Early Enhancement in Contrast-Enhanced Computed Tomography Is an Index of DUSP9, SLPI, ALDH1L2, and SLC1A1 Expression in Canine Hepatocellular Carcinoma: A Preliminary Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
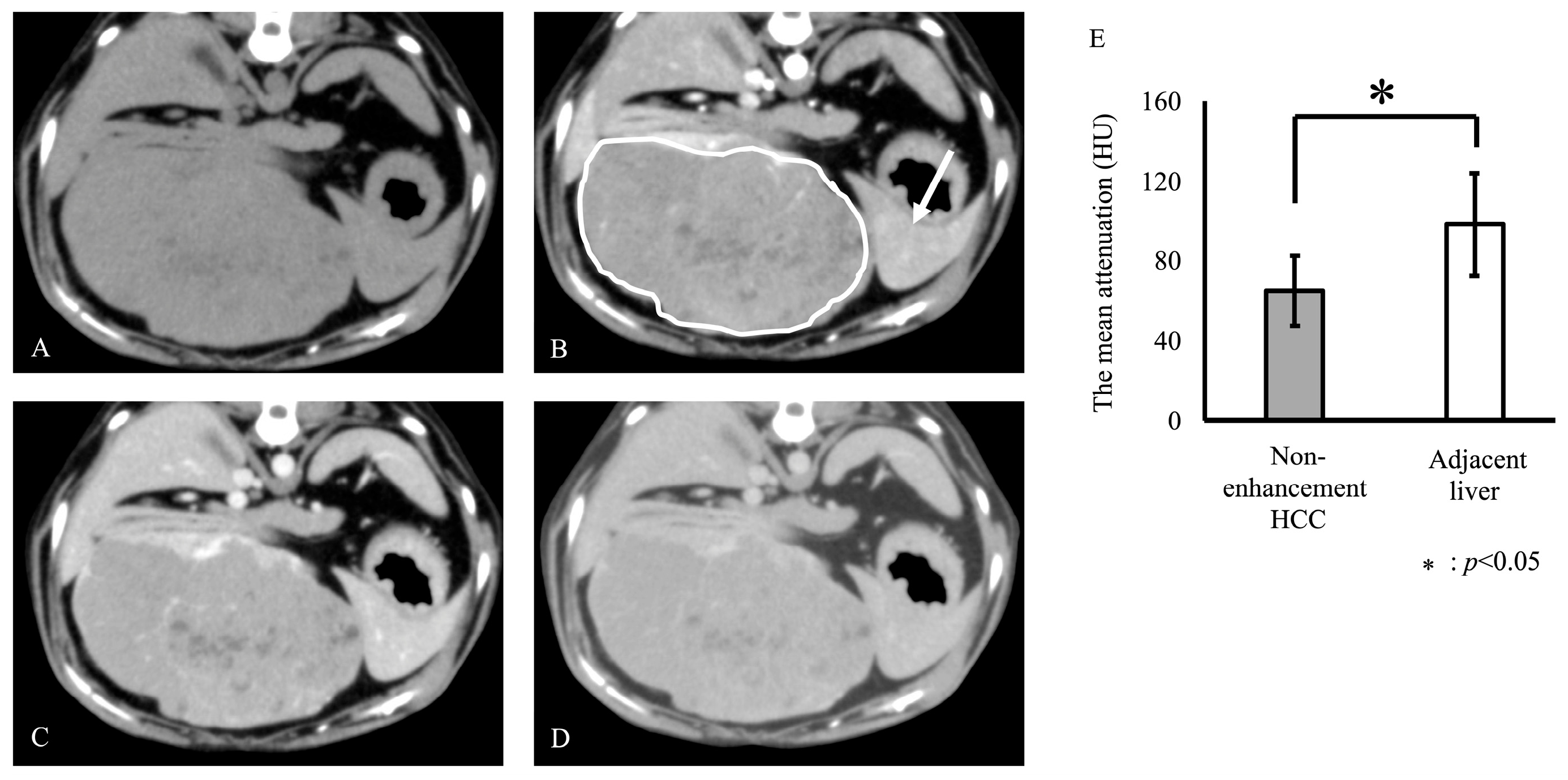
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| DEG | Differentially expressed gene |
| DUSPs | Dual-specificity phosphatases |
| HCC | Hepatocellular carcinoma |
| MAPK | Mitogen-activated protein kinases |
| MRI | Magnetic resonance imaging |
| RNA-Seq | RNA sequencing |
| SLC1 | Solute carrier family 1 |
| SLPIs | Secretory leukocyte protease inhibitors |
References
- Kurokawa, S.; Tanaka, T.; Yamazaki, H.; Noguchi, S.; Wada, Y.; Nishida, H.; Akiyoshi, H. Comparing the CT and MRI findings for canine primary hepatocellular lesions. VetRecord 2022, 190, e1083. [Google Scholar] [CrossRef] [PubMed]
- Kutara, K.; Seki, M.; Ishikawa, C.; Sakai, M.; Kagawa, Y.; Iida, G.; Ishigaki, K.; Teshima, K.; Edamura, K.; Nakayama, T.; et al. Triple-phase helical computed tomography in dogs with hepatic masses. Vet. Radiol. Ultrasound 2014, 55, 7–15. [Google Scholar] [CrossRef]
- Fukushima, K.; Kanemoto, H.; Ohno, K.; Takahashi, M.; Nakashima, K.; Fujino, Y.; Uchida, K.; Fujiwara, R.; Nishimura, R.; Tsujimoto, H. CT characteristics of primary hepatic mass lesions in dogs. Vet. Radiol. Ultrasound 2012, 53, 252–257. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Ouhmich, F.; Gonzalez-Cabrera, C.; Felli, E.; Saviano, A.; Agnus, V.; Savadjiev, P.; Baumert, T.F.; Pessaux, P.; Marescaux, J.; et al. Radiomics in hepatocellular carcinoma: A quantitative review. Hepatol. Int. 2019, 13, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, O.; Echegaray, S.; Khuong, A.; Hoang, C.D.; Shrager, J.B.; Jensen, K.C.; Berry, G.J.; Guo, H.H.; Lau, C.; Plevritis, S.K.; et al. Predictive radiogenomics modeling of EGFR mutation status in lung cancer. Sci. Rep. 2017, 7, 41674. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015, 149, 1226–1239.e4. [Google Scholar] [CrossRef]
- Bai, H.X.; Lee, A.M.; Yang, L.; Zhang, P.; Davatzikos, C.; Maris, J.M.; Diskin, S.J. Imaging genomics in cancer research: Limitations and promises. Br. J. Radiol. 2016, 89, 20151030. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Sirlin, C.B.; Ooi, C.; Adler, A.S.; Gollub, J.; Chen, X.; Chan, B.K.; Matcuk, G.R.; Barry, C.T.; Chang, H.Y.; et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat. Biotechnol. 2007, 25, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Hectors, S.J.; Wagner, M.; Bane, O.; Besa, C.; Lewis, S.; Remark, R.; Chen, N.; Fiel, M.I.; Zhu, H.; Gnjatic, S.; et al. Quantification of hepatocellular carcinoma heterogeneity with multiparametric magnetic resonance imaging. Sci. Rep. 2017, 7, 2452. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Ushio, N.; Rahman, M.M.; Katanoda, Y.; Ogihara, K.; Naya, Y.; Moriyama, A.; Iwanaga, T.; Saitoh, Y.; Sogawa, T.; et al. Aberrant expression of microRNAs and the miR-1/MET pathway in canine hepatocellular carcinoma. Vet. Comp. Oncol. 2018, 16, 288–296. [Google Scholar] [CrossRef]
- Polak, K.Z.; Schaffer, P.; Donaghy, D.; Zenk, M.C.; Olver, C.S. Iron, hepcidin, and microcytosis in canine hepatocellular carcinoma. Vet. Clin. Pathol. 2022, 51, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Iida, G.; Asano, K.; Seki, M.; Sakai, M.; Kutara, K.; Ishigaki, K.; Kagawa, Y.; Yoshida, O.; Teshima, K.; Edamura, K.; et al. Gene expression of growth factors and growth factor receptors for potential targeted therapy of canine hepatocellular carcinoma. J. Vet. Med. Sci. 2014, 76, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef]
- Tajima, T.; Honda, H.; Taguchi, K.; Asayama, Y.; Kuroiwa, T.; Yoshimitsu, K.; Irie, H.; Aibe, H.; Shimada, M.; Masuda, K. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am. J. Roentgenol. 2002, 178, 885–897. [Google Scholar] [CrossRef]
- Matsui, O.; Kobayashi, S.; Sanada, J.; Kouda, W.; Ryu, Y.; Kozaka, K.; Kitao, A.; Nakamura, K.; Gabata, T. Hepatocelluar nodules in liver cirrhosis: Hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom. Imaging 2011, 36, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Iimori, Y.; Yamazaki, H.; Nishida, H.; Akiyoshi, H. Contrast-enhanced computed tomography characterization of canine rectal neoplasms. Jpn. J. Vet. Res. 2021, 69, 163–173. [Google Scholar] [CrossRef]
- Maeda, S.; Motegi, T.; Iio, A.; Kaji, K.; Goto-Koshino, Y.; Eto, S.; Ikeda, N.; Nakagawa, T.; Nishimura, R.; Yonezawa, T.; et al. Anti-CCR4 treatment depletes regulatory T cells and leads to clinical activity in a canine model of advanced prostate cancer. J. Immunother. Cancer 2022, 10, e003731. [Google Scholar] [CrossRef] [PubMed]
- Zender, L.; Villanueva, A.; Tovar, V.; Sia, D.; Chiang, D.Y.; Llovet, J.M. Cancer gene discovery in hepatocellular carcinoma. J. Hepatol. 2010, 52, 921–929. [Google Scholar] [CrossRef]
- Sugino, T.; Yamaguchi, T.; Ogura, G.; Kusakabe, T.; Goodison, S.; Homma, Y.; Suzuki, T. The secretory leukocyte protease inhibitor (SLPI) suppresses cancer cell invasion but promotes blood-borne metastasis via an invasion-independent pathway. J. Pathol. 2007, 212, 152–160. [Google Scholar] [CrossRef]
- Zhong, Q.Q.; Wang, X.; Li, Y.F.; Peng, L.J.; Jiang, Z.S. Secretory leukocyte protease inhibitor promising protective roles in obesity-associated atherosclerosis. Exp. Biol. Med. 2017, 242, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, S.S.; Ma, J.; Qu, W. Secretory leukocyte protease inhibitor (SLPI) in cancer pathophysiology: Mechanisms of action and clinical implications. Pathol. Res. Pract. 2023, 248, 154633. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Wu, Z.; Liang, Y.; Duan, R.; Zheng, M.; Wang, J.; Kong, D. SLPI suppresses hepatocellular carcinoma progression via endoplasmic reticulum stress induced apoptosis. Int. J. Biol. Sci. 2022, 18, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.I.; Brummer, T.; O’Brien, P.M.; Daly, R.J. Dual-specificity phosphatases: Critical regulators with diverse cellular targets. Biochem. J. 2009, 418, 475–489. [Google Scholar] [CrossRef]
- Kidger, A.M.; Keyse, S.M. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs). Semin. Cell Dev. Biol. 2016, 50, 125–132. [Google Scholar] [CrossRef]
- Seternes, O.M.; Kidger, A.M.; Keyse, S.M. Dual-specificity MAP kinase phosphatases in health and disease. Biochim Biophys Acta Mol. Cell Res. 2019, 1866, 124–143. [Google Scholar] [CrossRef]
- Petrochilos, D.; Shojaie, A.; Gennari, J.; Abernethy, N. Using random walks to identify cancer-associated modules in expression data. BioData Min. 2013, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gorgen, A.; Ding, A.; Du, L.; Jiang, K.; Ding, Y.; Sapisochin, G.; Ghanekar, A. Dual-specificity phosphatase 9 regulates cellular proliferation and predicts recurrence after surgery in hepatocellular carcinoma. Hepatol. Commun. 2021, 5, 1310–1328. [Google Scholar] [CrossRef]
- Liu, J.; Ni, W.; Xiao, M.; Jiang, F.; Ni, R. Decreased expression and prognostic role of mitogen-activated protein kinase phosphatase 4 in hepatocellular carcinoma. J. Gastrointest. Surg. 2013, 17, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Chen, W.; Zuo, H.; Bi, Z.; Zhang, X.; Pang, L.; Jing, Y.; Yin, X.; Cheng, H. Comprehensive analysis of aldehyde dehydrogenases (ALDHs) and its significant role in hepatocellular carcinoma. Biochem. Genet. 2022, 60, 1274–1297. [Google Scholar] [CrossRef]
- Avallone, G.; Rasotto, R.; Chambers, J.K.; Miller, A.D.; Behling-Kelly, E.; Monti, P.; Berlato, D.; Valenti, P.; Roccabianca, P. Review of histological grading systems in veterinary medicine. Vet. Pathol. 2021, 58, 809–828. [Google Scholar] [CrossRef]
- Hennequart, M.; Pilley, S.E.; Labuschagne, C.F.; Coomes, J.; Mervant, L.; Driscoll, P.C.; Legrave, N.M.; Lee, Y.; Kreuzaler, P.; Macintyre, B.; et al. ALDH1L2 regulation of formate, formyl-methionine, and ROS controls cancer cell migration and metastasis. Cell Rep. 2023, 42, 112562. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.; Lopez, D.J.; Balkman, C.E.; Sumner, J.P. Factors associated with survival in dogs with a histopathological diagnosis of hepatocellular carcinoma: 94 cases (2007–2018). Open Vet. J. 2021, 11, 144–153. [Google Scholar] [CrossRef]
- Bjørn-Yoshimoto, W.E.; Underhill, S.M. The importance of the excitatory amino acid transporter 3 (EAAT3). Neurochem. Int. 2016, 98, 4–18. [Google Scholar] [CrossRef]
- Schmitt, A.; Zink, M.; Petroianu, G.; May, B.; Braus, D.F.; Henn, F.A. Decreased gene expression of glial and neuronal glutamate transporters after chronic antipsychotic treatment in rat brain. Neurosci. Lett. 2003, 347, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Schniepp, R.; Kohler, K.; Ladewig, T.; Guenther, E.; Henke, G.; Palmada, M.; Boehmer, C.; Rothstein, J.D.; Bröer, S.; Lang, F. Retinal colocalization and in vitro interaction of the glutamate transporter EAAT3 and the serum- and glucocorticoid-inducible kinase SGK1 [correction]. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1442–1449. [Google Scholar] [CrossRef]
- Maragakis, N.J.; Dietrich, J.; Wong, V.; Xue, H.; Mayer-Proschel, M.; Rao, M.S.; Rothstein, J.D. Glutamate transporter expression and function in human glial progenitors. Glia 2004, 45, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Yao, L.; Xu, L.; Ma, Q.; Huang, G.; Yang, M.; Gao, C.; Cheng, J.; Zhou, X.; Li, Q.; et al. Comprehensive analysis of potential correlation between solute Carrier 1A (SLC1A) family and lung adenocarcinoma. Int. J. Gen. Med. 2022, 15, 2101–2117. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Tang, Y.; Wang, C.; Landman, B.A.; Zhou, S.K. Transforming medical imaging with Transformers? A comparative review of key properties, current progresses, and future perspectives. Med. Image Anal. 2023, 85, 102762. [Google Scholar] [CrossRef] [PubMed]

| Group | CT Examination | Age | Sex | Bleed | Recurrence | Metastasis |
|---|---|---|---|---|---|---|
| G1 | NL | 3.3 | IF | beagle | N/A | N/A |
| G1 | NL | 4.8 | IF | beagle | N/A | N/A |
| G1 | NL | 1.4 | IF | beagle | N/A | N/A |
| G1 | NL | 3.3 | IF | beagle | N/A | N/A |
| G2 | enhancement | 13 | IF | Dachshund | - | - |
| G2 | enhancement | 12 | IM | West Highland White Terrier | - | - |
| G2 | enhancement | 7.5 | CM | Toy Poodle | - | - |
| G3 | non-enhancement | 14.4 | IF | Shiba | - | - |
| G3 | non-enhancement | 12 | IF | Border Collies | - | - |
| G3 | non-enhancement | 9 | SF | Brussels griffon | - | - |
| G3 | non-enhancement | 8 | IM | Shiba | - | - |
| G3 | non-enhancement | 8.6 | IF | Shiba | - | - |
| Ensembl Gene ID | Gene Symbol | Counts per Million Mapped Reads | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | ||||||||||
| NL | NL | NL | NL | E-HCC | E-HCC | E-HCC | |||||
| ENSCAFG00000019241 | DUSP9 | 0 | 0 | 1 | 0 | 699 | 2 | 139 | |||
| ENSCAFG00000028626 | SLPI | 18 | 2 | 3 | 2 | 269 | 3006 | 546 | |||
| ENSCAFG00000001911 | ALDH1L2 | 9 | 5 | 5 | 9 | 305 | 16 | 1775 | |||
| ENSCAFG00000047783 | N/A | 43.7 | 123.42 | 60.84 | 70.61 | 1.4 | 0 | 4.94 | |||
| ENSCAFG00000016090 | TOP2A | 16 | 11 | 6 | 18 | 116 | 175 | 620 | |||
| ENSCAFG00000025465 | TRPV6 | 317 | 123 | 191 | 153 | 55 | 1 | 56 | |||
| ENSCAFG00000012593 | CENPF | 4 | 4 | 4 | 4 | 24 | 90 | 280 | |||
| ENSCAFG00000002067 | SLC1A1 | 1879 | 1818 | 1332 | 2516 | 682 | 815 | 1204 | |||
| G3 | q-value | DEG order | |||||||||
| NE-HCC | NE-HCC | NE-HCC | NE-HCC | NE-HCC | |||||||
| 0 | 0 | 1 | 0 | 0 | 0.004863 | G2 > other | |||||
| 33 | 66 | 28 | 18 | 128 | 0.004863 | G2 > other | |||||
| 22 | 14 | 33 | 18 | 14 | 0.005376 | G2 > other | |||||
| 1.56 | 0 | 1.44 | 3.71 | 2.1 | 0.005387 | 0 | |||||
| 254 | 176 | 673 | 398 | 166 | 0.008041 | other > G1 | |||||
| 0 | 3 | 1 | 0 | 0 | 0.009765 | G1 > other | |||||
| 143 | 131 | 213 | 75 | 59 | 0.009765 | other > G1 | |||||
| 0 | 4 | 44 | 0 | 0 | 0.009828 | other > G3 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Motegi, T.; Sumikawa, N.; Mori, M.; Kurokawa, S.; Akiyoshi, H. Early Enhancement in Contrast-Enhanced Computed Tomography Is an Index of DUSP9, SLPI, ALDH1L2, and SLC1A1 Expression in Canine Hepatocellular Carcinoma: A Preliminary Study. Vet. Sci. 2025, 12, 137. https://doi.org/10.3390/vetsci12020137
Tanaka T, Motegi T, Sumikawa N, Mori M, Kurokawa S, Akiyoshi H. Early Enhancement in Contrast-Enhanced Computed Tomography Is an Index of DUSP9, SLPI, ALDH1L2, and SLC1A1 Expression in Canine Hepatocellular Carcinoma: A Preliminary Study. Veterinary Sciences. 2025; 12(2):137. https://doi.org/10.3390/vetsci12020137
Chicago/Turabian StyleTanaka, Toshiyuki, Tomoki Motegi, Nanami Sumikawa, Misaki Mori, Shohei Kurokawa, and Hideo Akiyoshi. 2025. "Early Enhancement in Contrast-Enhanced Computed Tomography Is an Index of DUSP9, SLPI, ALDH1L2, and SLC1A1 Expression in Canine Hepatocellular Carcinoma: A Preliminary Study" Veterinary Sciences 12, no. 2: 137. https://doi.org/10.3390/vetsci12020137
APA StyleTanaka, T., Motegi, T., Sumikawa, N., Mori, M., Kurokawa, S., & Akiyoshi, H. (2025). Early Enhancement in Contrast-Enhanced Computed Tomography Is an Index of DUSP9, SLPI, ALDH1L2, and SLC1A1 Expression in Canine Hepatocellular Carcinoma: A Preliminary Study. Veterinary Sciences, 12(2), 137. https://doi.org/10.3390/vetsci12020137






