Feline Calicivirus Infection Manipulates Central Carbon Metabolism
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Viral Infection
2.2. Reagents
2.3. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
2.4. Western Blotting Assay
2.5. The 50% Tissue Culture Infectious Dose (TCID50) Assay
2.6. Metabolite Extraction and LC-MS Analysis
2.7. Cell Viability Assay
2.8. Statistical Analysis
3. Results
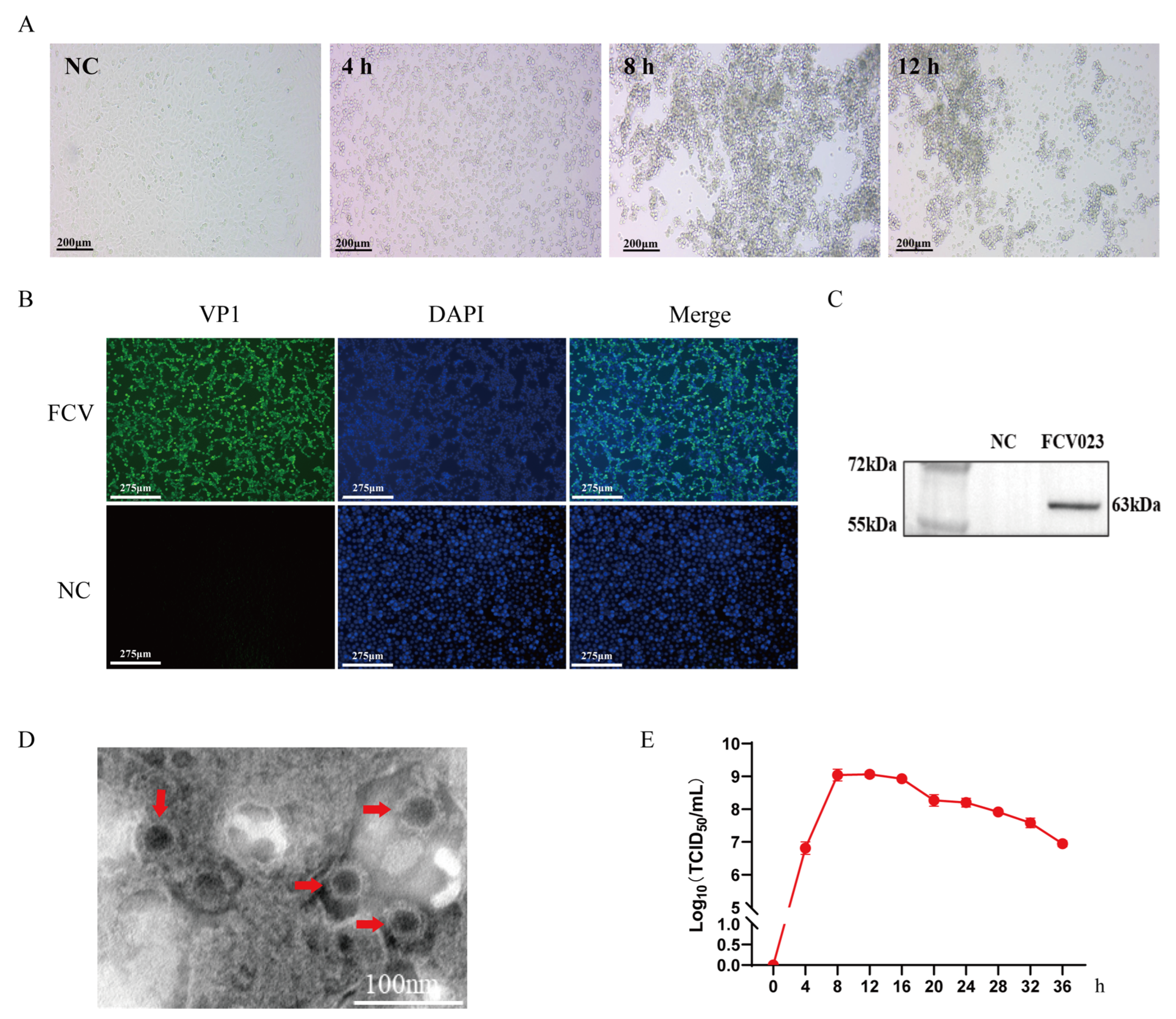
3.1. FCV023 Isolation Identification and One-Step Growth Curve Determination
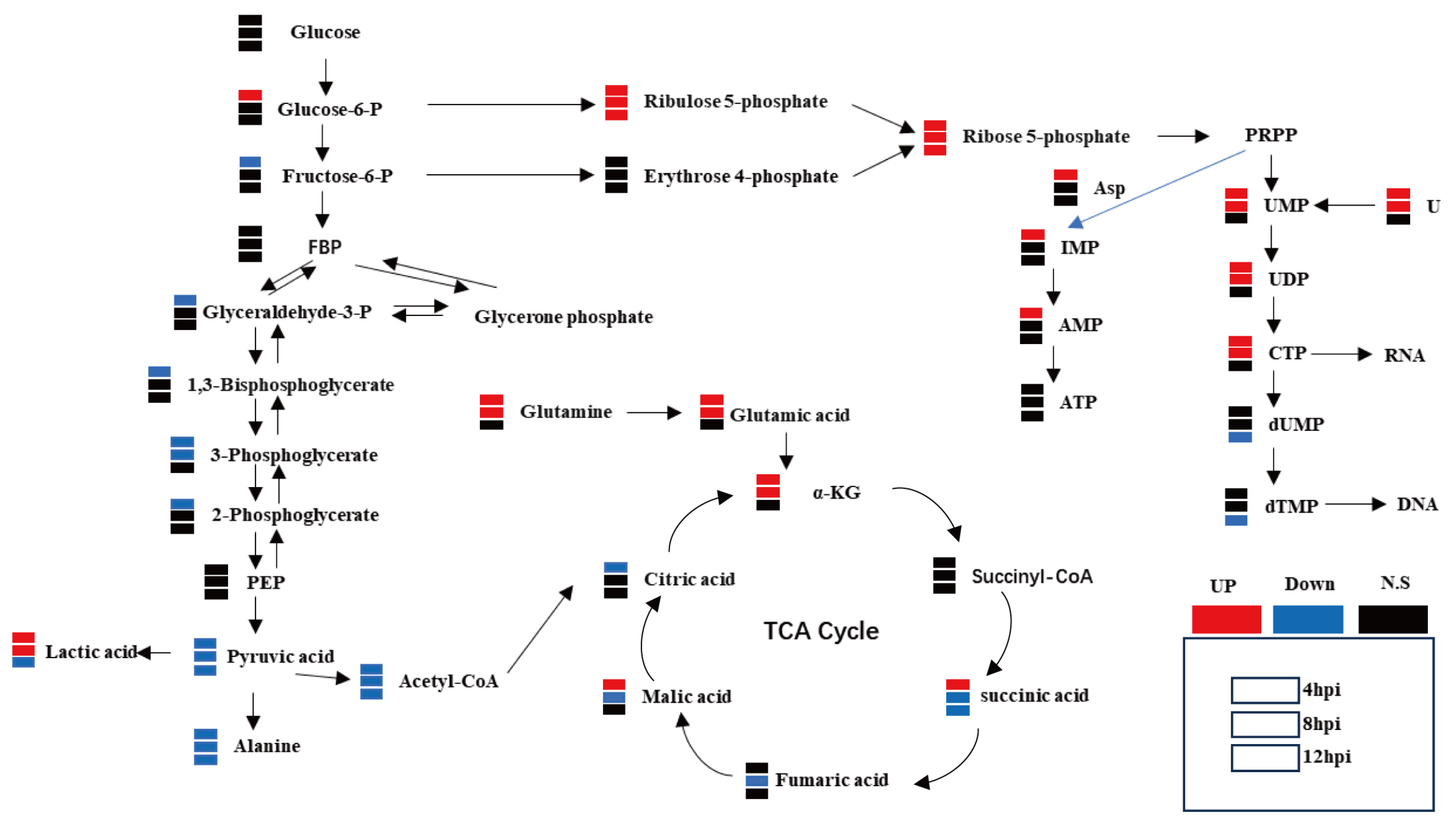
3.2. Metabolomic Studies Have Identified That FCV023 Infection Affects Central Carbon Metabolism in CRFK Cells
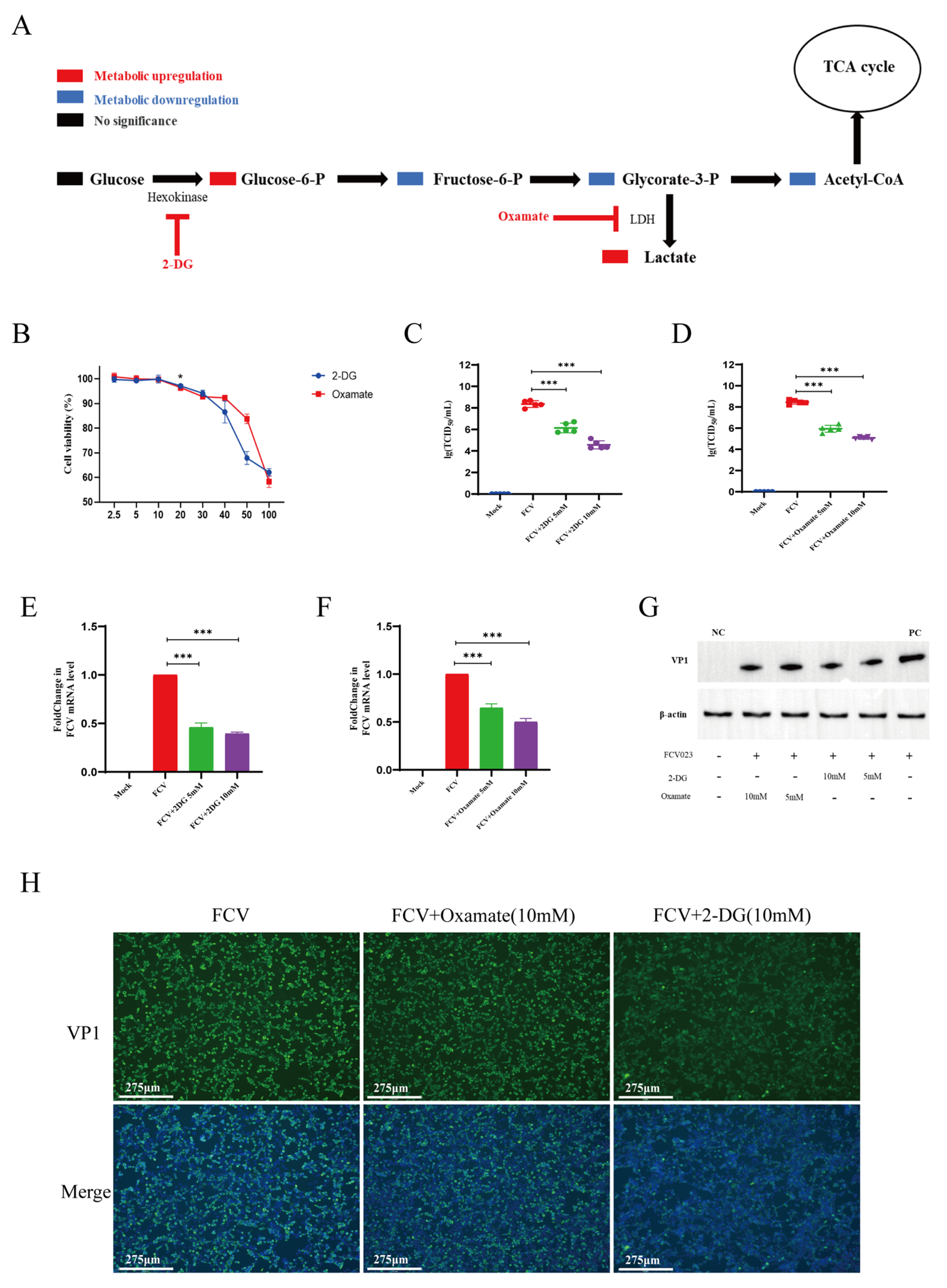
3.3. 2-DG and Sodium Oxamate Reduces FCV023 Infection in CRFK Cell
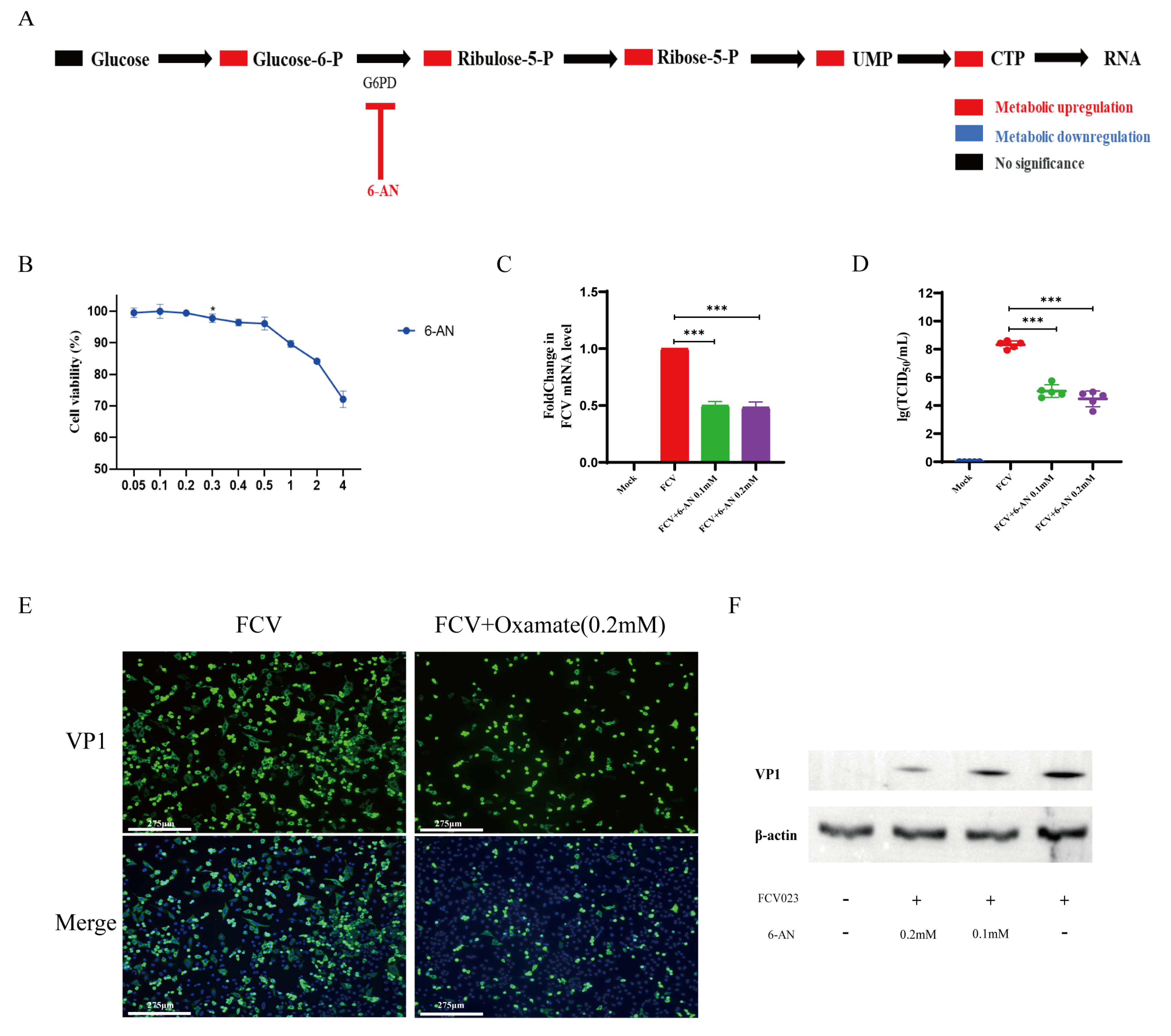
3.4. FCV023 Infection Enhances the PPP in CRFK Cells, and the Unobstructed PP Facilitates FCV Infection
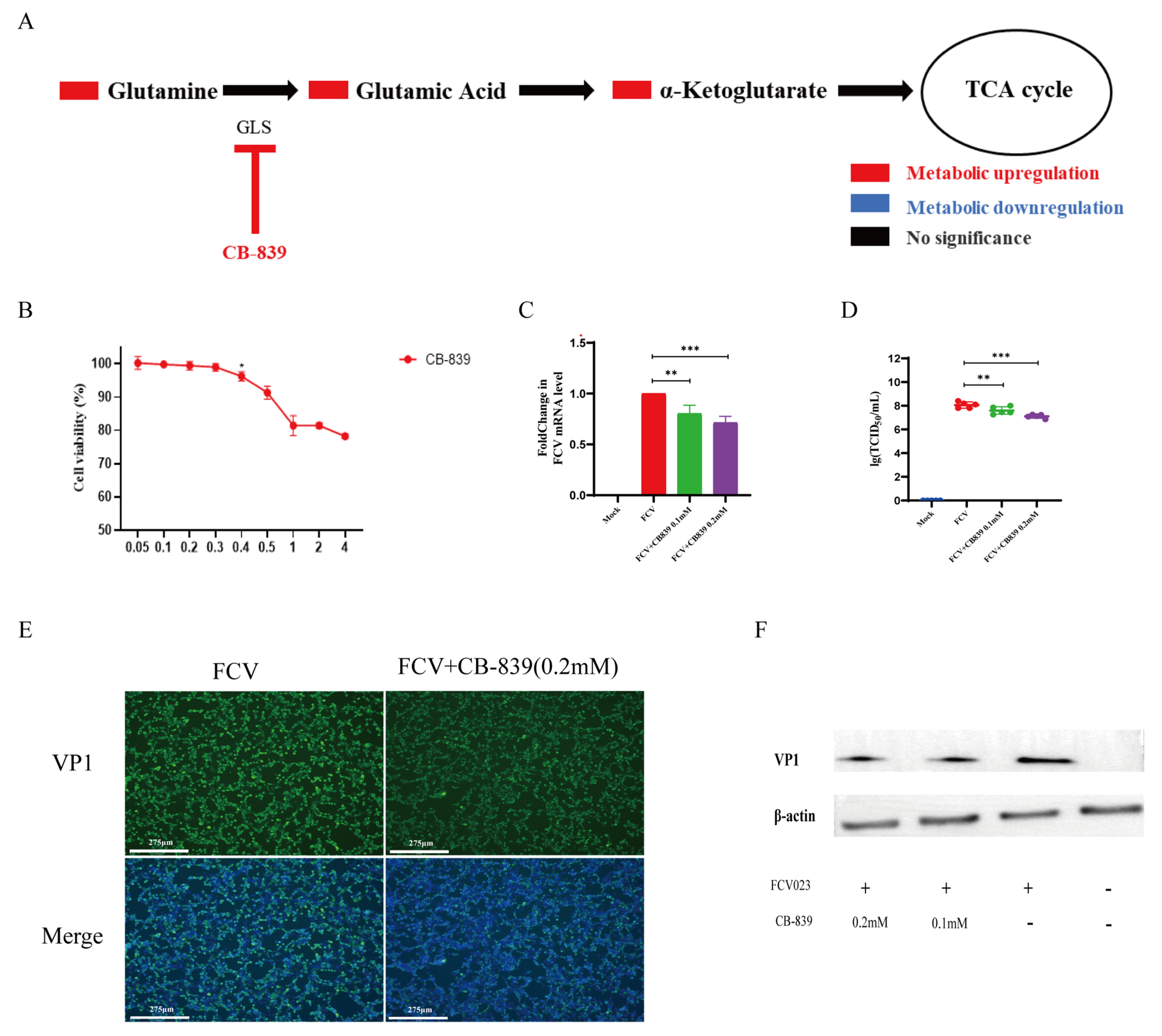
3.5. Glutamine Metabolism Is Crucial for the Proliferation of FCV023
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hofmann-Lehmann, R.; Hosie, M.J.; Hartmann, K.; Egberink, H.; Truyen, U.; Tasker, S.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Calicivirus Infection in Cats. Viruses 2022, 14, 937. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, P.A.; Chang, K.O.; Parker, J.S. Molecular virology of feline calicivirus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.D.; Coyne, K.P.; Dawson, S.; Porter, C.J.; Gaskell, R.M. Feline calicivirus. Vet. Res. 2007, 38, 319–335. [Google Scholar] [CrossRef]
- Lanave, G.; Buonavoglia, A.; Pellegrini, F.; Di Martino, B.; Di Profio, F.; Diakoudi, G.; Catella, C.; Omar, A.H.; Vasinioti, V.I.; Cardone, R.; et al. An Outbreak of Limping Syndrome Associated with Feline Calicivirus. Anim. Open Access J. MDPI 2023, 13, 1778. [Google Scholar] [CrossRef]
- Baratta, M.G. Virus-mediated hijack of one-carbon metabolism. Nat. Rev. Cancer 2019, 19, 486. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, Q.; Li, D.; Zhao, S.; Zhang, Q.; Tan, Y.; Gong, Q.; Liu, T.; Shao, J.; Zhang, S.; et al. Icariin, Formononetin and Caffeic Acid Phenethyl Ester Inhibit Feline Calicivirus Replication In Vitro. Arch. Virol. 2021, 166, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.M.; Xu, S.; Munger, J. Stealing the Keys to the Kitchen: Viral Manipulation of the Host Cell Metabolic Network. Trends Microbiol. 2015, 23, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Girdhar, K.; Powis, A.; Raisingani, A.; Chrudinová, M.; Huang, R.; Tran, T.; Sevgi, K.; Dogus Dogru, Y.; Altindis, E. Viruses and Metabolism: The Effects of Viral Infections and Viral Insulins on Host Metabolism. Annu. Rev. Virol. 2021, 8, 373–391. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Deng, H.; Li, S.; Qiu, H.J. Cellular metabolism hijacked by viruses for immunoevasion: Potential antiviral targets. Front. Immunol. 2023, 14, 1228811. [Google Scholar] [CrossRef]
- Cheng, K.; Martin-Sancho, L.; Pal, L.R.; Pu, Y.; Riva, L.; Yin, X.; Sinha, S.; Nair, N.U.; Chanda, S.K.; Ruppin, E. Genome-scale metabolic modeling reveals SARS-CoV-2-induced metabolic changes and antiviral targets. Mol. Syst. Biol. 2021, 17, e10260. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. SDS-Polyacrylamide Gel Electrophoresis of Proteins. CSH Protoc. 2006, 2006, pdb-prot4540. [Google Scholar] [CrossRef]
- Xie, Q.; Sun, Z.; Xue, X.; Pan, Y.; Zhen, S.; Liu, Y.; Zhan, J.; Jiang, L.; Zhang, J.; Zhu, H.; et al. China-origin G1 group isolate FPV072 exhibits higher infectivity and pathogenicity than G2 group isolate FPV027. Front. Vet. Sci. 2024, 11, 1328244. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H.; et al. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.J.; Guo, L.Y.; Li, P.; Zhao, Z.; Zhou, H.; Di, L.J. Molecular link between glucose and glutamine consumption in cancer cells mediated by CtBP and SIRT4. Oncogenesis 2018, 7, 26. [Google Scholar] [CrossRef]
- Prasad, A.; Roy, A.C.; Priya, K.; Meena, R.; Ghosh, I. Effect of differential deprivation of nutrients on cellular proliferation, oxidative stress, mitochondrial function, and cell migration in MDA-MB-231, HepG2, and HeLa cells. 3 Biotech 2023, 13, 339. [Google Scholar] [CrossRef]
- Howard-Varona, C.; Lindback, M.M.; Bastien, G.E.; Solonenko, N.; Zayed, A.A.; Jang, H.; Andreopoulos, B.; Brewer, H.M.; Glavina Del Rio, T.; Adkins, J.N.; et al. Phage-specific metabolic reprogramming of virocells. ISME J. 2020, 14, 881–895. [Google Scholar] [CrossRef]
- Yu, Y.; Clippinger, A.J.; Alwine, J.C. Viral effects on metabolism: Changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol. 2011, 19, 360–367. [Google Scholar] [CrossRef]
- Dekhne, A.S.; Hou, Z.; Gangjee, A.; Matherly, L.H. Therapeutic Targeting of Mitochondrial One-Carbon Metabolism in Cancer. Mol. Cancer Ther. 2020, 19, 2245–2255. [Google Scholar] [CrossRef]
- Jin, J.; Byun, J.K.; Choi, Y.K.; Park, K.G. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp. Mol. Med. 2023, 55, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zeng, H.; Fan, J.; Wang, F.; Xu, C.; Li, Y.; Tu, J.; Nephew, K.P.; Long, X. Glutamine metabolism in breast cancer and possible therapeutic targets. Biochem. Pharmacol. 2023, 210, 115464. [Google Scholar] [CrossRef]
- Amoedo, N.D.; Obre, E.; Rossignol, R. Drug discovery strategies in the field of tumor energy metabolism: Limitations by metabolic flexibility and metabolic resistance to chemotherapy. Biochim. Biophys. Acta. Bioenerg. 2017, 1858, 674–685. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Hafner, A.; Meurs, N.; Garner, A.; Azar, E.; Kannan, A.; Passalacqua, K.D.; Nagrath, D.; Wobus, C.E. Norovirus NS1/2 protein increases glutaminolysis for efficient viral replication. PLoS Pathog. 2024, 20, e1011909. [Google Scholar] [CrossRef]
- Passalacqua, K.D.; Lu, J.; Goodfellow, I.; Kolawole, A.O.; Arche, J.R.; Maddox, R.J.; Carnahan, K.E.; O’Riordan, M.X.D.; Wobus, C.E. Glycolysis Is an Intrinsic Factor for Optimal Replication of a Norovirus. mBio 2019, 10, e02175-18. [Google Scholar] [CrossRef]
- Pang, Y.; Li, C.; Wang, Y.; Liu, J.; Su, G.; Duan, C.; Fang, L.; Zhou, Y.; Xiao, S. Porcine reproductive and respiratory syndrome virus infection manipulates central carbon metabolism. Vet. Microbiol. 2023, 279, 109674. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Xu, Z.G.; Tu, H.; Hu, F.; Dai, J.; Chang, Y.; Chen, Y.; Lu, Y.; Zeng, H.; et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 2019, 178, 176–189.e115. [Google Scholar] [CrossRef]
- Li, H.; Lin, C.; Qi, W.; Sun, Z.; Xie, Z.; Jia, W.; Ning, Z. Senecavirus A-induced glycolysis facilitates virus replication by promoting lactate production that attenuates the interaction between MAVS and RIG-I. PLoS Pathog. 2023, 19, e1011371. [Google Scholar] [CrossRef]
- Xue, Q.; Liu, H.; Zhu, Z.; Yang, F.; Song, Y.; Li, Z.; Xue, Z.; Cao, W.; Liu, X.; Zheng, H. African Swine Fever Virus Regulates Host Energy and Amino Acid Metabolism To Promote Viral Replication. J. Virol. 2022, 96, e0191921. [Google Scholar] [CrossRef]
- He, Q.Q.; Huang, Y.; Nie, L.; Ren, S.; Xu, G.; Deng, F.; Cheng, Z.; Zuo, Q.; Zhang, L.; Cai, H.; et al. MAVS integrates glucose metabolism and RIG-I-like receptor signaling. Nat. Commun. 2023, 14, 5343. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Y.; Sun, H.; Yang, X.; Hao, S.; Liu, B.; Zhou, H.; Wang, Y.; Xu, Z.X. Human papillomavirus-16 E6 activates the pentose phosphate pathway to promote cervical cancer cell proliferation by inhibiting G6PD lactylation. Redox Biol. 2024, 71, 103108. [Google Scholar] [CrossRef]
| Primer | Sequence (5′ to 3′) | Size |
|---|---|---|
| FCV-F | ATACCCGCCAATCAACATGT | 84 bp |
| FCV-R | AGTCAATGTCAGGTGTCGGC | |
| Beta-actin-F | AAAGTCCAGGGCCACATAGC | 400 bp |
| Beta-actin-R | AGGACTGCTATGTGGGGGAT |
| Stage 1 | Pre-denaturation | Rep:1 | 95 °C | 5 min |
| Stage 2 | Cycling reaction | Rep:40 | 95 °C | 10 s |
| 60 °C | 30 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Zhu, H.; Xue, X.; Zhao, C.; Yu, X.; Jiang, L.; Cong, J.; Liu, Y.; He, Y.; Zhang, J.; et al. Feline Calicivirus Infection Manipulates Central Carbon Metabolism. Vet. Sci. 2025, 12, 138. https://doi.org/10.3390/vetsci12020138
Zhao G, Zhu H, Xue X, Zhao C, Yu X, Jiang L, Cong J, Liu Y, He Y, Zhang J, et al. Feline Calicivirus Infection Manipulates Central Carbon Metabolism. Veterinary Sciences. 2025; 12(2):138. https://doi.org/10.3390/vetsci12020138
Chicago/Turabian StyleZhao, Guangrong, Hongwei Zhu, Xiu Xue, Chenpei Zhao, Xin Yu, Linlin Jiang, Jingxian Cong, Yang Liu, Yuanlong He, Jianlong Zhang, and et al. 2025. "Feline Calicivirus Infection Manipulates Central Carbon Metabolism" Veterinary Sciences 12, no. 2: 138. https://doi.org/10.3390/vetsci12020138
APA StyleZhao, G., Zhu, H., Xue, X., Zhao, C., Yu, X., Jiang, L., Cong, J., Liu, Y., He, Y., Zhang, J., & Zhang, X. (2025). Feline Calicivirus Infection Manipulates Central Carbon Metabolism. Veterinary Sciences, 12(2), 138. https://doi.org/10.3390/vetsci12020138







