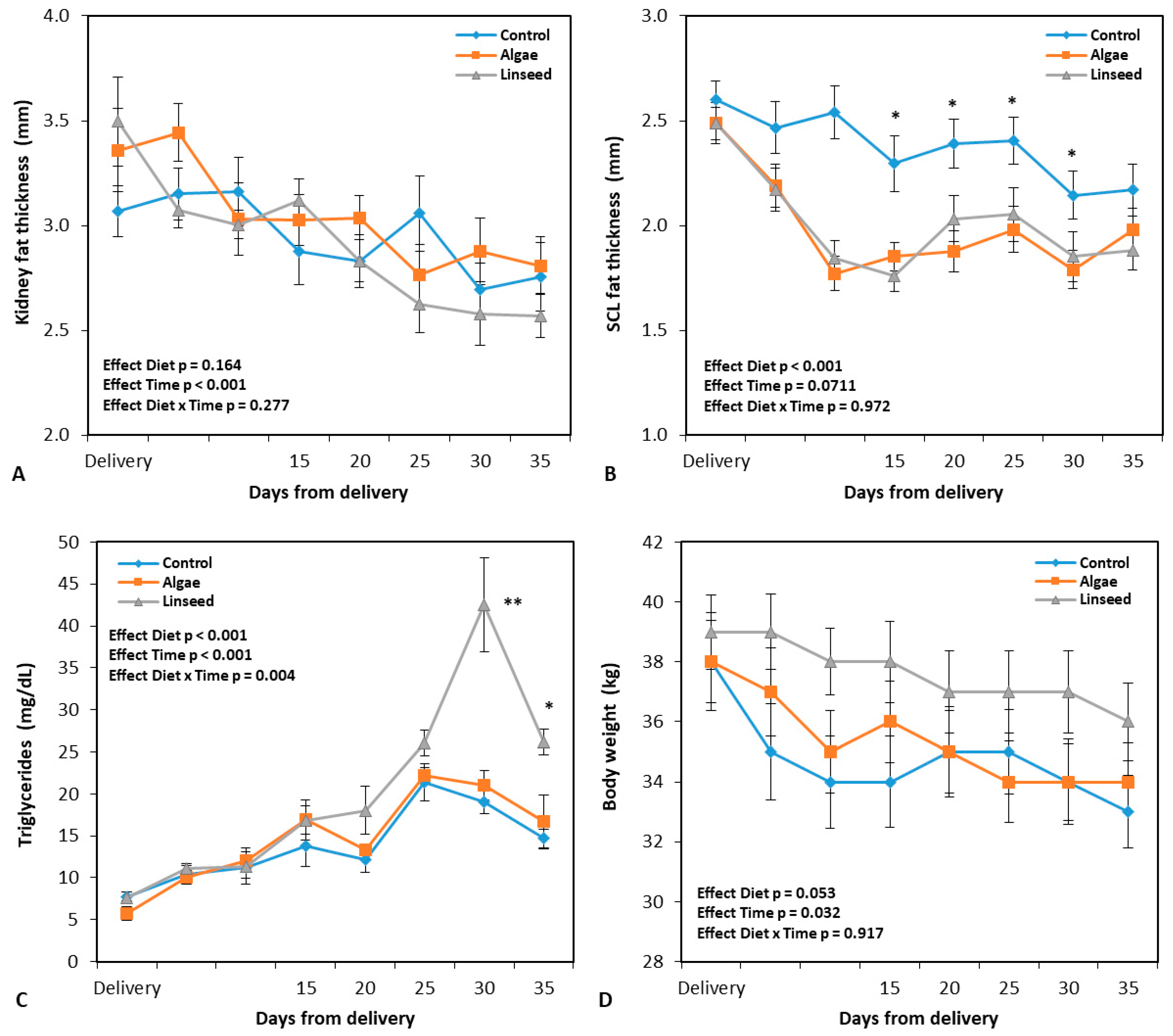
3.1.1. Changes in Feed Intake, Body Weight, and Carcass Markers
3.1.2. Metabolic and Oxidative Damage Markers
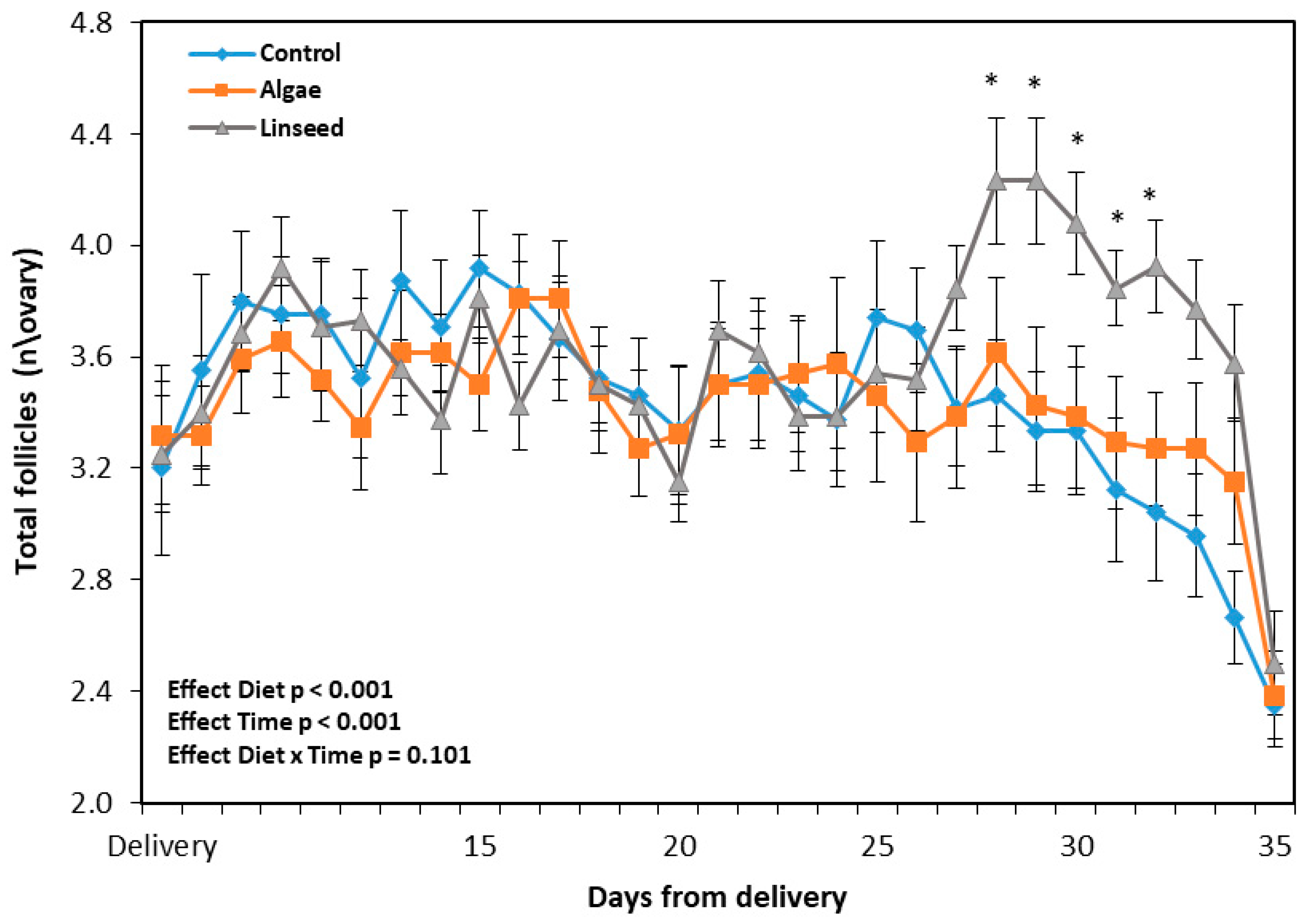
3.1.3. Ovarian and Luteal Function
3.2. Response After Ovulation Induction
3.2.1. Responsiveness to Estrus Synchronization
3.2.2. Follicles, Ovulatory Response, BHB, and GPx
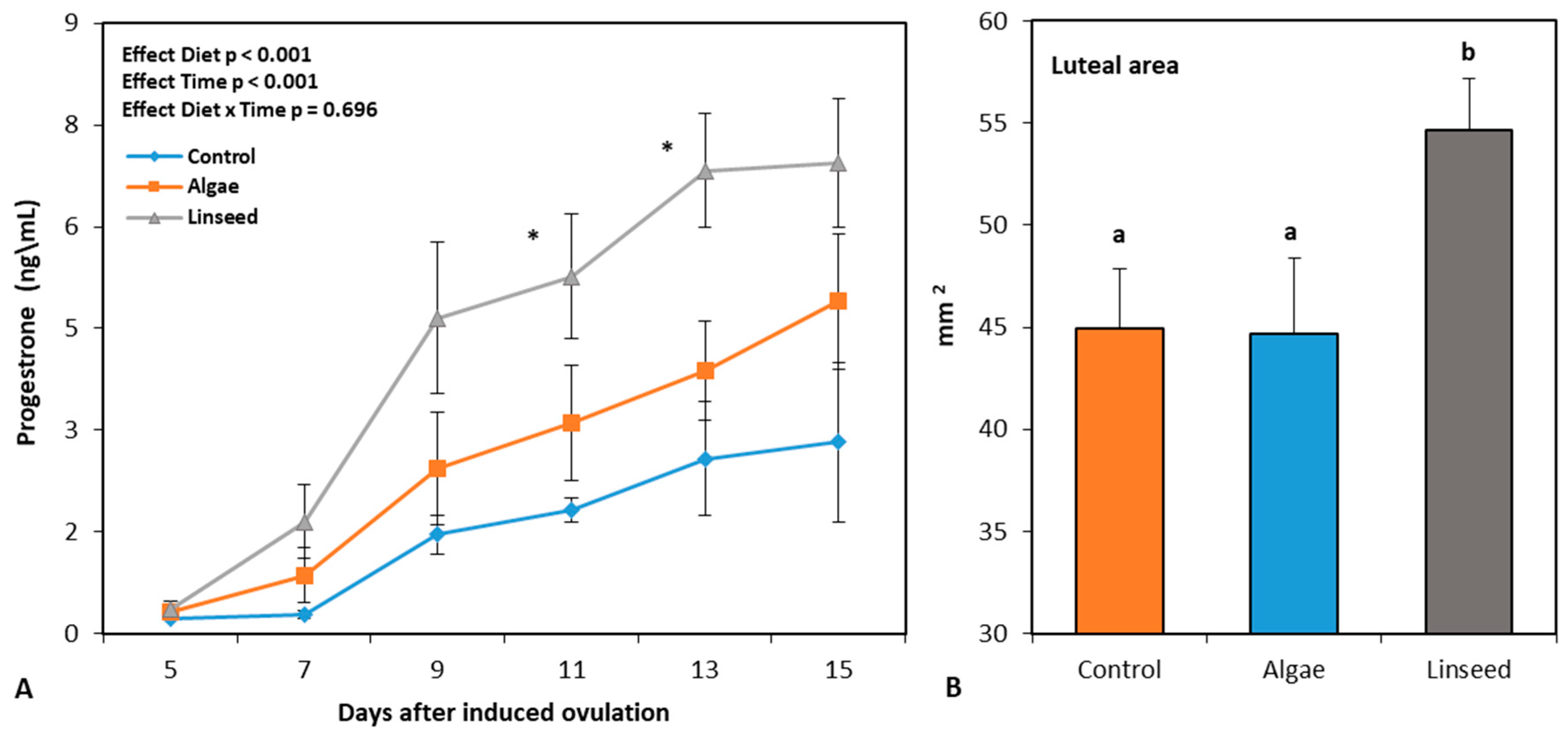
3.2.3. Luteal Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banuelos, S.; Stevenson, J.S. Transition cow metabolites and physical traits influence days to first postpartum ovulation in dairy cows. Theriogenology 2021, 173, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, M.; Castellini, C.; Dall’Aglio, C.; Petrucci, L.; Mattioli, S.; Boiti, C.; Zerani, M. Effects of PUFAs on animal reproduction: Male and female performances and endocrine mechanisms. Phytochem. Rev. 2018, 17, 801–814. [Google Scholar] [CrossRef]
- Sordillo, L.M. Nutritional strategies to optimize dairy cattle immunity. J. Dairy Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, M.; Bionaz, M.; Trevisi, E. Interaction between inflammation and metabolism in periparturient dairy cows. J. Anim. Sci. 2020, 98 (Suppl. S1), S155–S174. [Google Scholar] [CrossRef]
- Kuhla, B. Pro-inflammatory cytokines and hypothalamic inflammation: Implications for insufficient feed intake of transition dairy cows. Animal 2020, 14, s65–s77. [Google Scholar] [CrossRef]
- Robertson, S.M.; Atkinson, T.; Friend, M.A.; Allworth, M.B.; Refshauge, G. Reproductive performance in goats and causes of perinatal mortality: A review. Anim. Prod. Sci. 2020, 60, 1669–1680. [Google Scholar] [CrossRef]
- Menezes, L.M.; Sousa, W.H.; Cavalcanti-Filho, E.P.; Gama, L.T. Genetic parameters for reproduction and growth traits in Boer goats in Brazil. Small Rumin. Res. 2016, 136, 247–256. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Jenkins, T.C. A 100-Year Review: Fat feeding of dairy cows. J. Dairy Sci. 2017, 100, 10061–10077. [Google Scholar] [CrossRef]
- Hristov, A.N.; Melgar, A.; Wasson, D.; Arndt, C. Symposium review: Effective nutritional strategies to mitigate enteric methane in dairy cattle. J. Dairy Sci. 2022, 105, 8543–8557. [Google Scholar] [CrossRef]
- Roque-Jiménez, J.A.; Rosa-Velázquez, M.; Pinos-Rodríguez, J.M.; Vicente-Martínez, J.G.; Mendoza-Cervantes, G.; Flores-Primo, A.; Relling, A.E. Role of long chain fatty acids in developmental programming in ruminants. Animals 2021, 11, 762. [Google Scholar] [CrossRef]
- Yadav, D.; Singh, A.K.; Kumar, B.; Mahla, A.S.; Singh, S.K.; Patra, M.K.; Krishnaswamy, N. Effect of n-3 PUFA-rich fish oil supplementation during late gestation on kidding, uterine involution and resumption of follicular activity in goat. Reprod. Domest. Anim. 2019, 54, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Mahla, A.S.; Bunkar, S.K.; Kumawat, B.L.; Saxena, V.K.; Selvaraju, S.; Bhatt, R.S.; Kumar, A. Dietary n-3 PUFA augments pre-ovulatory follicle turnover and prolificacy in well-fed ewes. Anim. Reprod. Sci. 2023, 252, 107231. [Google Scholar] [CrossRef] [PubMed]
- Elis, S.; Freret, S.; Desmarchais, A.; Maillard, V.; Cognié, J.; Briant, E.; Dupont, J. Effect of a long chain n-3 PUFA-enriched diet on production and reproduction variables in Holstein dairy cows. Anim. Reprod. Sci. 2016, 164, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.S.; Greco, L.F.; Bisinotto, R.S.; Lima, F.S.; Thatcher, W.W.; Santos, J.E. Biology of Preimplantation Conceptus at the Onset of Elongation in Dairy Cows1. Biol. Reprod. 2016, 94, 97. [Google Scholar] [CrossRef]
- Cavalcanti, C.M.; Silva, M.R.L.; Conde, A.J.H.; Bezerra, A.F.; Alves, J.P.M.; Fernandes, C.C.L.; Rondina, D. Effect of peri-conception high-fat diets on maternal ovarian function, foetal and placentome growth, and vascular umbilical development in goats. Reprod. Domest. Anim. 2022, 57, 1481–1492. [Google Scholar] [CrossRef]
- Senosy, W.; Kassab, A.Y.; Mohammed, A.A. Effects of feeding green microalgae on ovarian activity, reproductive hormones and metabolic parameters of Boer goats in arid subtropics. Theriogenology 2017, 96, 16–22. [Google Scholar] [CrossRef]
- Silva, M.R.L.; Alves, J.P.M.; Fernandes, C.C.L.; Cavalcanti, C.M.; Conde, A.J.H.; Bezerra, A.F.; Rondina, D. Use of green microalgae Chlorella as a nutritional supplement to support oocyte and embryo production in goats. Anim. Reprod. Sci. 2023, 256, 107296. [Google Scholar] [CrossRef]
- Silva, M.R.L.; Alves, J.P.M.; Fernandes, C.C.L.; Cavalcanti, C.M.; Conde, A.J.H.; Bezerra, A.F.; Rondina, D. Effect of short-term nutritional supplementation of green microalgae on some reproductive indicators of Anglo-Nubian crossbred goats. Vet. World 2023, 16, 464. [Google Scholar] [CrossRef]
- Nudda, A.; Bee, G.; Correddu, F.; Lunesu, M.F.; Cesarani, A.; Rassu, S.P.G.; Battacone, G. Linseed supplementation during uterine and early post-natal life markedly affects fatty acid profiles of brain, liver and muscle of lambs. Ital. J. Anim. Sci. 2022, 21, 361–377. [Google Scholar] [CrossRef]
- Akhtar, P.; Rajoriya, J.S.; Singh, A.K.; Ojha, B.K.; Jha, A.K.; Bisen, A.; Singh, M. Effects of dietary supplementation with omega-3 fatty acid-rich linseed on the reproductive performance of ewes in subtropical climates. Front. Vet. Sci. 2024, 11, 1398961. [Google Scholar] [CrossRef]
- Till, B.E.; Huntington, J.A.; Posri, W.; Early, R.; Taylor-Pickard, J.; Sinclair, L.A. Influence of rate of inclusion of microalgae on the sensory characteristics and fatty acid composition of cheese and performance of dairy cows. J. Dairy Sci. 2019, 102, 10934–10946. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.S. Symposium review: Lipids as regulators of conceptus development: Implications for metabolic regulation of reproduction in dairy cattle 1. J. Dairy Sci. 2018, 101, 3630–3641. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, D.; Roura, M.; Pérez-Trujillo, M.; Soto-Heras, S.; Paramio, M.T. Fatty acids and metabolomic composition of follicular fluid collected from environments associated with good and poor oocyte competence in goats. Int. J. Mol. Sci. 2022, 23, 4141. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Nutrient requirements of small ruminants: Sheep, goats, cervids, and new world camelids. In Nutrient Requirements of Small Ruminants; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 39, 911–917. [Google Scholar] [CrossRef]
- Sniffen, C.J.; O’connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- Teixeira, A.; Joy, M.; Delfa, R. In vivo estimation of goat carcass composition and body fat partition by real-time ultrasonography. J. Anim. Sci. 2008, 86, 369–2376. [Google Scholar] [CrossRef]
- Harter, C.J.; Silva, H.G.O.; Lima, L.D.; Castagnino, D.S.; Rivera, A.R.; Neto, O.B.; Gomes, R.A.; Canola, J.C.; Resende, K.T.; Teixeira, I.A.M.A. Ultrasonographic measurements of kidney fat thickness and Longissimus muscle area in predicting body composition of pregnant goats. Anim. Prod. Sci. 2014, 54, 1481–1485. [Google Scholar] [CrossRef]
- Guido, S.I.; Andrade, J.C.O.; Guido, F.C.L.; Oliveira, M.A.L.; Lima, P.F.; Moura, R.T.D.; Santos, V.F.; Cavalcanti, C.C. Avaliação de corpos lúteos de receptoras caprinas. Rev. Bras. Reprodução Anim. 2023, 27, 491–492. [Google Scholar]
- Balaro, M.F.A.; Santos, A.S.; Moura, L.F.G.M.; Fonseca, J.F.; Brandão, F.Z. Luteal dynamic and functionality assessment in dairy goats by luteal blood flow, luteal biometry, and hormonal assay. Theriogenology 2017, 95, 118–126. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Boeckaert, C.; Vlaeminck, B.; Dijkstra, J.; Issa-Zacharia, A.; Van Nespen, T.; Van Straalen, W.; Fievez, V. Effect of dietary starch or micro algae supplementation on rumen fermentation and milk fatty acid composition of dairy cows. J. Dairy Sci. 2008, 91, 4714–4727. [Google Scholar] [CrossRef] [PubMed]
- Pajor, F.; Egerszegi, I.; Szűcs, Á.; Póti, P.; Bodnár, Á. Effect of marine algae supplementation on somatic cell count, prevalence of udder pathogens, and fatty acid profile of dairy goats’ milk. Animals 2021, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Zeron, Y.; Sklan, D.; Arav, A. Effect of polyunsaturated fatty acid supplementation on biophysical parameters and chilling sensitivity of ewe oocytes. Mol. Reprod. Dev. 2002, 61, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, M.; De Matteis, L.; Vernay, M.C.M.B.; Wellnitz, O.; Van Dorland, H.A.; Bruckmaier, R.M. Long-term elevation of β-hydroxybutyrate in dairy cows through infusion: Effects on feed intake, milk production, and metabolism. J. Dairy Sci. 2013, 96, 2960–2972. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Zhao, C.; Bai, Y.; Xia, C.; Xu, C. Effect of negative energy balance on plasma metabolites, minerals, hormones, cytokines and ovarian follicular growth rate in Holstein dairy cows. J. Vet. Res. 2021, 65, 361. [Google Scholar] [CrossRef]
- Pereira, G.; Simões, P.; Bexiga, R.; Silva, E.; Mateus, L.; Fernandes, T.; Lopes-da-Costa, L. Effects of feeding rumen-protected linseed fat to postpartum dairy cows on plasma n-3 polyunsaturated fatty acid concentrations and metabolic and reproductive parameters. J. Dairy Sci. 2022, 105, 361–374. [Google Scholar] [CrossRef]
- Oliveira, F.B.B.D.; Fernandes, C.C.L.; Montenegro, A.R.; Oliveira, I.T.M.; Silva, C.P.; LIMA, F.W.R.; Rondina, D. Cured dry smoked shoulder meat quality from culled adult goats fed a high lipid diet. Food Sci. Technol. 2021, 42, e19521. [Google Scholar] [CrossRef]
- Silva, J.R.V.; Lima, F.E.O.; Souza, A.L.P.; Silva, A.W.B. Interleukin-1β and TNF-α systems in ovarian follicles and their roles during follicular development, oocyte maturation and ovulation. Zygote 2020, 28, 270–277. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kuwahara, A.; Taniguchi, Y.; Yamasaki, M.; Tanaka, Y.; Mukai, Y.; Irahara, M. Tumor necrosis factor alpha inhibits ovulation and induces granulosa cell death in rat ovaries. Reprod. Med. Biol. 2014, 14, 107–115. [Google Scholar] [CrossRef]
- Crespo, D.; Mañanós, E.L.; Roher, N.; MacKenzie, S.A.; Planas, J.V. Tumor necrosis factor alpha may act as an intraovarian mediator of luteinizing hormone-induced oocyte maturation in trout. Biol. Reprod. 2012, 86, 5. [Google Scholar] [CrossRef]
- Ambrose, D.J.; Kastelic, J.P.; Corbett, R.; Pitney, P.A.; Petit, H.V.; Small, J.A.; Zalkovic, P. Lower Pregnancy losses in lactating dairy cows fed a diet enriched in α-linolenic acid. J. Dairy Sci. 2006, 89, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.S.; Saraiva, M.V.A.; Celestino, J.J.H.; Bruno, J.B.; Almeida, A.P.; Cunha, R.M.S.; Figueiredo, J.R. Expression of protein and mRNA encoding Insulin Growth Factor-I (IGF-I) in goat ovarian follicles and the influence of IGF-I on in vitro development and survival of caprine preantral follicles. Anim. Reprod. (AR) 2018, 7, 349–361. [Google Scholar]
- Magalhães-Padilha, D.M.; Duarte, A.B.G.; Araújo, V.R.; Saraiva, M.V.A.; Almeida, A.P.; Rodrigues, G.Q.; Figueiredo, J.R. Steady-state level of insulin-like growth factor-I (IGF-I) receptor mRNA and the effect of IGF-I on the in vitro culture of caprine preantral follicles. Theriogenology 2012, 77, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.L.; Costa, E.P.; Pereira, E.; Benjamin, L.A.; Rodrigues, M.T.; Mendes, V.R.; Silva, T.F. Influence of Insulin-like Growth Factor I (IGF-I) on the survival and the in vitro development of caprine preantral follicles. Pesqui. Vet. Bras. 2014, 34, 1037–1044. [Google Scholar] [CrossRef]
- Luz, V.B.; Chaves, R.N.; Alves, A.M.C.V.; Pinheiro, A.S.; Figueiredo, J.R. Role of insulin-like growth factor-I (IGF-I) and kit ligand (KL) in ovarian function. Acta Sci. Vet. 2015, 43, 1300. [Google Scholar]
- Nudda, A.; Battacone, G.; Usai, M.G.; Fancellu, S.; Pulina, G. Supplementation with extruded linseed cake affects concentrations of conjugated linoleic acid and vaccenic acid in goat milk. J. Dairy Sci. 2006, 89, 277–282. [Google Scholar] [CrossRef]
- Luna, P.; Bach, A.; Juárez, M.; De La Fuente, M.A. Effect of a diet enriched in whole linseed and sunflower oil on goat milk fatty acid composition and conjugated linoleic acid isomer profile. J. Dairy Sci. 2008, 91, 20–28. [Google Scholar] [CrossRef]
- Bernard, L.; Bonnet, M.; Leroux, C.; Shingfield, K.J.; Chilliard, Y. Effect of sunflower-seed oil and linseed oil on tissue lipid metabolism, gene expression, and milk fatty acid secretion in alpine goats fed maize silage–based diets. J. Dairy Sci. 2009, 92, 6083–6094. [Google Scholar] [CrossRef]
- Dutra, P.A.; Pinto, L.F.B.; Neto, B.C.; Gobikrushanth, M.; Barbosa, A.M.; Barbosa, L.P. Flaxseed improves embryo production in Boer goats. Theriogenology 2019, 127, 26–31. [Google Scholar] [CrossRef]
- Zachut, M.; Dekel, I.; Lehrer, H.; Arieli, A.; Arav, A.; Livshitz, L.; Moallem, U. Effects of dietary fats differing in n-6:n-3 ratio fed to high-yielding dairy cows on fatty acid composition of ovarian compartments, follicular status, and oocyte quality. J. Dairy Sci. 2010, 93, 529–545. [Google Scholar] [CrossRef]
- Ghaffarilaleh, V.; Fouladi-Nashta, A.; Paramio, M.-T. Effect of α-linolenic acid on oocyte maturation and embryo development of prepubertal sheep oocytes. Theriogenology 2014, 82, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Fabjanowska, J.; Kowalczuk-Vasilev, E.; Klebaniuk, R.; Milewski, S.; Gümüş, H. N-3 Polyunsaturated Fatty Acids as a Nutritional Support of the Reproductive and Immune System of Cattle—A Review. Animals 2023, 13, 3589. [Google Scholar] [CrossRef] [PubMed]
- Surlis, C.; Cormican, P.; Waters, S.M.; Lonergan, P.; Keogh, K.; Doyle, D.N.; Kenny, D.A. Effects of dietary n-3-PUFA supplementation, post-insemination plane of nutrition and pregnancy status on the endometrial transcriptome of beef heifers. Sci. Rep. 2020, 10, 20798. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Diet | Algae | Linseed | ||
|---|---|---|---|---|---|
| Control | Algae | Linseed | |||
| Ingredients, g/kg DM | |||||
| Elephant grass | 600 | 600 | 600 | - | - |
| Ground corn grain | 120 | 120 | 60 | - | - |
| Soybean meal | 120 | 120 | 100 | - | - |
| Wheat bran | 140 | 140 | 100 | - | - |
| Ground linseed | - | - | 120 | - | - |
| Algae | - | 10 | - | - | - |
| Mineral mixture | 20 | 20 | 20 | - | - |
| Chemical fraction | |||||
| Dry matter, g/kg as-fed basis | 568 | 523 | 573 | 892 | 929 |
| Crude protein, g/kg of DM | 114 | 102 | 122 | 397 | 245 |
| Ether extract, g/kg of DM | 28 | 28 | 57 | 210 | 283 |
| Neutral detergent fiber, g/kg of DM | 546 | 507 | 555 | 178 | 331 |
| Acid detergent fiber, g/kg of DM | 310 | 297 | 329 | 50 | 231 |
| Ash, g/kg of DM | 89 | 83 | 88 | 44 | 34 |
| Non-fibrous carbohydrates, g/kg of DM | 324 | 430 | 420 | 917 | 910 |
| ME, Mcal/kg of DM | 2.23 | 2.27 | 2.35 | - | - |
| Fatty acids, % | |||||
| Saturated | - | - | - | 28.1 | 11.5 |
| Unsaturated | - | - | - | 71.9 | 88.5 |
| Monounsaturated | - | - | - | 45.0 | 23.1 |
| Polyunsaturated | - | - | - | 26.9 | 65.4 |
| UFA/SFA * | 2.6 | 7.7 |
| Parameters | Diet | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | Algae | Linseed | SEM | Diet | Time | D vs. T | |
| Feed intake | |||||||
| DMI *, kg/doe | 1.2 a | 1.0 b | 1.2 a | 0.015 | <0.001 | 0.231 | 0.997 |
| Metabolic markers and oxidative stress | |||||||
| Glutathione peroxidase, U/L | 127.3 | 120.5 | 147.8 | 2.169 | 0.058 | 0.947 | 0.702 |
| BHB **, mmol/L | 0.3 | 0.3 | 0.4 | 0.019 | 0.878 | - | - |
| Cholesterol, mg/dL | 53.6 | 63.2 | 62.9 | 1.774 | 0.107 | <0.001 | 0.180 |
| FPR ***, ratio | 0.8 a | 1.0 b | 1.1 b | 0.03 | 0.002 | 0.822 | 0.994 |
| Luteal function | |||||||
| Functional CL ****, n/doe | 0 B | 0 B | 0 B | - | - | - | - |
| Non-functional CL, n/doe | 0 B | 0 B | 1 B | - | - | - | - |
| CL absent, n/doe | 12 A | 13 A | 12 A | - | - | - | - |
| Progesterone, ng/mL | 0.2 a | 0.2 a | 0.3 b | 0.011 | 0.025 | 0.945 | 0.988 |
| Parameters | Diet | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Control | Algae | Linseed | SEM | Diet | Time | D vs. T | |
| Follicle after ovulation induction * | |||||||
| Follicles < 3 mm, n\ovary | 2.0 ab | 2.0 a | 1.6 b | 0.032 | 0.044 | 0.094 | 0.503 |
| Follicles ≥ 3 mm, n\ovary | 1.4 ab | 1.2 a | 1.6 b | 0.025 | 0.016 | 0.009 | 0.404 |
| Total follicles, n\ovary | 3.3 | 3.2 | 3.3 | 0.025 | 0.461 | 0.681 | 0.503 |
| Max follicle size, mm | 4.2 a | 4.6 ab | 5.0 b | 0.051 | 0.008 | 0.006 | 0.175 |
| Estrus and ovulatory response | |||||||
| No. of does in estrus, % (n\n) | 25.0 (3/12) ‡ | 38.5 (5/13) | 46.2 (6/13) | - | 0.367 | - | - |
| Onset estrus **, h | 38.6 | 41.7 | 21.5 | 6.278 | 0.344 | - | - |
| Estrus length, h | 16.3 | 31.5 | 37.7 | 6.036 | 0.473 | - | - |
| No. of does ovulated, % (n\n) | 25.0 (3/12) ‡ | 69.2 (9/13) | 61.5 (8/13) | - | 0.216 | - | - |
| Ovulatory rate, n\doe | 0.4 a | 0.8 ab | 1.0 b | 0.132 | 0.046 | - | - |
| Metabolic marker and oxidative stress *** | |||||||
| Glutathione peroxidase, U/L | 188.9 | 235.6 | 228.6 | 2.211 | 0.345 | - | - |
| BHB ****, mmol/L | 0.2 | 0.2 | 0.3 | 0.038 | 0.715 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.P.; Fernandes, C.C.L.; Alves, J.P.M.; Cavalcanti, C.M.; Oliveira, F.B.B.; Conde, A.J.H.; Pinheiro, D.C.S.N.; Teixeira, D.I.A.; Rego, A.C.; Rondina, D. Efficacy of Fat Supplements with Different Unsaturated/Saturated FA Ratios Undergoing First Postpartum Ovulation in Lactating Anovulatory Goats. Vet. Sci. 2025, 12, 60. https://doi.org/10.3390/vetsci12010060
Silva CP, Fernandes CCL, Alves JPM, Cavalcanti CM, Oliveira FBB, Conde AJH, Pinheiro DCSN, Teixeira DIA, Rego AC, Rondina D. Efficacy of Fat Supplements with Different Unsaturated/Saturated FA Ratios Undergoing First Postpartum Ovulation in Lactating Anovulatory Goats. Veterinary Sciences. 2025; 12(1):60. https://doi.org/10.3390/vetsci12010060
Chicago/Turabian StyleSilva, Caroline P., César C. L. Fernandes, Juliana P. M. Alves, Camila M. Cavalcanti, Felipe B. B. Oliveira, Alfredo J. H. Conde, Diana Celia S. N. Pinheiro, Darcio I. A. Teixeira, Anibal C. Rego, and Davide Rondina. 2025. "Efficacy of Fat Supplements with Different Unsaturated/Saturated FA Ratios Undergoing First Postpartum Ovulation in Lactating Anovulatory Goats" Veterinary Sciences 12, no. 1: 60. https://doi.org/10.3390/vetsci12010060
APA StyleSilva, C. P., Fernandes, C. C. L., Alves, J. P. M., Cavalcanti, C. M., Oliveira, F. B. B., Conde, A. J. H., Pinheiro, D. C. S. N., Teixeira, D. I. A., Rego, A. C., & Rondina, D. (2025). Efficacy of Fat Supplements with Different Unsaturated/Saturated FA Ratios Undergoing First Postpartum Ovulation in Lactating Anovulatory Goats. Veterinary Sciences, 12(1), 60. https://doi.org/10.3390/vetsci12010060







