Danggui Buxue Decoction Alleviates Inflammation and Oxidative Stress in Mice with Escherichia coli-Induced Mastitis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Strains
2.3. Animals
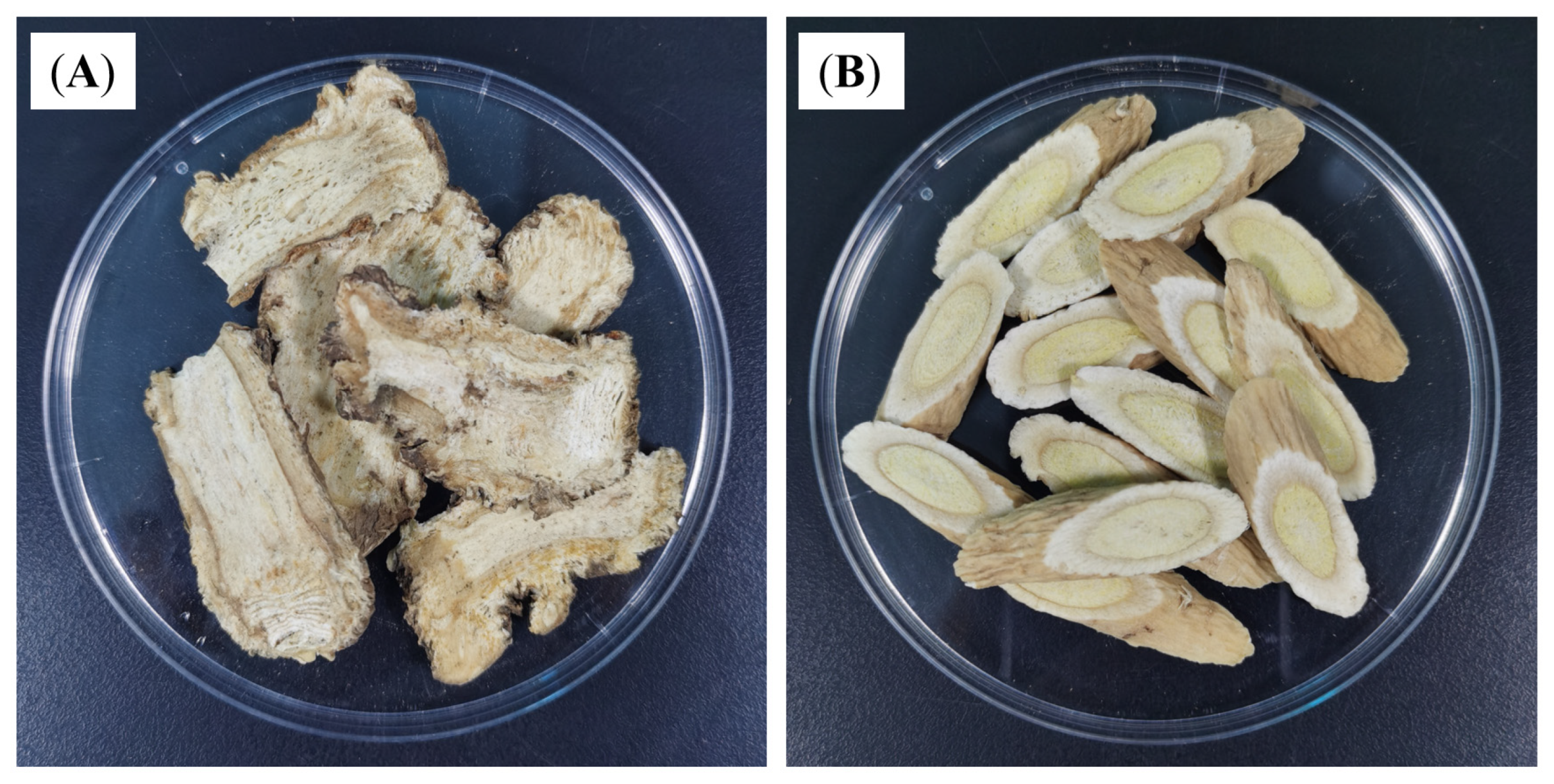
2.4. Preparation of DBD
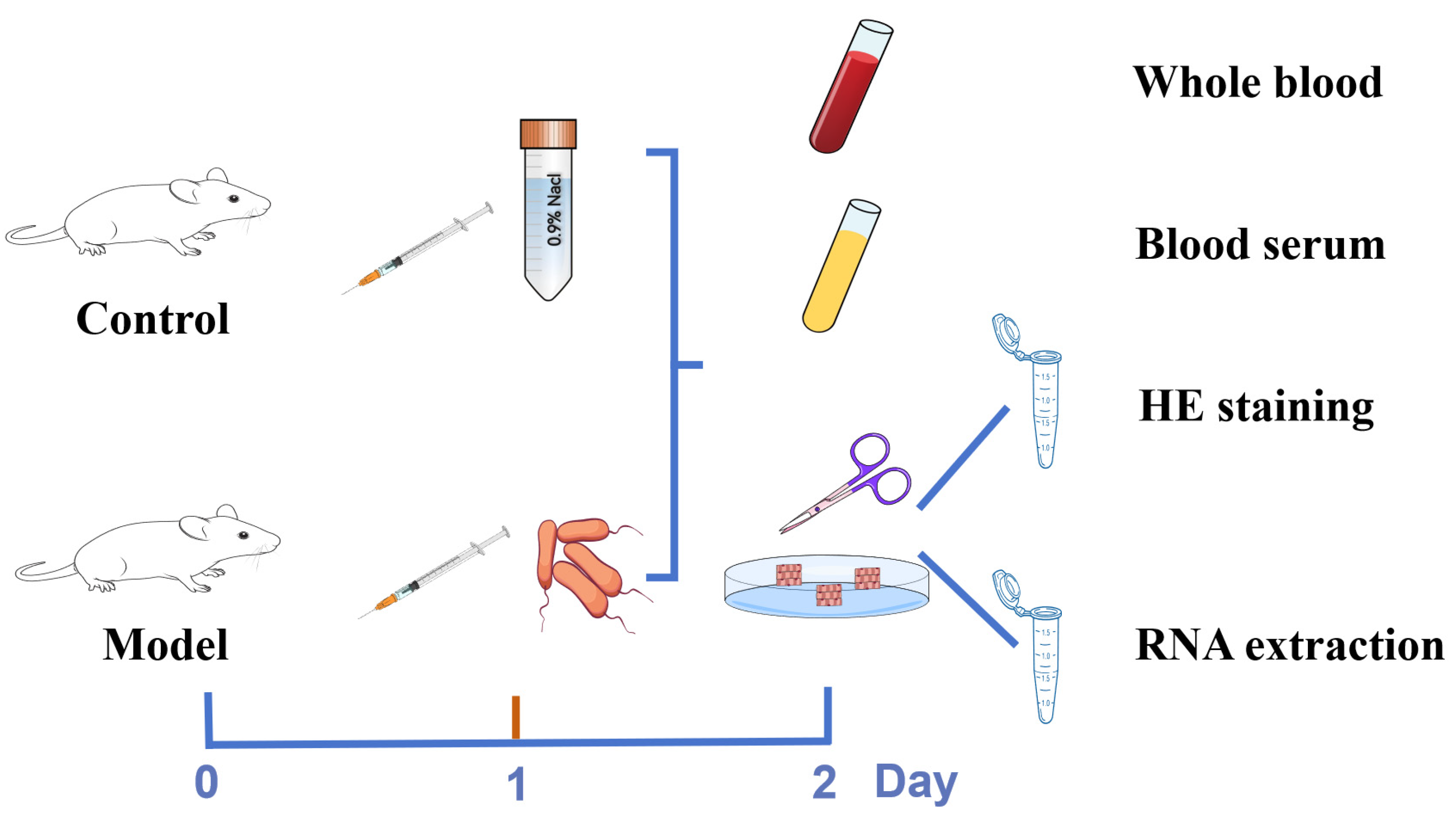
2.5. Establishment of the Mastitis Model
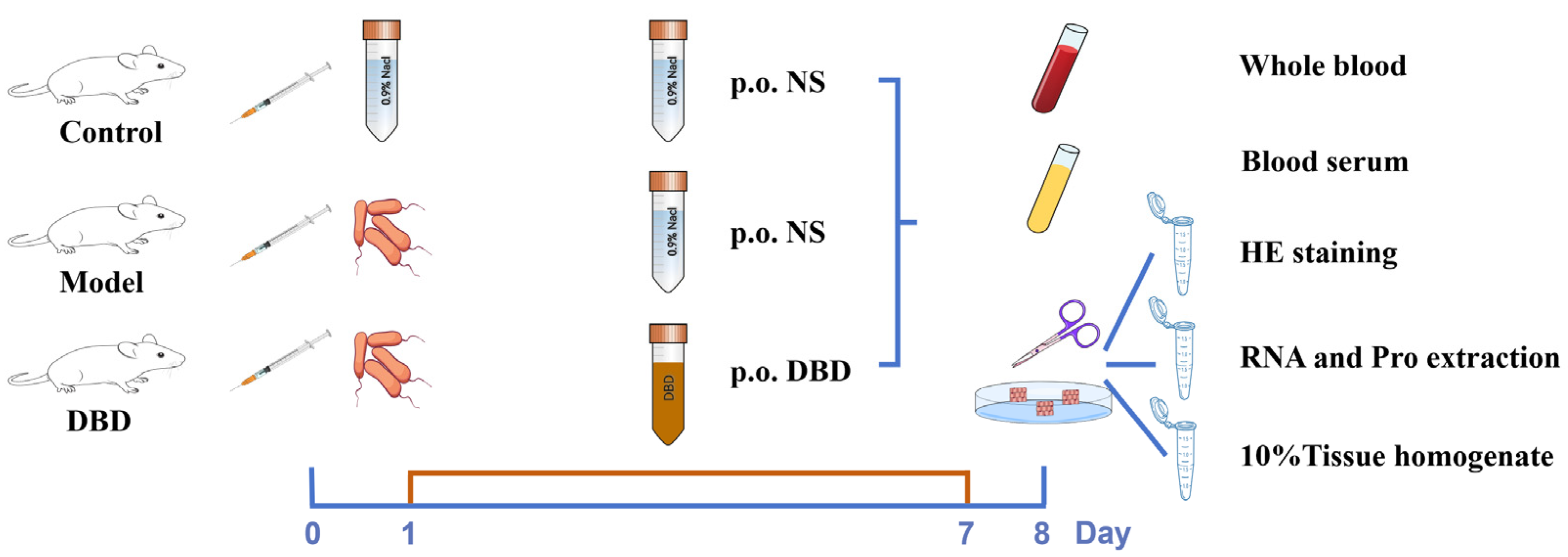
2.6. Grouping and Sample Collection for DBD Treatment of Mastitis in Mice
2.7. Body Weight and Organ Indices
2.8. Blood Routine
2.9. H&E Staining
2.10. ELISA
2.11. qRT-PCR and Western Blot (WB) Assays
2.12. Oxidation and Antioxidant Indices
2.13. Statistical Analysis
3. Results
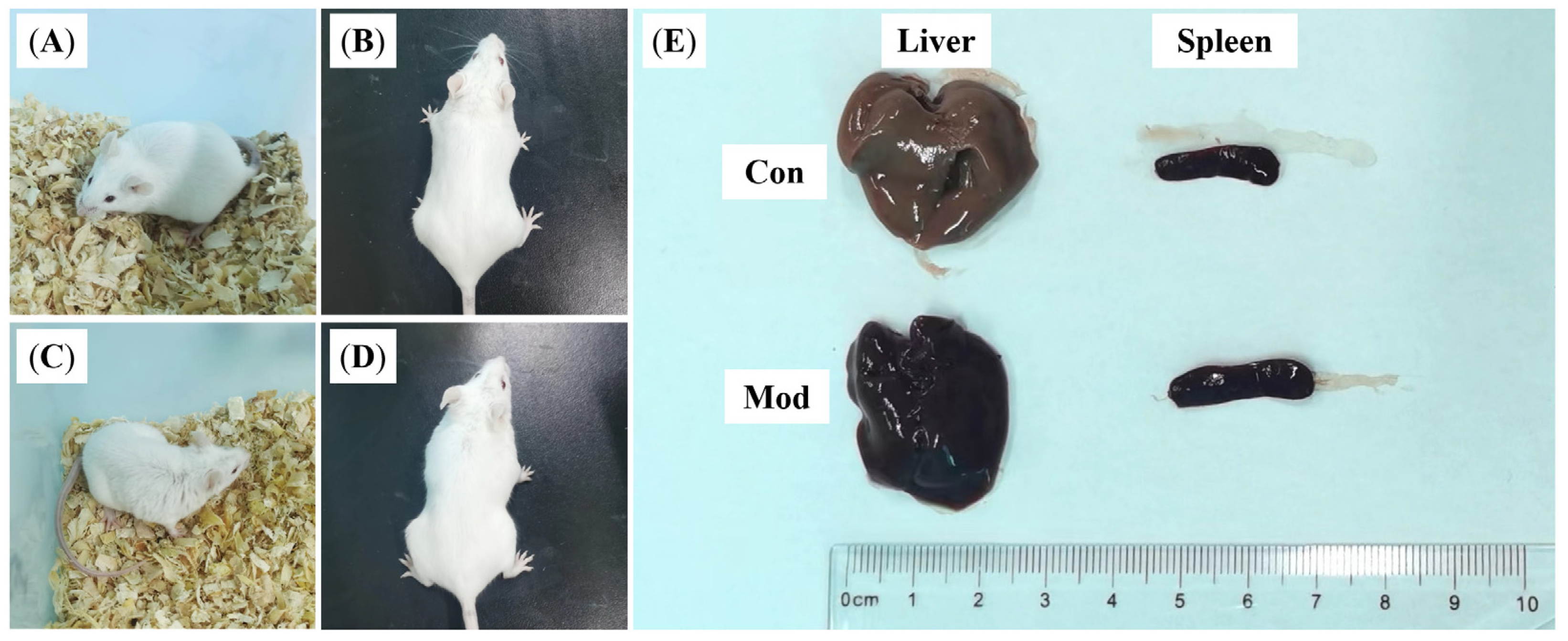
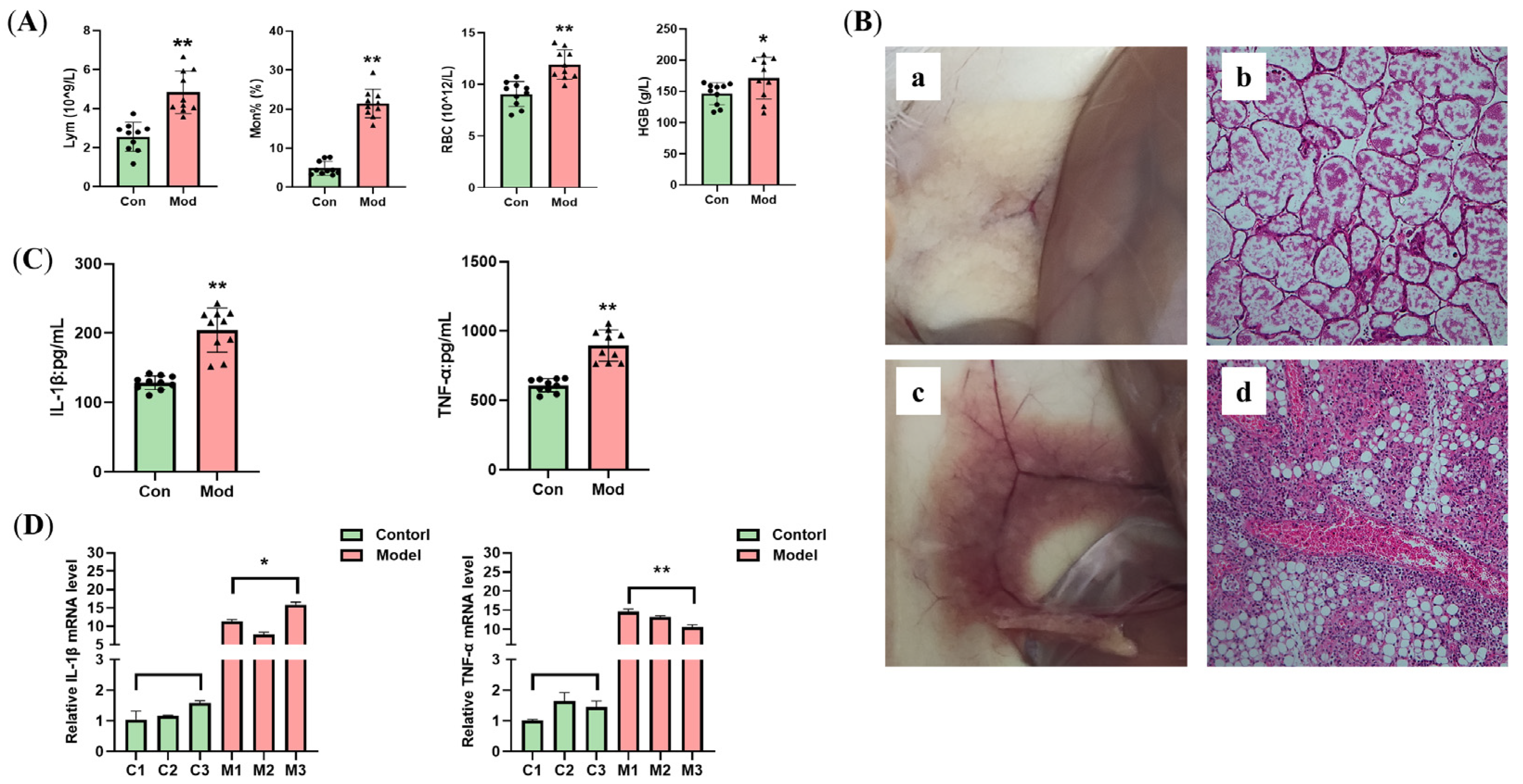
3.1. Establishing the Mastitis Model
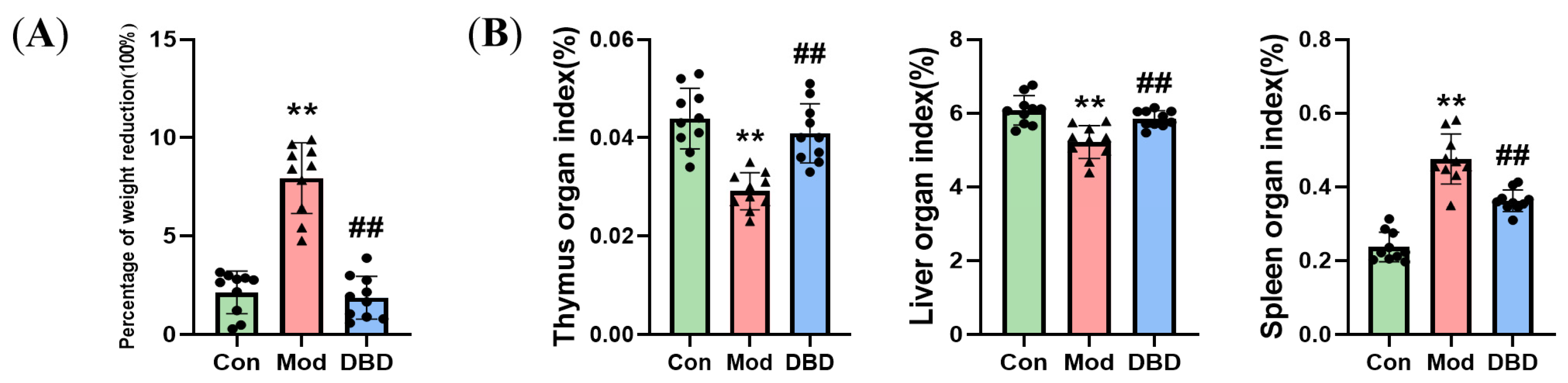
3.2. Effects of DBD on the Body Weight and Organ Indices of Mastitis Model Mice
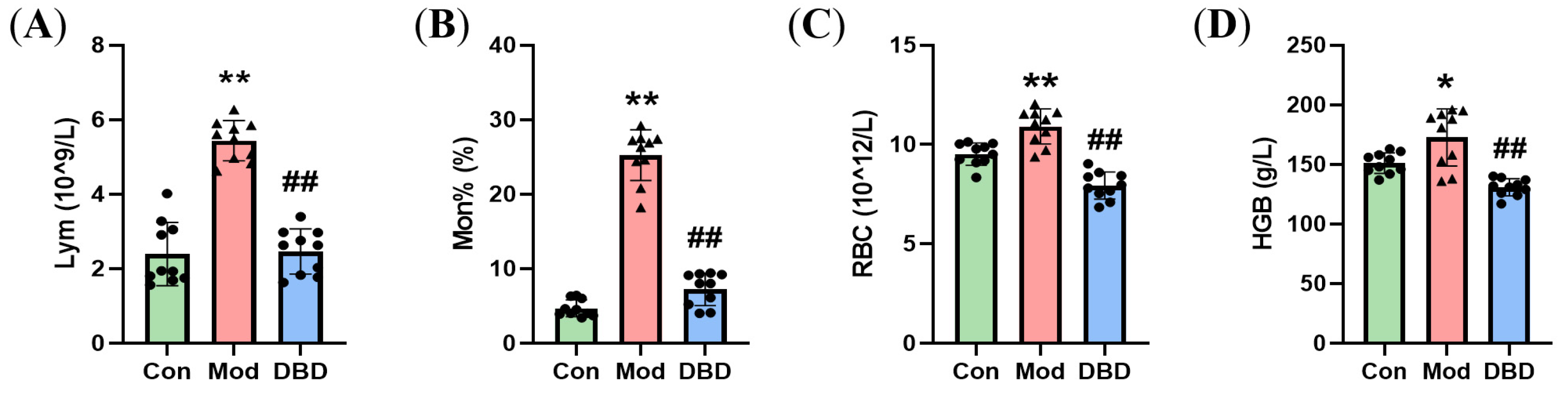
3.3. Effects of DBD on Hematological Parameters in Mastitis Model Mice
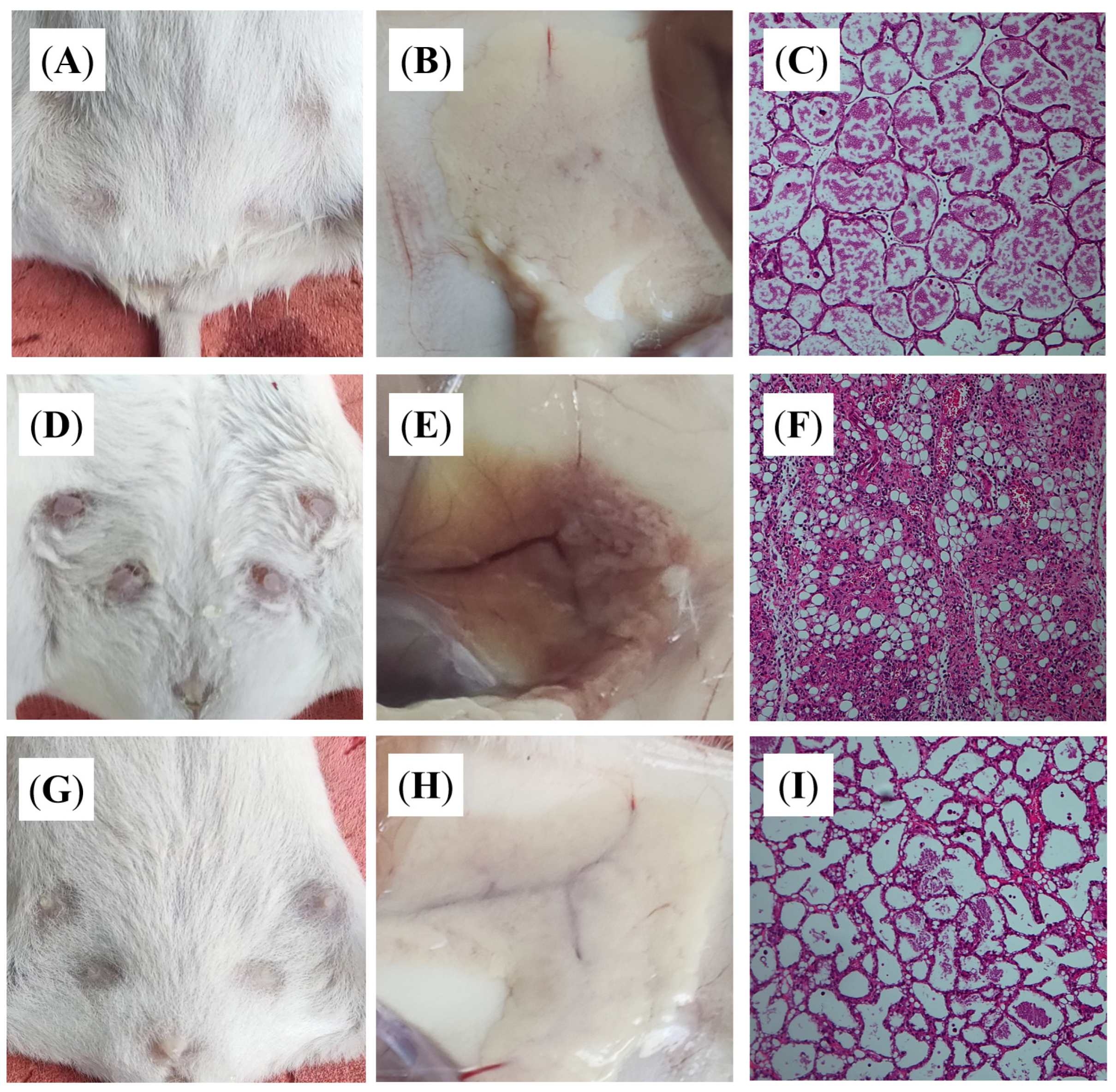
3.4. DBD Alleviates Histopathological Changes in Mastitis Model Mice
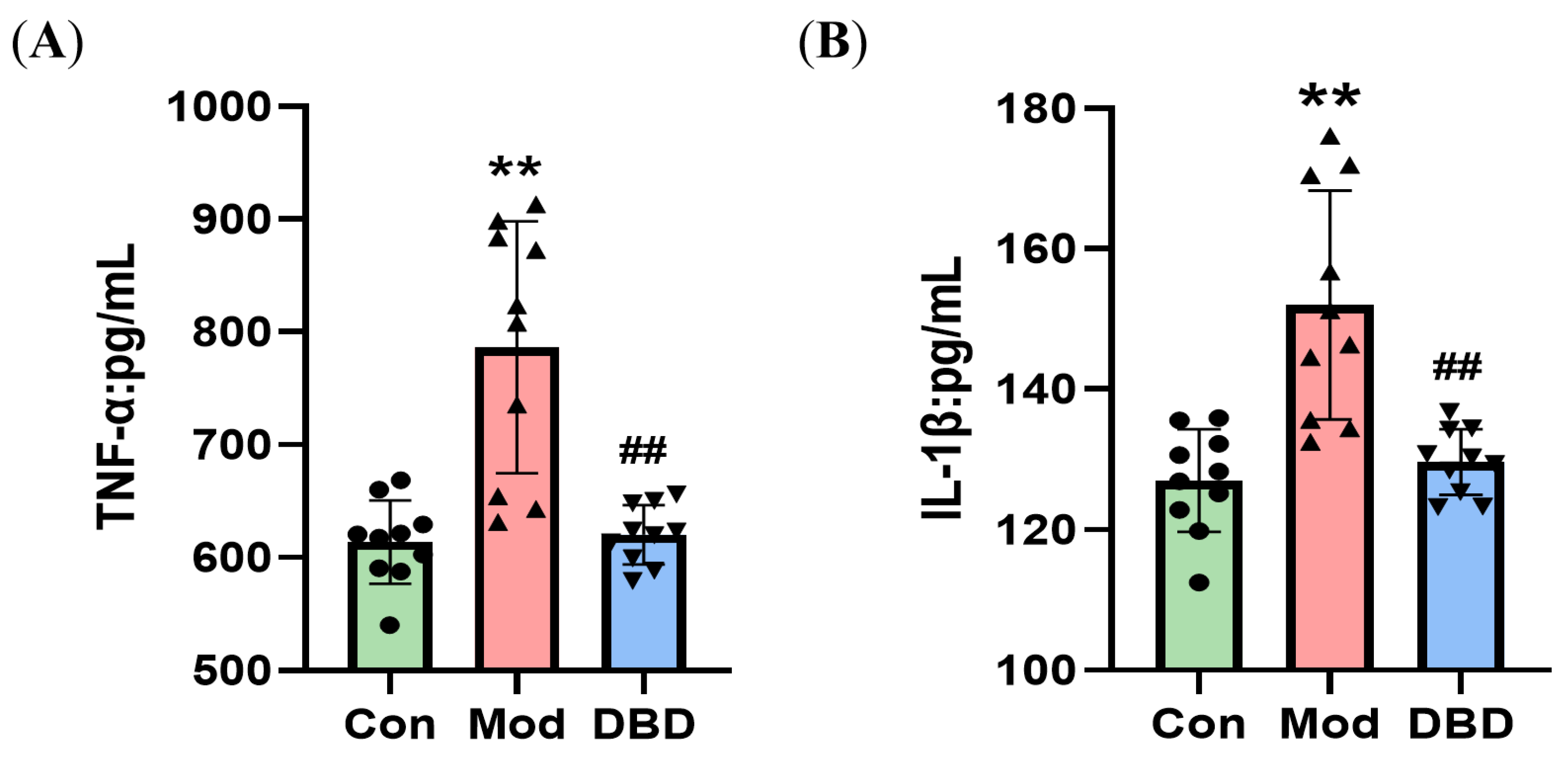
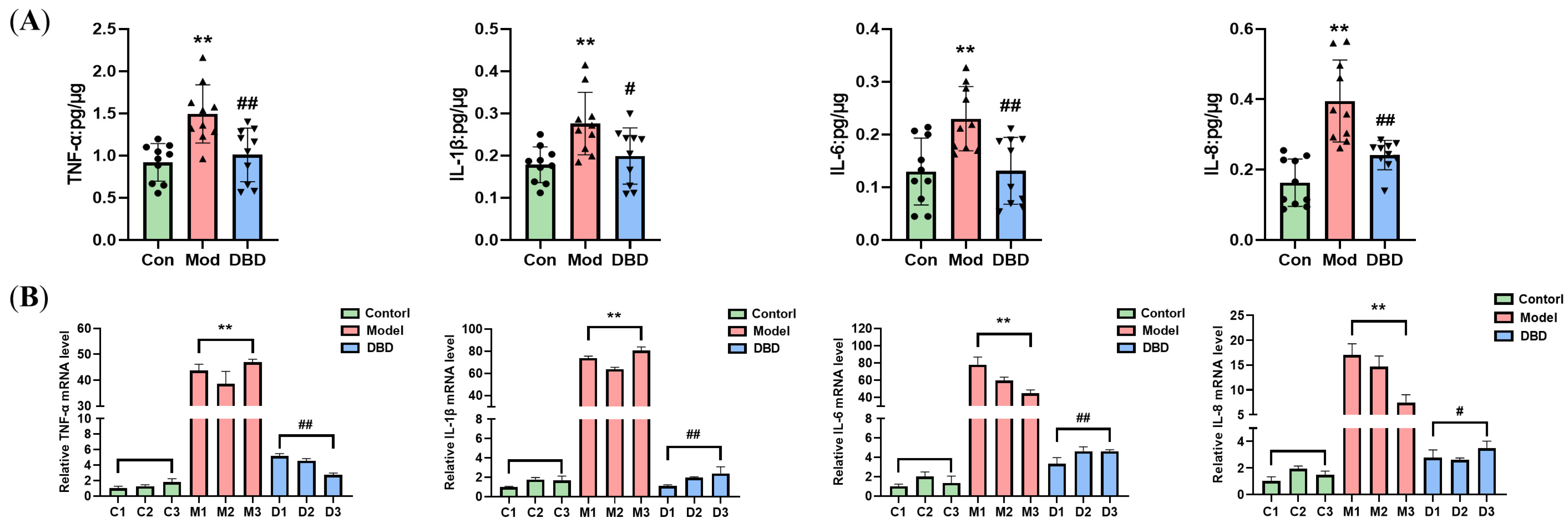
3.5. DBD Attenuates the Inflammatory Response in Mastitis Model Mice
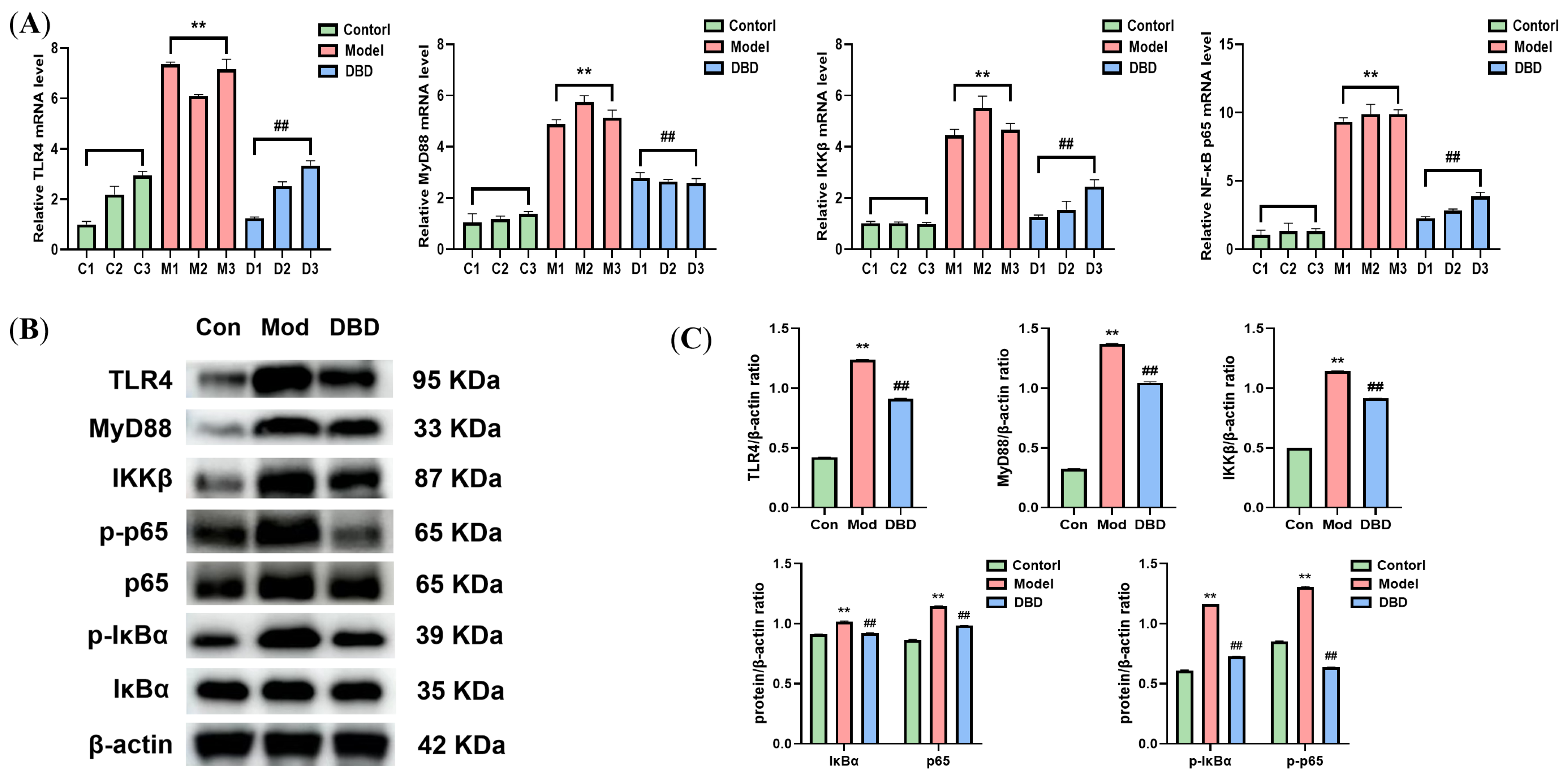
3.6. Effects of DBD on the TLR4/NF-κB Signaling Pathway
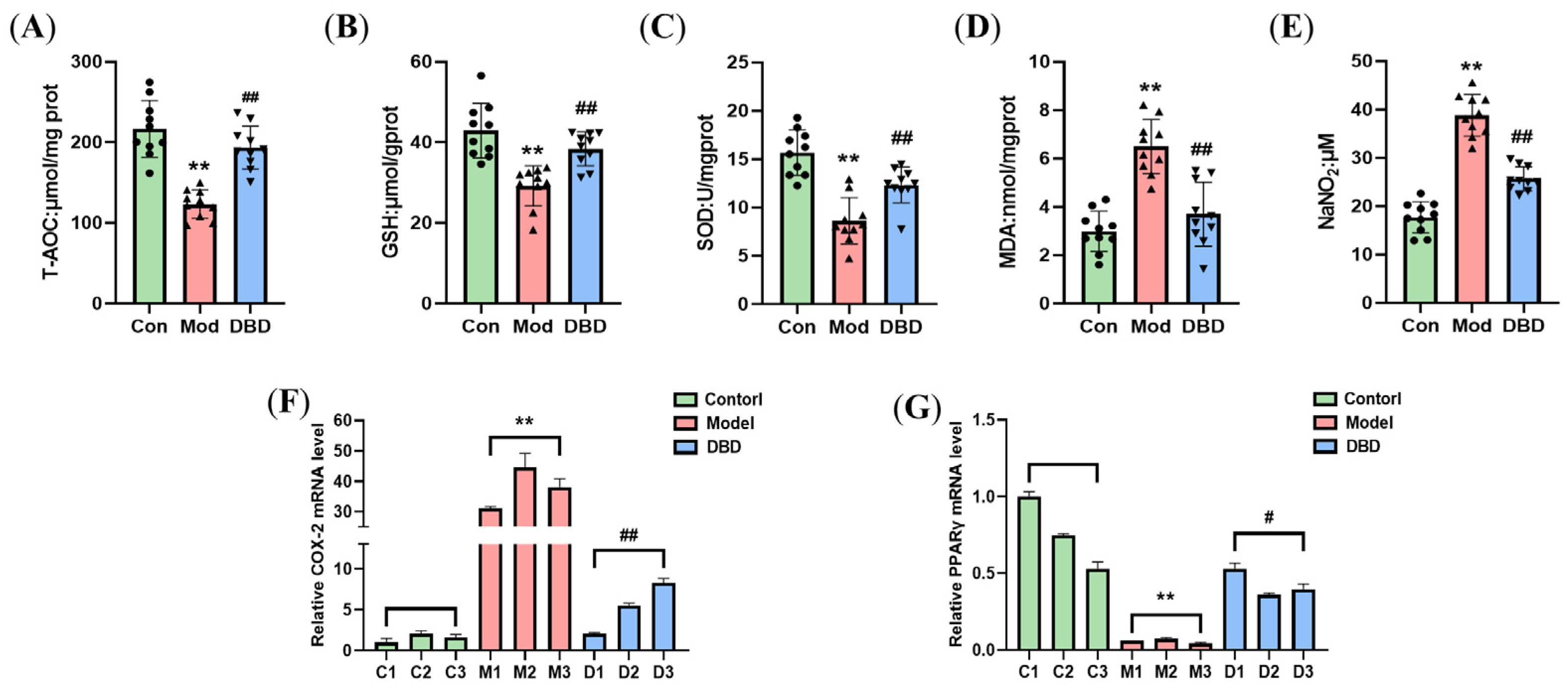
3.7. DBD Alleviates OS in Mastitis Model Mice
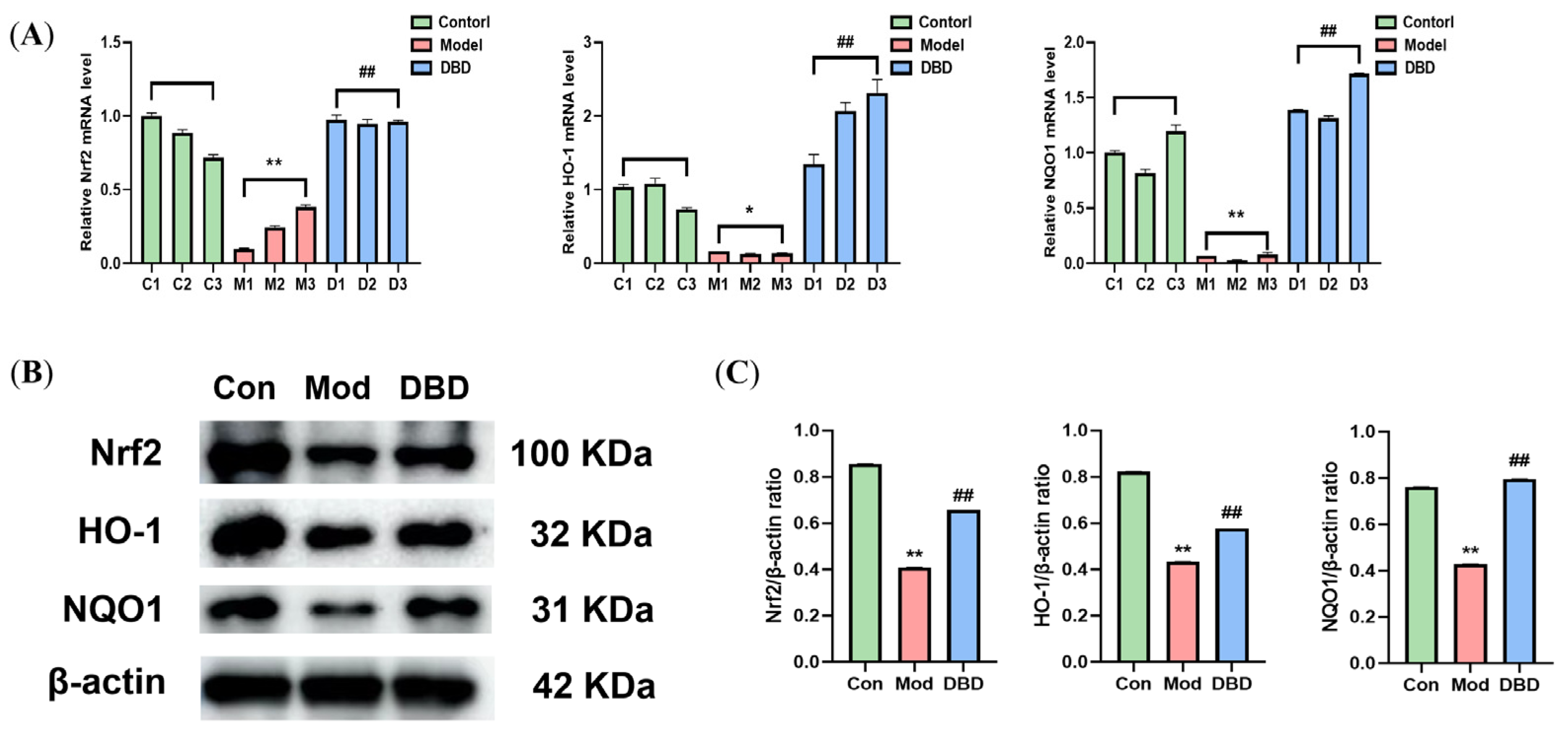
3.8. Effects of DBD on the Nrf2/HO-1 Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | Bovine mastitis |
| DBD | Danggui buxue decoction |
| E. coli | Escherichia coli |
| OS | Oxidative stress |
References
- Timonen, A.; Sammul, M.; Taponen, S.; Kaart, T.; Mõtus, K.; Kalmus, P. Antimicrobial Selection for the Treatment of Clinical Mastitis and the Efficacy of Penicillin Treatment Protocols in Large Estonian Dairy Herds. Antibiotics 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in Therapeutic and Managemental Approaches of Bovine Mastitis: A Comprehensive Review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Krebs, I.; Zhang, Y.; Wente, N.; Leimbach, S.; Krömker, V. Bacteremia in Severe Mastitis of Dairy Cows. Microorganisms 2023, 11, 1639. [Google Scholar] [CrossRef]
- Wei, G.; Qiu, M.; Pu, Z.; Long, N.; Yang, M.; Ren, K.; Ning, R.; Zhang, S.; Lin, L.; Guo, L.; et al. Geraniol-a Potential Alternative to Antibiotics for Bovine Mastitis Treatment Without Disturbing the Host Microbial Community or causing Drug Residues and Resistance. Front. Cell. Infect. Microbiol. 2023, 13, 1126409. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Zhang, H.; Hao, Z.; Zhou, G.; Wen, H.; Su, Q.; Tong, C.; Yang, X.; Wang, X. Forsythiaside A Attenuates Mastitis via PINK1/Parkin-Mediated Mitophagy. Phytomedicine 2024, 125, 155358. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Liu, W.; Zhang, N.; Gao, Q.; Shangguan, J.; Li, N.; Zhao, Y.; Jia, Y. Danggui Buxue Decoction Alleviates Cyclophosphamide-Induced Myelosuppression by Regulating β-Hydroxybutyric Acid Metabolism and Suppressing Oxidative Stress. Pharm. Biol. 2023, 61, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Si, J.; Guo, Y.; Liu, B.; Chen, X.; Qi, K.; Yang, S.; Ji, E. Danggui-Buxue Decoction Alleviated Vascular Senescence in Mice Exposed to Chronic Intermittent Hypoxia through Activating the Nrf2/HO-1 Pathway. Pharm. Biol. 2023, 61, 1041–1053. [Google Scholar] [CrossRef]
- Gong, G.; Ganesan, K.; Liu, Y.; Huang, Y.; Luo, Y.; Wang, X.; Zhang, Z.; Zheng, Y. Danggui Buxue Tang Improves Therapeutic Efficacy of Doxorubicin in Triple Negative Breast Cancer via Ferroptosis. J. Ethnopharmacol. 2024, 323, 117655. [Google Scholar] [CrossRef]
- Zhu, D.; Li, S.; Xu, L.; Ren, X.; Wang, S.; Chen, J.; Zhao, E.; Zheng, Z. Investigation of the Molecular Mechanism of Danggui Buxue Tang in Treating Lung Cancer Using Network Pharmacology and Molecular Docking Techniques. Nat. Prod. Res. 2024, 25, 1–4. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, C.; Huai, Y.; Liu, D.; Zhang, M.; Wang, H.; Zhao, X.; Bo, R.; Li, J.; Liu, M. The Inhibition Effect of Caffeic Acid on NOX/ROS-Dependent Macrophages M1-like Polarization Contributes to Relieve the LPS-Induced Mice Mastitis. Cytokine 2024, 174, 156471. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Guo, S.; Guo, Y.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated-Gene Expression of pro-Inflammatory Cytokines (TNF-α, IL-1β and IL-6) via TLRs Following NF-κB and MAPKs in Bovine Mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef]
- Nakov, D.; Kuzelov, A.; Hristov, S.; Nakova, V.V.; Stanković, B.; Miočinović, J. The Impact of Mastitis Pathogens on Antioxidant Enzyme Activity in Cows’ Milk. Contemp. Agric. 2023, 72, 199–206. [Google Scholar] [CrossRef]
- Li, C.; Zhu, F.; Wang, S.; Wang, J.; Wu, B. Danggui Buxue Decoction Ameliorates Inflammatory Bowel Disease by Improving Inflammation and Rebuilding Intestinal Mucosal Barrier. Evid. Based Complement. Alternat. Med. 2021, 2021, 8853141. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Fang, F.; Fang, C.; Wang, S.; Lu, C.; Liu, N. Danggui Buxue Tang Ameliorates Bleomycin-Induced Pulmonary Fibrosis by Suppressing the TLR4/NLRP3 Signaling Pathway in Rats. Evid. Based Complement. Alternat. Med. 2021, 2021, 8030143. [Google Scholar] [CrossRef]
- Jiang, X.; He, Y.; Zhao, Y.; Pan, Z.; Wang, Y. Danggui Buxue Decoction Exerts Its Therapeutic Effect on Rheumatoid Arthritis through the Inhibition of Wnt/β-Catenin Signaling Pathway. J. Orthop. Surg. 2023, 18, 944. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.; Guo, C.; Chen, C.; Yin, Z.; Zhang, X.; Chen, X. Danggui Buxue Tang Attenuates Tubulointerstitial Fibrosis via Suppressing NLRP3 Inflammasome in a Rat Model of Unilateral Ureteral Obstruction. BioMed Res. Int. 2016, 2016, 9368483. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, C.; Liu, X.; Zhang, L.; Yong, K.; Lv, Q.; Zhang, Y.; Chen, L.; Zhong, P.; Liu, Y. The Pharmacokinetics and Pharmacodynamics of Cefquinome against Streptococcus agalactiae in a Murine Mastitis Model. PLoS ONE 2023, 18, e0278306. [Google Scholar] [CrossRef]
- Ye, T.-S.; Zhang, Y.-W.; Zhang, X.-M. Protective Effects of Danggui Buxue Tang on Renal Function, Renal Glomerular Mesangium and Heparanase Expression in Rats with Streptozotocin-Induced Diabetes Mellitus. Exp. Ther. Med. 2016, 11, 2477–2483. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Cheng, C.; Li, Y.; Zhang, X.; Yao, D.; Zhang, Y. Dangguibuxue Decoction Protects Against Lipopolysaccharides-Induced Mastitis in Bovine Mammary Epithelial Cells in Vitro. J. Anim. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Yu, Y.; Fang, J.-T.; Sun, J.; Zheng, M.; Zhang, Q.; He, J.-S.; Liao, X.-P.; Liu, Y.-H. Efficacy of Cefquinome against Escherichia coli Environmental Mastitis Assessed by Pharmacokinetic and Pharmacodynamic Integration in Lactating Mouse Model. Front. Microbiol. 2017, 8, 1445. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zeng, Z.; Chen, J.; Wang, J.; Chen, Q.; Bo, H. Effect of Danggui Buxue Tang on Erythropoiesis in Acute Chemotherapy Injured Mice. Int. J. Pharmacol. 2017, 13, 583–592. [Google Scholar] [CrossRef]
- Ferchiou, A.; Ndiaye, Y.; Mandour, M.A.; Herman, N.; Lhermie, G.; Raboisson, D. The Marginal Abatement Cost of Antimicrobials for Dairy Cow Mastitis: A Bioeconomic Optimization Perspective. Vet. Sci. 2023, 10, 92. [Google Scholar] [CrossRef]
- Tomanić, D.; Samardžija, M.; Kovačević, Z. Alternatives to Antimicrobial Treatment in Bovine Mastitis Therapy: A Review. Antibiotics 2023, 12, 683. [Google Scholar] [CrossRef]
- Dou, Y.; Shu, Y.; Wang, Y.; Jia, D.; Han, Z.; Shi, B.; Chen, J.; Yang, J.; Qin, Z.; Huang, S. Combination Treatment of Danggui Buxue Decoction and Endothelial Progenitor Cells Can Enhance Angiogenesis in Rats with Focal Cerebral Ischemia and Hyperlipidemia. J. Ethnopharmacol. 2023, 314, 116563. [Google Scholar] [CrossRef]
- Hoque, M.N.; Faisal, G.M.; Jerin, S.; Moyna, Z.; Islam, M.A.; Talukder, A.K.; Alam, M.S.; Das, Z.C.; Isalm, T.; Hossain, M.A.; et al. Unveiling Distinct Genetic Features in Multidrug-Resistant Escherichia coli Isolated from Mammary Tissue and Gut of Mastitis Induced Mice. Heliyon 2024, 10, e26723. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Wang, B.; Li, J.L.; Yang, R.; Li, S.C.; Zhang, M.; Huang, W.; Cao, L. Effects of Dangguibuxue Tang, a Chinese Herbal Medicine, on Growth Performance and Immune Responses in Broiler Chicks. Biol. Res. 2013, 46, 183–188. [Google Scholar] [CrossRef]
- Liu, S.-P. Protective Effect of Angelica Sinensis Polysaccharide on Experimental Immunological Colon Injury in Rats. World J. Gastroenterol. 2003, 9, 2786. [Google Scholar] [CrossRef]
- Kerwin, A.L.; McCarthy, M.M.; Burhans, W.S.; Nydam, D.V.; Wall, S.K.; Schoenberg, K.M.; Perfield, K.L.; Overton, T.R. Associations of a Liver Health Index with Health, Milk Yield, and Reproductive Performance in Dairy Herds in the Northeastern United States. JDS Commun. 2022, 3, 446–450. [Google Scholar] [CrossRef]
- Reinaldo, L.G.C.; Araújo Júnior, R.J.C.; Diniz, T.M.; Moura, R.D.D.; Meneses Filho, A.J.; Furtado, C.V.V.D.M.; Dos Santos, W.L.C.; Costa, D.L.; Eulálio, K.D.; Ferreira, G.R.; et al. The Spleen Is the Graveyard of CD4+ Cells in Patients with Immunological Failure of Visceral Leishmaniasis and AIDS. Parasit. Vectors 2024, 17, 132. [Google Scholar] [CrossRef]
- Onur, H.; Onur, A.R. Diagnostic Performance of Routine Blood Parameters in Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Adenitis Syndrome. J. Clin. Lab. Anal. 2023, 37, e24934. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yin, C.; Liu, Q.; Wang, F.; Yuan, C. Clinical Significance of Routine Blood Test-Associated Inflammatory Index in Breast Cancer Patients. Med. Sci. Monit. 2017, 23, 5090–5095. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, X.; Xu, J.; Wang, S.; Li, S.; Zhu, Y.; Wang, J.Z. Morio Hemolymph Relieves E. coli-Induced Mastitis by Inhibiting Inflammatory Response and Repairing the Blood–Milk Barrier. Int. J. Mol. Sci. 2022, 23, 13279. [Google Scholar] [CrossRef]
- Li, K.; Ran, X.; Zeng, Y.; Li, S.; Hu, G.; Wang, X.; Li, Y.; Yang, Z.; Liu, J.; Fu, S. Maslinic Acid Alleviates LPS-Induced Mice Mastitis by Inhibiting Inflammatory Response, Maintaining the Integrity of the Blood-Milk Barrier and Regulating Intestinal Flora. Int. Immunopharmacol. 2023, 122, 110551. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, L.; Yang, B. Saikosaponin A Alleviates Staphylococcus aureus-induced Mastitis in Mice by Inhibiting Ferroptosis via SIRT1/Nrf2 Pathway. J. Cell. Mol. Med. 2023, 27, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Du, M.; Liu, N.; Shan, Q.; Zou, Y.; Wang, J.; Zhu, Y. A Novel Glycine-Rich Peptide from Zophobas atratus, Coleoptericin B, Targets Bacterial Membrane and Protects against Klebsiella pneumoniae-Induced Mastitis in Mice. J. Antimicrob. Chemother. 2024, 79, 417–428. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, W.; Wen, H.; Su, Q.; Hao, Z.; Liu, J.; Gao, Y.; Zhang, H.; Ge, B.; Tong, C.; et al. Esculetin Improves Murine Mastitis Induced by Streptococcus Isolated from Bovine Mammary Glands by Inhibiting NF-κB and MAPK Signaling Pathways. Microb. Pathog. 2023, 185, 106393. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xue, G.; Dai, A.; Wu, H. Protective Effects of Lentinan Against Lipopolysaccharide-Induced Mastitis in Mice. Front. Pharmacol. 2021, 12, 755768. [Google Scholar] [CrossRef]
- Liu, K.; Ren, X.; You, Q.; Gu, M.-M.; Wang, F.; Wang, S.; Ma, C.-H.; Li, W.-N.; Ye, Q. Ameliorative Effect of Dangguibuxue Decoction against Cyclophosphamide-Induced Heart Injury in Mice. BioMed Res. Int. 2018, 2018, 8503109. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Li, W.; Ma, H.; Gong, Q.; Kan, X.; Cao, Y.; Wang, J.; Fu, S. Niacin Alleviates Dairy Cow Mastitis by Regulating the GPR109A/AMPK/NRF2 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 3321. [Google Scholar] [CrossRef]
- Zhou, M.; Barkema, H.W.; Gao, J.; Yang, J.; Wang, Y.; Kastelic, J.P.; Khan, S.; Liu, G.; Han, B. MicroRNA miR-223 Modulates NLRP3 and Keap1, Mitigating Lipopolysaccharide-Induced Inflammation and Oxidative Stress in Bovine Mammary Epithelial Cells and Murine Mammary Glands. Vet. Res. 2023, 54, 78. [Google Scholar] [CrossRef] [PubMed]
- Píšťková, K.; Illek, J.; Kadek, R. Determination of Antioxidant Indices in Dairy Cows during the Periparturient Period. Acta Vet. Brno 2019, 88, 3–9. [Google Scholar] [CrossRef]
- Wang, X. Nervonic Acid Suppresses MPTP-Induced Parkinson’s Disease in an Adult Zebrafish Model by Regulating the MAPK/NF-κB Signaling Pathway, Inflammation, Apoptosis, and Oxidative Stress. Food Biosci. 2024, 59, 103777. [Google Scholar] [CrossRef]
- Wang, H.; Guo, J.; Yang, Y.; Wang, N.; Yang, X.; Deng, L.; Cao, X.; Ran, Z.; Fang, D.; Xu, K.; et al. CuFeS2 Nanozyme Regulating ROS/GSH Redox Induces Ferroptosis-like Death in Bacteria for Robust Anti-Infection Therapy. Mater. Des. 2024, 239, 112809. [Google Scholar] [CrossRef]
- Pan, X.; Shao, Y.; Wang, F.; Cai, Z.; Liu, S.; Xi, J.; He, R.; Zhao, Y.; Zhuang, R. Protective Effect of Apigenin Magnesium Complex on H2O2-Induced Oxidative Stress and Inflammatory Responses in Rat Hepatic Stellate Cells. Pharm. Biol. 2020, 58, 553–560. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, L.; Cui, C.; Zhao, H.; Zhang, Y.; Tian, Z.; Guan, W.; Chen, F. Maternal Supplementation with Artemisia annua L. Ameliorates Intestinal Inflammation via Inhibiting the TLR4/NF-κB and MAPK Pathways and Improves the Oxidative Stability of Offspring. Food Funct. 2022, 13, 9311–9323. [Google Scholar] [CrossRef]
- Kamila, S.; Dey, K.K.; Islam, S.; Chattopadhyay, A. Arsenic and Chromium Induced Hepatotoxicity in Zebrafish (Danio rerio) at Environmentally Relevant Concentrations: Mixture Effects and Involvement of Nrf2-Keap1-ARE Pathway. Sci. Total Environ. 2024, 921, 171221. [Google Scholar] [CrossRef]
- Li, W.; Yang, S.; Zhao, Y.; Di Nunzio, G.; Ren, L.; Fan, L.; Zhao, R.; Zhao, D.; Wang, J. Ginseng-Derived Nanoparticles Alleviate Alcohol-Induced Liver Injury by Activating the Nrf2/HO-1 Signalling Pathway and Inhibiting the NF-κB Signalling Pathway In Vitro and In Vivo. Phytomedicine 2024, 127, 155428. [Google Scholar] [CrossRef]
- Al-Sa’Doni, H.; Ferro, A. S-Nitrosothiols: A Class of Nitric Oxide-Donor Drugs. Clin. Sci. 2000, 98, 507–520. [Google Scholar]
- Zhang, Y.; Xu, Y.; Chen, B.; Zhao, B.; Gao, X. Selenium Deficiency Promotes Oxidative Stress-Induced Mastitis via Activating the NF-κB and MAPK Pathways in Dairy Cow. Biol. Trace Elem. Res. 2022, 200, 2716–2726. [Google Scholar] [CrossRef]
- Alba, D.F.; Da Rosa, G.; Hanauer, D.; Saldanha, T.F.; Souza, C.F.; Baldissera, M.D.; Da Silva Dos Santos, D.; Piovezan, A.P.; Girardini, L.K.; Schafer Da Silva, A. Subclinical Mastitis in Lacaune Sheep: Causative Agents, Impacts on Milk Production, Milk Quality, Oxidative Profiles and Treatment Efficacy of Ceftiofur. Microb. Pathog. 2019, 137, 103732. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Wang, T.; Cobo, E.R.; Liang, B.; Khan, M.A.; Xu, M.; Qu, W.; Gao, J.; Barkema, H.W.; Kastelic, J.P.; et al. Antioxidative Sirt1 and the Keap1-Nrf2 Signaling Pathway Impair Inflammation and Positively Regulate Autophagy in Murine Mammary Epithelial Cells or Mammary Glands Infected with Streptococcus Uberis. Antioxidants 2024, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Muralidhar, Y.; Alpha Raj, M.; Prasad, T.N.K.; Chaitanya Kumar, T.V.; Adilaxmamma, K.; Srilatha, C.; Sreeenivasa Rao, G.; Sreevani, P.; Aparna, N. Antibacterial, Anti-inflammatory and Antioxidant Effects of Acetyl-11-α-keto-β-boswellic Acid Mediated Silver Nanoparticles in Experimental Murine Mastitis. IET Nanobiotechnol. 2017, 11, 682–689. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; D’Amico, R.; Licata, P.; Crupi, R.; Gugliandolo, E. Effects of Hydroxytyrosol against Lipopolysaccharide-Induced Inflammation and Oxidative Stress in Bovine Mammary Epithelial Cells: A Natural Therapeutic Tool for Bovine Mastitis. Antioxidants 2020, 9, 693. [Google Scholar] [CrossRef]
| Gene | Primers Sequences (5′→3′) | Gene Accession Number |
|---|---|---|
| TNF-α | F:ACGGCATGGATCTCAAAGAC R:GTGGGTGAGGAGCACGTAGT | NC_000083.7 |
| IL-1β | F:GCCACCTTTTGACAGTGATGAG R:ATGTGCTGCTGCGAGATTTG | NC_000068.7 |
| IL-6 | F:CCCCAATTTCCAATGCTCTCC R:CGCACTAGGTTTGCCGAGTA | NC_000071.7 |
| IL-8 | F:GTCCTTCCTACCCCAATTTCCA R:TAACGCACTAGGTTTGCCGA | NC_000075.7 |
| TLR4 | F:GATCTGAGCTTCAACCCCTTG R:TGCCATGCCTTGTCTTCAAT | NC_000070.7 |
| NF-κB p65 | F:CGAGTCTCCATGCAGCTACG R:TTTCGGGTAGGCACAGCAATA | NC_000085.7 |
| MyD88 | F:CCCACTCGCAGTTTGTTG R:TGCCTCCCAGTTCCTTTG | NC_034599.1 |
| IKKβ | F:TCAAGCAATGCCGACAGGAG R:CGATGTCACTCAGCAGGGTAAG | NC_000074.7 |
| COX-2 | F:ATAGACGAAATCAACAACCCCG R:GGATTGGAAGTTCTATTGGCAG | NC_000067.7 |
| PPARγ | F:AAGAAGCGGTGAACCACTGA R:GGAATGCGAGTGGTCTTCCA | NC_000072.7 |
| Nrf2 | F:CAGCATGTTACGTGATGAGG R:GCTCAGAAAAGGCTCCATCC | NC_000068.8 |
| HO-1 | F:ACATTGAGCTGTTTGAGGAG R:TACATGGCATAAATTCCCACTG | NC_000074.6 |
| NQO1 | F:TGAGCCCGGATATTGTAGCTGA R:GCATACGTGTAGGCGAATCCTG | NC_058382.1 |
| GAPDH | F:AGGTCGGTGTGAACGGATTTG R:TGTAGACCATGTAGTTGAGGTCA | NC_034591.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Cheng, C.; Gao, Y.; Li, Y.; Zhang, X.; Yao, D.; Zhang, Y. Danggui Buxue Decoction Alleviates Inflammation and Oxidative Stress in Mice with Escherichia coli-Induced Mastitis. Vet. Sci. 2025, 12, 227. https://doi.org/10.3390/vetsci12030227
Wang J, Cheng C, Gao Y, Li Y, Zhang X, Yao D, Zhang Y. Danggui Buxue Decoction Alleviates Inflammation and Oxidative Stress in Mice with Escherichia coli-Induced Mastitis. Veterinary Sciences. 2025; 12(3):227. https://doi.org/10.3390/vetsci12030227
Chicago/Turabian StyleWang, Jiamian, Chen Cheng, Yujin Gao, Yina Li, Xijun Zhang, Dan Yao, and Yong Zhang. 2025. "Danggui Buxue Decoction Alleviates Inflammation and Oxidative Stress in Mice with Escherichia coli-Induced Mastitis" Veterinary Sciences 12, no. 3: 227. https://doi.org/10.3390/vetsci12030227
APA StyleWang, J., Cheng, C., Gao, Y., Li, Y., Zhang, X., Yao, D., & Zhang, Y. (2025). Danggui Buxue Decoction Alleviates Inflammation and Oxidative Stress in Mice with Escherichia coli-Induced Mastitis. Veterinary Sciences, 12(3), 227. https://doi.org/10.3390/vetsci12030227






