Development of an RT-qPCR Assay for the Detection of an Emerging Duck Egg-Reducing Syndrome
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Viral Strains and Clinical Samples
2.2. Primers and Probe Design
2.3. RNA Extraction and cDNA Synthesis
2.4. Construction of Standard Plasmid
2.5. RT-qPCR Assay
2.6. Specificity Analysis
2.7. Sensitivity and Repeatability of the RT-qPCR Assays
2.8. Detection of the Clinical Sample
3. Results
3.1. Optimization for the RT-qPCR
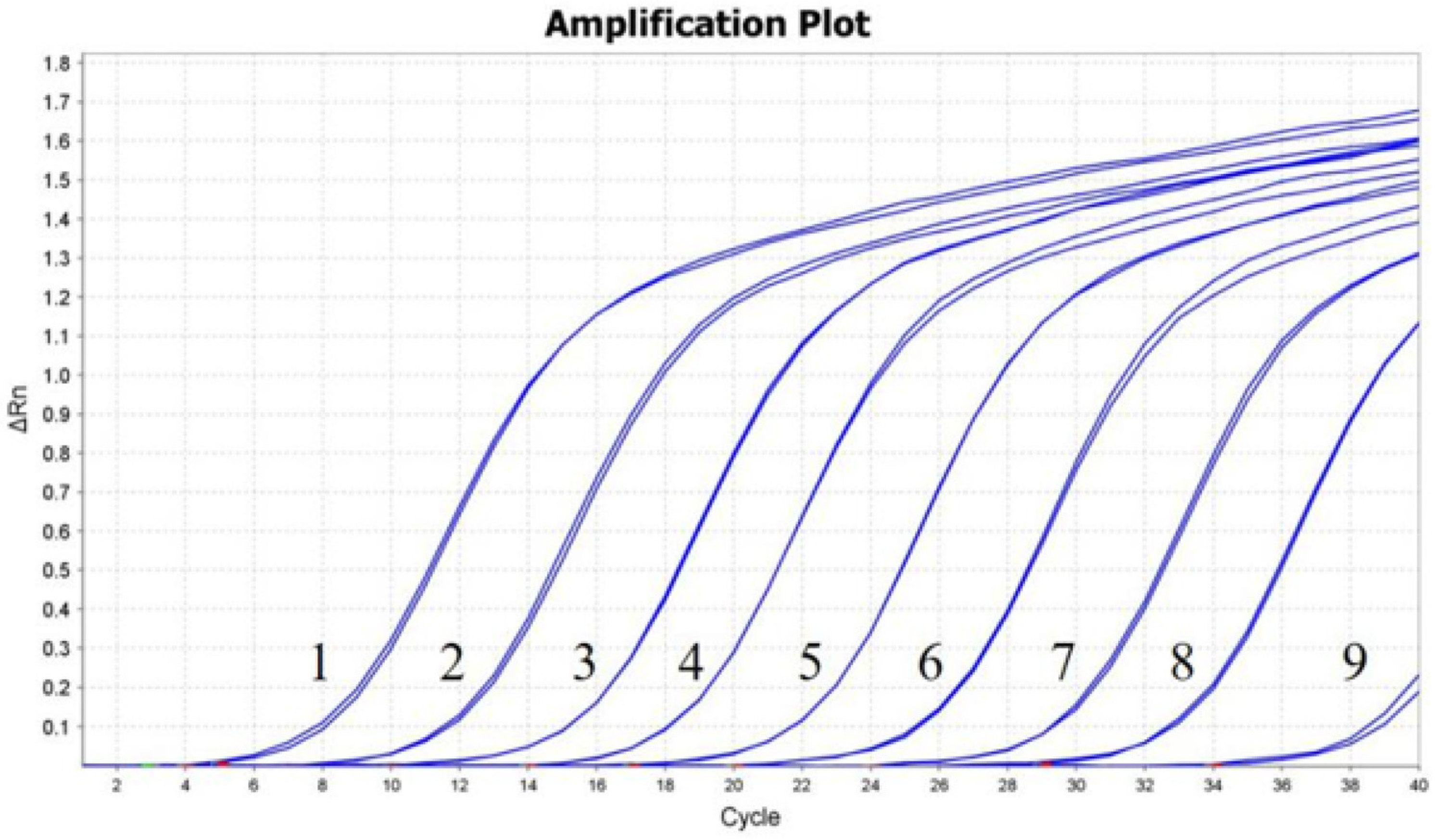
3.2. Standard Curve
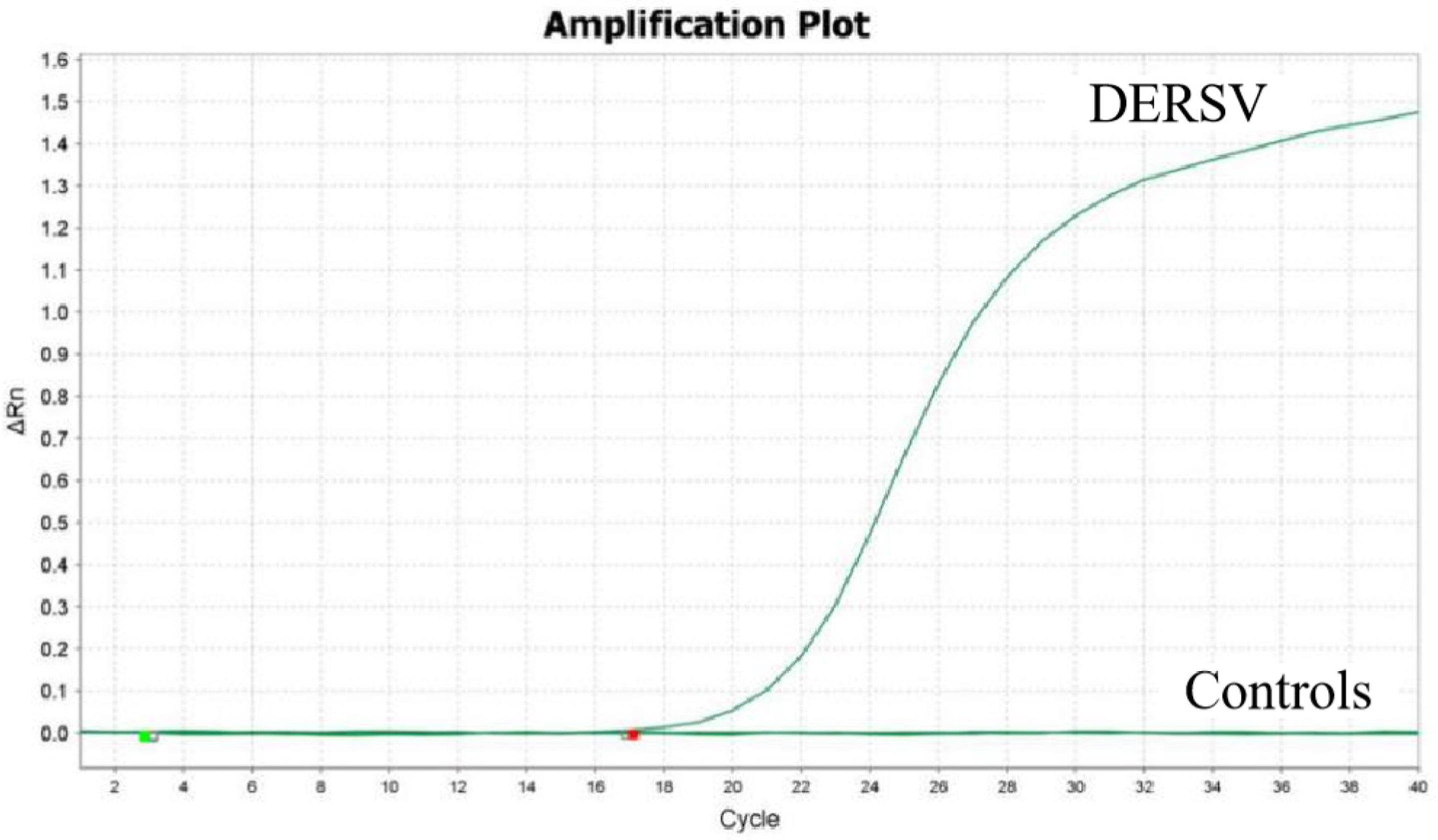
3.3. Specificity of the RT-qPCR Assay
3.4. Sensitivity and Repeatability of the RT-qPCR Assay
3.5. Clinical Sample Test Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, X.; Shuo, D.; Luo, Y.; Pan, X.; Yan, D.; Li, X.; Lin, W.; Huang, D.; Yang, J.; Yuan, C.; et al. An Emerging Duck Egg-Reducing Syndrome Caused by a Novel Picornavirus Containing Seven Putative 2A Peptides. Viruses 2022, 14, 932. [Google Scholar] [CrossRef] [PubMed]
- Lizcano-Perret, B.; Michiels, T. Nucleocytoplasmic Trafficking Perturbation Induced by Picornaviruses. Viruses 2021, 13, 1210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Paget, M.; Wang, C.; Zhu, Z.; Zheng, H. Innate Immune Evasion by Picornaviruses. Eur. J. Immunol. 2020, 50, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Haji Zamani, N.; Hosseini, H.; Ziafati Kafi, Z.; Sadri, N.; Hojabr Rajeoni, A.; Esmaeelzadeh Dizaji, R.; Molouki, A.; FallahMehrabadi, M.H.; Abdoshah, M.; Ghalyanchilangeroudi, A. Whole-Genome Characterization of Avian Picornaviruses from Diarrheic Broiler Chickens Co-Infected with Multiple Picornaviruses in Iran. Virus Genes 2023, 59, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef]
- Zell, R. Picornaviridae—The Ever-Growing Virus Family. Arch. Virol. 2018, 163, 299–317. [Google Scholar] [CrossRef]
- Holmes, A.C.; Semler, B.L. Picornaviruses and RNA Metabolism: Local and Global Effects of Infection. J. Virol. 2019, 93, e02088-17. [Google Scholar] [CrossRef]
- Kim, M.-C.; Kwon, Y.-K.; Joh, S.-J.; Lindberg, A.M.; Kwon, J.-H.; Kim, J.-H.; Kim, S.-J. Molecular Analysis of Duck Hepatitis Virus Type 1 Reveals a Novel Lineage Close to the Genus Parechovirus in the Family Picornaviridae. J. Gen. Virol. 2006, 87, 3307–3316. [Google Scholar] [CrossRef]
- Zhang, G.; Li, S.; Shen, Z.; Wang, F. Progress in Research on the Molecular Biological Detection Techniques of Avian Encephalomyelitis. Res. Vet. Sci. 2023, 159, 232–236. [Google Scholar] [CrossRef]
- Buh Gašparič, M.; Tengs, T.; La Paz, J.L.; Holst-Jensen, A.; Pla, M.; Esteve, T.; Žel, J.; Gruden, K. Comparison of Nine Different Real-Time PCR Chemistries for Qualitative and Quantitative Applications in GMO Detection. Anal. Bioanal. Chem. 2010, 396, 2023–2029. [Google Scholar] [CrossRef]
- Bustin, S. Absolute Quantification of mRNA Using Real-Time Reverse Transcription Polymerase Chain Reaction Assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Xu, W.; Wang, Y.; Wang, H.; Wang, Y.; Li, Y.; Shi, C.; Bergmann, S.M.; Mo, X.; et al. Development of a Rapid and Sensitive Reverse Transcription Real-Time Quantitative PCR Assay for Detection and Quantification of Grass Carp Reovirus II. J. Virol. Methods 2023, 312, 114663. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.; Menzo, S.; Bagnarelli, P.; Manzin, A.; Valenza, A.; Varaldo, P.E. Quantitative PCR and RT-PCR in Virology. Arch. Virol. 1995, 140, 1523–1539. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Wang, J.; Liu, L.; Pang, X.; Yuan, W. Development of a TaqMan-Based Real-Time PCR Assay for the Specific Detection of Porcine Circovirus 3. J. Virol. Methods 2017, 248, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Guo, X.; Zhang, D.; Cui, Y.; Li, W.; Liu, G.; Li, Y.; Jiang, S. TaqMan-Based Real-Time Polymerase Chain Reaction Assay for Specific Detection of Bocavirus-1 in Domestic Cats. Mol. Cell. Probes 2020, 53, 101647. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, X.; Shuo, D.; Luo, Y.; Yan, D.; Pan, X.; Huang, D.; Teng, Q.; Liu, Q.; Yuan, C.; et al. Establishment and Application of RT-PCR Method for Duck Egg-Reducing Syndrome Virus. Chin. J. Anim. Infect. Dis. 2023. [Google Scholar] [CrossRef]
- Yan, L.; Yan, P.; Zhou, J.; Teng, Q.; Li, Z. Establishing a TaqMan-Based Real-Time PCR Assay for the Rapid Detection and Quantification of the Newly Emerged Duck Tembusu Virus. Virol. J. 2011, 8, 464. [Google Scholar] [CrossRef]
- Hoffmann, E.; Stech, J.; Guan, Y.; Webster, R.G.; Perez, D.R. Universal Primer Set for the Full-Length Amplification of All Influenza A Viruses. Arch. Virol. 2001, 146, 2275–2289. [Google Scholar] [CrossRef]
- Wen, X.J.; Cheng, A.C.; Wang, M.S.; Jia, R.Y.; Zhu, D.K.; Chen, S.; Liu, M.F.; Liu, F.; Chen, X.Y. Detection, Differentiation, and VP1 Sequencing of Duck Hepatitis A Virus Type 1 and Type 3 by a 1-Step Duplex Reverse-Transcription PCR Assay. Poult. Sci. 2014, 93, 2184–2192. [Google Scholar] [CrossRef]
- Yang, F.-L.; Jia, W.-X.; Yue, H.; Luo, W.; Chen, X.; Xie, Y.; Zen, W.; Yang, W.-Q. Development of Quantitative Real-Time Polymerase Chain Reaction for Duck Enteritis Virus DNA. Avian Dis. 2005, 49, 397–400. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Liu, X.; Li, X.; Gao, B.; Diao, Y.; Tang, Y. A TaqMan-Based Real-Time PCR Assay for Specific Detection of Novel Duck Reovirus in China. BMC Vet. Res. 2020, 16, 306. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Fockler, C.; Dollinger, G.; Watson, R. Kinetic PCR Analysis: Real-Time Monitoring of DNA Amplification Reactions. Nat. Biotechnol. 1993, 11, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yue, H.; Zhang, B.; Nie, P.; Tang, C. Development of a Real-Time Quantitative PCR for Detecting Duck Hepatitis A Virus Genotype C. J. Clin. Microbiol. 2012, 50, 3318–3323. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Liu, H.; Wang, J.; Li, Y.; Wang, Y. Establishment of Duplex SYBR Green I-Based Real-Time PCR Assay for Simultaneous Detection of Duck Hepatitis A Virus-1 and Duck Astrovirus-3. Avian Dis. 2021, 65, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Z.; Hao, H.; Cheng, S.; Fan, W.; Du, E.; Xiao, S.; Wang, X.; Zhang, S. Development of a SYBR Green Real-Time RT-PCR Assay for the Detection of Avian Encephalomyelitis Virus. J. Virol. Methods 2014, 206, 46–50. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primers | Sequence (5′-3′) | Position a |
|---|---|---|---|
| 3D | Forward | TGGGACTCAATGATGGAGAATG | 7992–8013 |
| Reverse | TGTTTATGGAAGCAGGCTAAGA | 8095–8116 | |
| Probe | FAM-TCAAGTCATGGAGGCTGCAGTTGA-BHQ2 | 8069–8092 |
| Concentrations of Standard Plasmid | Intra-Assay Variability | Inter-Assay Variability | ||
|---|---|---|---|---|
| ± SD | CV(%) | ± SD | CV(%) | |
| 1 × 107 copies/μL | 18.795 ± 0.051 | 0.27% | 18.823 ± 0.060 | 0.32% |
| 1 × 106 copies/μL | 22.52 ± 0.085 | 0.38% | 22.22 ± 0.411 | 1.85% |
| 1 × 105 copies/μL | 26.15 ± 0.114 | 0.44% | 26.06 ± 0.229 | 0.88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Su, X.; Shuo, D.; Yan, D.; Pan, X.; Xu, B.; Yan, M.; Ren, S.; Liu, Q.; Yuan, C.; et al. Development of an RT-qPCR Assay for the Detection of an Emerging Duck Egg-Reducing Syndrome. Vet. Sci. 2025, 12, 241. https://doi.org/10.3390/vetsci12030241
Zhang Z, Su X, Shuo D, Yan D, Pan X, Xu B, Yan M, Ren S, Liu Q, Yuan C, et al. Development of an RT-qPCR Assay for the Detection of an Emerging Duck Egg-Reducing Syndrome. Veterinary Sciences. 2025; 12(3):241. https://doi.org/10.3390/vetsci12030241
Chicago/Turabian StyleZhang, Zhifei, Xin Su, Dun Shuo, Dawei Yan, Xue Pan, Bangfeng Xu, Minghao Yan, Shuxuan Ren, Qinfang Liu, Chunxiu Yuan, and et al. 2025. "Development of an RT-qPCR Assay for the Detection of an Emerging Duck Egg-Reducing Syndrome" Veterinary Sciences 12, no. 3: 241. https://doi.org/10.3390/vetsci12030241
APA StyleZhang, Z., Su, X., Shuo, D., Yan, D., Pan, X., Xu, B., Yan, M., Ren, S., Liu, Q., Yuan, C., Teng, Q., & Li, Z. (2025). Development of an RT-qPCR Assay for the Detection of an Emerging Duck Egg-Reducing Syndrome. Veterinary Sciences, 12(3), 241. https://doi.org/10.3390/vetsci12030241







