Development of Recombinant Follicle-Stimulating Hormone for the Superovulation of Cattle: A Review
Simple Summary
Abstract
1. Introduction
2. Molecular Characteristics of Natural FSH
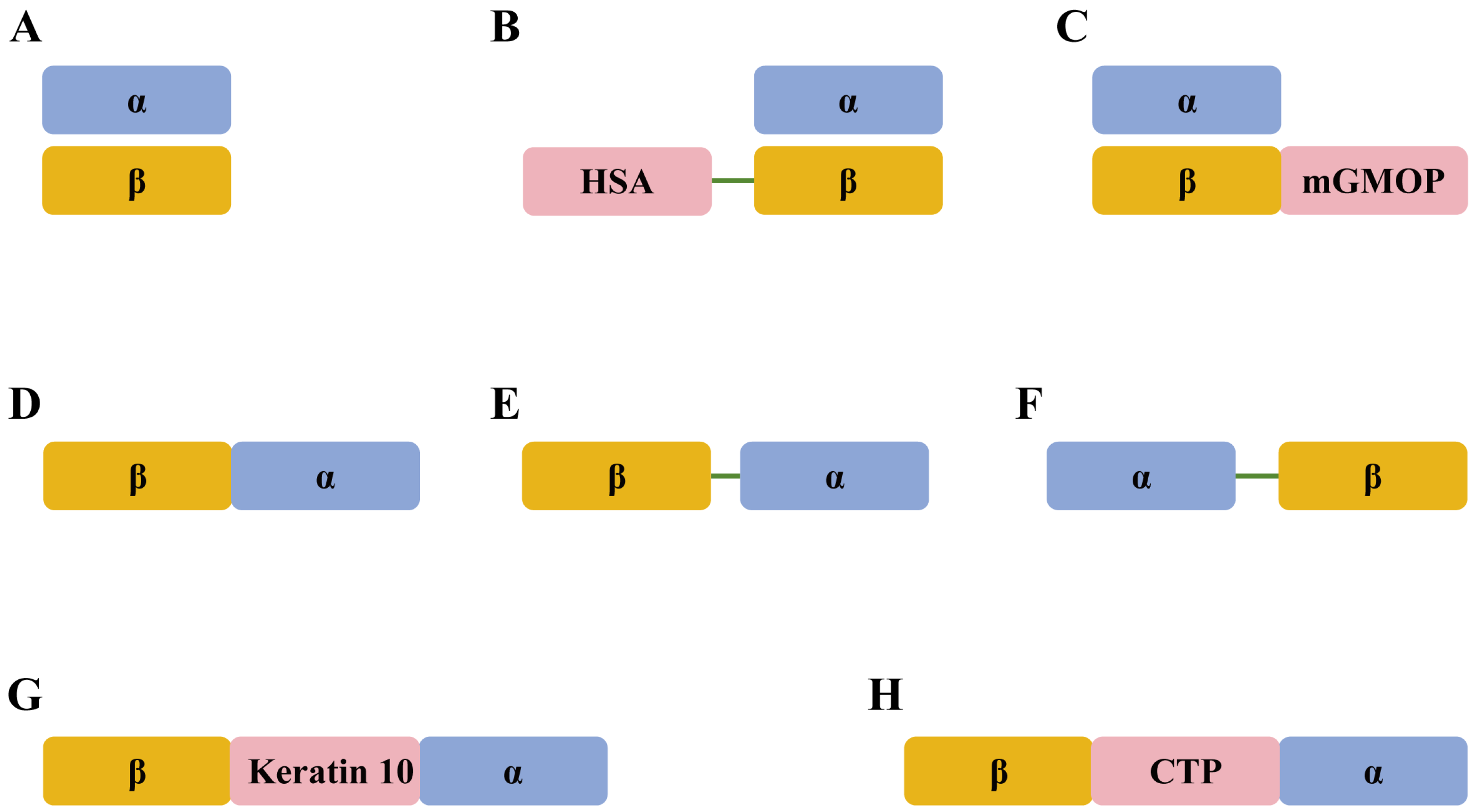
3. Molecular Design of Recombinant FSH Proteins
4. Expression Systems for Recombinant FSH Proteins Production
5. Biological Activity of Recombinant FSH Proteins
6. Application of Recombinant FSH in the Superovulation of Cattle
| Year | Species | Molecular Design | Expression System | Viable Embryos | Reference |
|---|---|---|---|---|---|
| 1996 | bovine | NR | NR | 2.4 ± 0.9 and 3.0 ± 0.9 | [98] |
| 2014 | bovine | NR | NR | 4.3 ± 1.5 (type A) | [101] |
| 2014 | bovine | NR | NR | 7.6 ± 2.4 (type B) | [101] |
| 2020 | ovine | NR | NR | averages of 6.1 | [102] |
| 2022 | human | NR | NR | 5.1 ± 0.86 | [99] |
| 2022 | bovine | α and β linked by a flexible spacer peptide | CHO | 8.65 ± 0.67 | [70] |
| 2023 | bovine | α and β linked by a flexible spacer peptide | CHO | unsorted (7.60 ± 1.27) and sex-sorted semen (4.10 ± 0.88) | [100] |
| 2024 | human | α and β-CTP non-covalently linked | CHO | 5.8 ± 1.6 and 6.4 ± 1.0 | [104] |
| 2024 | bovine | β and α linked by a 33-amino acid spacer peptide | CHO | average of 8–12 | [2] |
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choudhary, K.K.; Kavya, K.M.; Jerome, A.; Sharma, R.K. Advances in reproductive biotechnologies. Vet. World 2016, 9, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, O.I.; Parra, N.; Cerro, R.; Mansilla, R.; Sanchez, R.Z.; Gutierrez-Reinoso, M.; Escribano, E.H.; Castillo, R.; Rodriguez-Alvarez, L.; Tavares, K.; et al. Development and characterization of a novel variant of long-acting bovine follicle-stimulating hormone (brscFSH). Theriogenology 2024, 226, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Sairam, M.R.; Jiang, L.G.; Yarney, T.A.; Khan, H. Follitropin signal transduction: Alternative splicing of the FSH receptor gene produces a dominant negative form of receptor which inhibits hormone action. Biochem. Biophys. Res. Commun. 1996, 226, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Manuela, S.; Jörg, G.; Eberhard, N. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997, 18, 739–773. [Google Scholar] [CrossRef]
- Steelman, S.L.; Lamont, W.A.; Baltes, B.J. Preparation of highly active follicle stimulating hormone from swine pituitary glands. Acta Endocrinol. 1956, 22, 186. [Google Scholar] [CrossRef]
- Donaldson, L.E.; Ward, D.N.; Glenn, S.D. Use of porcine follicle stimulating hormone after chromatographic purification in superovulation of cattle. Theriogenology 1986, 25, 747–757. [Google Scholar] [CrossRef]
- Gonzalez, A.; Lussier, I.G.; Carruthers, T.D.; Murphy, B.D.; Mapletoft, R.J. Superovulation of beef heifers with Folltropin: A new FSH preparation containing reduced LH activity. Theriogenology 1990, 33, 519–529. [Google Scholar] [CrossRef]
- Bó, G.A.; Mapletoft, R.J. Superstimulation of ovarian follicles in cattle: Gonadotropin treatment protocols and FSH profiles. Theriogenology 2020, 150, 353–359. [Google Scholar] [CrossRef]
- Bó, G.A.; Mapletoft, R.J. Historical perspectives and recent research on superovulation in cattle. Theriogenology 2014, 81, 38–48. [Google Scholar] [CrossRef]
- Armstrong, D.T. Recent advances in superovulation of cattle. Theriogenology 1993, 39, 7–24. [Google Scholar] [CrossRef]
- Mapletoft, R.J. History and perspectives on bovine embryo transfer. Anim. Reprod. 2013, 10, 168–173. [Google Scholar]
- Mapletoft, R.J.; Bó, G.A. The evolution of improved and simplified superovulation protocols in cattle. Reprod. Fertil. Dev. 2011, 24, 278–283. [Google Scholar] [CrossRef]
- Friedman, E.; Glick, G.; Lavon, Y.; Roth, Z. Effects of low-dose follicle-stimulating hormone administration on follicular dynamics and preovulatory follicle characteristics in dairy cows during the summer. Domest. Anim. Endocrinol. 2010, 39, 106–115. [Google Scholar] [CrossRef]
- De Roover, R.; Feugang, J.M.; Bols, P.E.; Genicot, G.; Hanzen, C. Effects of ovum pick-up frequency and FSH stimulation: A retrospective study on seven years of beef cattle In Vitro embryo production. Reprod. Domest. Anim. 2008, 43, 239–245. [Google Scholar] [CrossRef]
- Laster, D.B. Disappearance and uptake of (125 I)FSH in the rat, rabbit, ewe and cow. J. Reprod. Fertil. 1972, 30, 407–415. [Google Scholar] [CrossRef]
- Demoustier, M.M.; Beckers, J.F.; Zwalmen, P.V.D.; Closset, J.; Ectors, F. Determination of porcine plasma follitropin levels during superovulation treatment in cows. Theriogenology 1988, 30, 379–386. [Google Scholar] [CrossRef]
- Donaldson, L.E.; Ward, D.N. Effects of luteinising hormone on embryo production in superovulated cows. Vet. Rec. 1986, 119, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Viudes-de-Castro, M.P.; Pomares, A.; Saenz de Juano I Ribes, M.D.; Marco-Jiménez, F.; Vicente, J.S. Variability in gonadotrophin preparations as a factor in the superovulatory response. Theriogenology 1984, 21, 117–125. [Google Scholar] [CrossRef]
- Tribulo, H.; Bo, G.A.; Jofre, F.; Carcedo, J.; Mapletoft, R.J. The effect of LH concentration in a porcine pituitary extract and season on superovulatory response of Bos indicus heifers. Theriogenology 1991, 35, 286. [Google Scholar] [CrossRef]
- Herrler, A.; Elsaesser, F.; Parvizi, N.; Niemann, H. Superovulation of dairy cows with purified FSH supplemented with defined amounts of LH. Theriogenology 1991, 35, 633–643. [Google Scholar] [CrossRef]
- Monniaux, D.; Chupin, D.; Saumande, J. Superovulatory responses of cattle. Theriogenology 1983, 19, 55–81. [Google Scholar] [CrossRef]
- Beckers, J.F. Isolation and use of a porcine FSH to improve the quality of superovulation in cattle. Theriogenology 1987, 27, 213. [Google Scholar] [CrossRef]
- Remy, B.; Baril, G.; Vallet, J.C.; Dufour, R.; Beckers, J.F. Are Antibodies Responsible for a Decreased Superovulatory Response in Goats Which Have Been Treated Repeatedly with Porcine Follicle-Stimulating Hormone? Theriogenology 1991, 36, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Farmer, S.F.; Hyare, H.; Jaunmuktane, Z.; Mead, S.; Ryan, N.S.; Schott, J.M.; Werring, D.J.; Rudge, P.; Collinge, J. Iatrogenic Alzheimer’s disease in recipients of cadaveric pituitary-derived growth hormone. Nat. Med. 2024, 30, 394–402. [Google Scholar] [CrossRef]
- Walsh, J.H.; Mantovani, R.; Duby, R.T.; Overstrom, E.W.; Boland, M.P. The effects of once or twice daily injections of pFSH on superovulatory response in heifers. Theriogenology 1993, 40, 313–321. [Google Scholar] [CrossRef]
- Looney, C.R.; Boutte, B.W.; Archbald, L.F.; Godke, R.A. Comparison of once daily and twice daily FSH injections for superovulating beef cattle. Theriogenology 1981, 15, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Chasombat, J.; Sakhong, D.; Nagai, T.; Parnpai, R.; Vongpralub, T. Superstimulation of Follicular Growth in Thai Native Heifers by a Single Administration of Follicle Stimulating Hormone Dissolved in Polyvinylpyrrolidone. J. Reprod. Dev. 2013, 59, 214–218. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ooe, M.; Kawaguchi, M.; Suzuki, T. Superovulation in the cow with a single intramuscular injection of FSH dissolved in polyvinylpyrrolidone. Theriogenology 1994, 41, 747–755. [Google Scholar] [CrossRef]
- Kimura, K.; Hirako, M.; Iwata, H.; Aoki, M.; Kawaguchi, M.; Seki, M. Successful superovulation of cattle by a single administration of FSH in aluminum hydroxide gel. Theriogenology 2007, 68, 633–639. [Google Scholar] [CrossRef]
- Bó, G.A.; Rogan, D.R.; Mapletoft, R.J. Pursuit of a method for single administration of pFSH for superstimulation in cattle: What we have learned. Theriogenology 2017, 112, 26–33. [Google Scholar] [CrossRef]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.A.; Kong, J.H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef]
- Biancucci, A.; Sbaragli, T.; Comin, A.; Sylla, L.; Monaci, M.; Peric, T.; Stradaioli, G. Reducing treatments in cattle superovulation protocols by combining a pituitary extract with a 5% hyaluronan solution: Is it able to diminish activation of the hypothalamic pituitary adrenal axis compared to the traditional protocol? Theriogenology 2016, 85, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Tríbulo, A.; Rogan, D.; Tribulo, H.; Tribulo, R.; Alasino, R.V.; Beltramo, D.; Bianco, I.; Mapletoft, R.J.; Bó, G.A. Superstimulation of ovarian follicular development in beef cattle with a single intramuscular injection of Folltropin-V. Anim. Reprod. Sci. 2011, 129, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Tríbulo, A.; Rogan, D.; Tríbulo, H.; Tríbulo, R.; Mapletoft, R.J.; Bó, G.A. Superovulation of beef cattle with a split-single intramuscular administration of Folltropin-V in two concentrations of hyaluronan. Theriogenology 2012, 77, 1679–1685. [Google Scholar] [CrossRef]
- Hasler, J.F. Forty years of embryo transfer in cattle: A review focusing on the journal Theriogenology, the growth of the industry in North America, and personal reminisces. Theriogenology 2014, 81, 152–169. [Google Scholar] [CrossRef]
- Leibowitz, D.; Hoffman, J. Fertility Drug Therapies: Past, Present, and Future. J. Obstet. Gynecol. Neonatal Nurs. 2000, 29, 201–210. [Google Scholar] [CrossRef]
- He, T.P.; Tang, J.Y.; Xu, L.; Song, C.L.; Lei, T. Expression of recombinant human follicle-stimulating hormone in CHO cells. Chin. J. Biol. 2011, 24, 1409–1412. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M.; Mannaerts, B.M.J.L.; Devroey, P.; Leader, A.; Boime, I.; Baird, D.T. Advances in recombinant DNA technology: Corifollitropin alfa, a hybrid molecule with sustained follicle-stimulating activity and reduced injection frequency. Hum. Reprod. Update 2009, 15, 309–321. [Google Scholar] [CrossRef]
- Pouwer, A.W.; Farquhar, C.; Kremer, J.A. Long-acting FSH versus daily FSH for women undergoing assisted reproduction. Cochrane Database Syst. Rev. 2015, 2015, Cd009577. [Google Scholar] [CrossRef]
- Meduri, G.; Bachelot, A.; Cocca, M.P.; Vasseur, C.; Misrahi, M. Molecular pathology of the FSH receptor: New insights into FSH physiology. Mol. Cell. Endocrinol. 2008, 282, 130–142. [Google Scholar] [CrossRef]
- De La Llosa, P.; Jutisz, M. Reversible dissociation into subunits and biological activity of ovine luteinizing hormone. BBA Protein Struct. 1969, 181, 426–436. [Google Scholar] [CrossRef]
- Volkin, D.B.; Sanyal, G.; Burke, C.J.; Middaugh, C.R. Preformulation Studies as an Essential Guide to Formulation Development and Manufacture of Protein Pharmaceuticals. Pharm. Biotechnol. 2002, 14, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.G.; Bahl, O.P.; Cornell, J.S.; Swaminathan, N. Biologically Active Hormones Prepared by Recombination of the α Chain of Human Chorionic Gonadotropin and the Hormone-Specific Chain of Bovine Thyrotropin or of Bovine Luteinizing Hormone. J. Biol. Chem. 1971, 246, 2321–2324. [Google Scholar] [CrossRef] [PubMed]
- Reichert, L.E. Biological evidence for the subunit structure of human follicle-stimulating hormone. Endocrinology 1971, 89, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.N.; Reichart, L.E., Jr.; Fitak, B.A.; Nahm, H.S.; Sweeney, C.M.; Neill, J.D. Isolation and properties of subunits of rat pituitary luteinizing hormone. Biochemistry 1971, 10, 1796–1802. [Google Scholar] [CrossRef]
- Closset, J.; Hennen, G.; Lequin, R.M. Isolation and properties of human luteinizing hormone subunits. FEBS Lett. 1972, 21, 325–329. [Google Scholar] [CrossRef]
- Yves, C. Molecular Basis of the Specificity of Binding of Glycoprotein Hormones to Their Receptors. Endocr. Rev. 1992, 13, 670–691. [Google Scholar] [CrossRef]
- Fidler, A.E.; Lin, J.S.; Lun, S.; Ng Chie, W.; Western, A.; Stent, V.; McNatty, K.P. Production of biologically active tethered ovine FSHbetaalpha by the methylotrophic yeast Pichia pastoris. J. Mol. Endocrinol. 2003, 30, 213–225. [Google Scholar] [CrossRef]
- Fox, K.M.; Dias, J.A.; Van Roey, P. Three-Dimensional Structure of Human Follicle-Stimulating Hormone. Mol. Endocrinol. 2001, 15, 378–389. [Google Scholar] [CrossRef]
- Bousfield, G.R.; Harvey, D.J. Follicle-Stimulating Hormone Glycobiology. Endocrinology 2019, 60, 1515–1535. [Google Scholar] [CrossRef]
- Bishop, L.A.; Nguyen, T.V.; Schofield, P.R. Both of the beta-subunit carbohydrate residues of follicle-stimulating hormone determine the metabolic clearance rate and In Vivo potency. Endocrinology 1995, 136, 2635–2640. [Google Scholar] [CrossRef]
- Bishop, L.A.; Robertson, D.M.; Cahir, N.; Schofield, P.R. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. Mol. Endocrinol. 1994, 8, 722–731. [Google Scholar] [CrossRef]
- Valove, F.M.; Finch, C.; Anasti, J.N.; Froehlich, J.; Flack, M.R. Receptor binding and signal transduction are dissociable functions requiring different sites on follicle stimulating hormone. Endocrinology 1995, 135, 2657–2661. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Boime, I. The role of the asparagine-linked oligosaccharides of the alpha subunit in the secretion and assembly of human chorionic gonadotrophin. J. Cell Biol. 1988, 106, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- van Zuylen, C.W.; Kamerling, J.P.; Vliegenthart, J.F. Glycosylation beyond the Asn78-linked GlcNAc residue has a significant enhancing effect on the stability of the α subunit of human chorionic gonadotropin. Biochem. Biophys. Res. Commun. 1997, 232, 117–120. [Google Scholar] [CrossRef]
- Bousfield, G.R.; Butnev, V.Y.; Butnev, V.Y.; Nguyen, V.T.; Gray, C.M.; Dias, J.A.; MacColl, R.; Eisele, L.; Harvey, D.J. Differential Effects of α Subunit Asparagine56 Oligosaccharide Structure on Equine Lutropin and Follitropin Hybrid Conformation and Receptor-Binding Activity. Biochemistry 2004, 43, 10817–10833. [Google Scholar] [CrossRef] [PubMed]
- Flack, M.R.; Froehlich, J.; Bennet, A.P.; Anasti, J.; Nisula, B.C. Site-directed mutagenesis defines the individual roles of the glycosylation sites on follicle-stimulating hormone. J. Biol. Chem. 1994, 269, 14015–14020. [Google Scholar] [CrossRef]
- Ulloa-Aguirre, A.; Timossi, C.; Damián-Matsumura, P.; Dias, J.A. Role of Glycosylation in Function of Follicle-Stimulating Hormone. Endocrine 2000, 11, 205–215. [Google Scholar] [CrossRef]
- Deechongkit, S.; Aoki, K.H.; Park, S.S.; Kerwin, B.A. Biophysical comparability of the same protein from different manufacturers: A case study using Epoetin alfa from Epogen and Eprex. J. Pharm. Sci. 2006, 95, 1931–1943. [Google Scholar] [CrossRef]
- Nguyen, T.M.D.; Klett, D.; Combarnous, Y. Undissociable chemically cross-linked and single-chain gonadotropins. Theriogenology 2023, 198, 250–255. [Google Scholar] [CrossRef]
- Mountford, P.S.; Brandon, M.R.; Adams, T.E. Expression and characterization of biologically active ovine FSH from mammalian cell lines. J. Mol. Endocrinol. 1994, 12, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Mori, J.; Ohmura, M.; Kato, Y.; Ueda, S. Baculovirus-insect cell production of bioactive porcine FSH. Theriogenology 1997, 47, 491–499. [Google Scholar] [CrossRef]
- Richard, F.; Robert, P.; Remy, J.J.; Martinat, N.; Bidart, J.M.; Salesse, R.; Combarnous, Y. High-level secretion of biologically active recombinant porcine follicle-stimulating hormone by the methylotrophic yeast Pichia pastoris. Biochem. Biophys. Res. Commun. 1998, 245, 847–852. [Google Scholar] [CrossRef]
- van de Wiel, D.F.; van Rijn, P.A.; Meloen, R.H.; Moormann, R.J. High-level expression of biologically active recombinant bovine follicle stimulating hormone in a baculovirus system. J. Mol. Endocrinol. 1998, 20, 83–98. [Google Scholar] [CrossRef]
- Inaba, T.; Mori, J.; Ohmura, M.; Tani, H.; Ueda, S. Recombinant porcine follicle stimulating hormone produced in baculovirus-insect cells induces rat ovulation In Vivo and gene expression of tissue plasminogen activator In Vitro. Res. Vet. Sci. 1998, 64, 25–29. [Google Scholar] [CrossRef]
- Kato, Y.; Sato, I.; Ihara, T.; Tomizawa, K.; Mori, J.; Geshi, M.; Nagai, T.; Okuda, K.; Kato, T.; Ueda, S. Expression and purification of biologically active porcine follicle-stimulating hormone in insect cells bearing a baculovirus vector. J. Mol. Endocrinol. 1998, 20, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Dirnberger, D.; Steinkellner, H.; Abdennebi, L.; Remy, J.J.; van de Wiel, D. Secretion of biologically active glycoforms of bovine follicle stimulating hormone in plants. Eur. J. Biochem. 2001, 268, 4570–4579. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Tian, J.; Huang, H.; An, L. Improving heterologous expression of porcine follicle-stimulating hormone in Pichia pastoris by integrating molecular strategies and culture condition optimization. Appl. Microbiol. Biotechnol. 2018, 102, 8867–8882. [Google Scholar] [CrossRef]
- Villarraza, J.; Antuña, S.; Tardivo, M.B.; Rodríguez, M.C.; Díaz, P.U.; Notaro, U.S.; Ortega, H.H.; Prieto, C.; Ceaglio, N. Development of a biotechnology process for the production of a novel hyperglycosylated long-acting recombinant bovine follicle-stimulating hormone. Biotechnol. J. 2024, 19, e2400260. [Google Scholar] [CrossRef]
- Gutiérrez-Reinoso, M.A.; Aguilera, C.J.; Navarrete, F.; Cabezas, J.; Castro, F.O.; Cabezas, I.; Sánchez, O.; García-Herreros, M.; Rodríguez-Alvarez, L. Effects of Extra-Long-Acting Recombinant Bovine FSH (bscrFSH) on Cattle Superovulation. Animals 2022, 12, 153. [Google Scholar] [CrossRef]
- Abreu, C.; Grunberg, K.; Bonilla, M.; Crispo, M.; Pantano, S.; Jaeschke, J.; Comini, M.A.; Bollati-Fogolín, M. Expression and functional characterization of chimeric recombinant bovine follicle-stimulating hormone produced in Leishmania tarentolae. Microb. Biotechnol. 2024, 17, e14444. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Mulders, A.G.; Fauser, B.C.; Schoot, B.C.; Renier, M.A.; Devroey, P.; Struijs, M.J.; Mannaerts, B.M. Pharmacodynamics of a single low dose of long-acting recombinant follicle-stimulating hormone (FSH-carboxy terminal peptide, corifollitropin alfa) in women with World Health Organization group II anovulatory infertility. J. Clin. Endocrinol. Metab. 2004, 89, 6297. [Google Scholar] [CrossRef] [PubMed]
- Devroey, P.; Fauser, B.C.; Platteau, P.; Beckers, N.G.; Dhont, M.; Mannaerts, B.M. Induction of Multiple Follicular Development by a Single Dose of Long-Acting Recombinant Follicle-Stimulating Hormone (FSH-CTP, Corifollitropin Alfa) for Controlled Ovarian Stimulation before In Vitro Fertilization. J. Clin. Endocrinol. Metab. 2004, 89, 2062–2070. [Google Scholar] [CrossRef]
- Lemke, E.P.; Adams, B.M.; Jablonka-Shariff, A.; Boime, I.; Adams, T.E. Single-chain human gonadotropin analogs induce follicle development in sheep. J. Endocrinol. 2008, 196, 593–600. [Google Scholar] [CrossRef]
- Loutradis, D.; Drakakis, P.; Vlismas, A.; Antsaklis, A. Corifollitropin alfa, a long-acting follicle-stimulating hormone agonist for the treatment of infertility. Curr. Opin. Investig. Drugs 2009, 10, 372–380. [Google Scholar]
- Loutradis, D.; Vlismas, A.; Drakakis, P. Corifollitropin alfa: A novel long-acting recombinant follicle-stimulating hormone agonist for controlled ovarian stimulation. Womens Health 2010, 6, 655–664. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Bilger, W.; Longobardi, S.; Alam, V.; D′Hooghe, T.; Sunkara, S.K. The Development of Gonadotropins for Clinical Use in the Treatment of Infertility. Front. Endocrinol. 2019, 10, 429. [Google Scholar] [CrossRef]
- van Rosmalen, M.; Krom, M.; Merk, M. Tuning the Flexibility of Glycine-Serine Linkers to Allow Rational Design of Multidomain Proteins. Biochemistry 2017, 56, 6565–6574. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Karadag, M.; Onal, E.; Gelinci, E.; Cakan-Akdogan, G.; Kalyoncu, S. Effect of non-repetitive linker on In Vitro and In Vivo properties of an anti-VEGF scFv. Sci. Rep. 2022, 12, 5449. [Google Scholar] [CrossRef]
- Schütz, A.; Bernhard, F.; Berrow, N.; Buyel, J.F.; Ferreira-da-Silva, F.; Haustraete, J.; van den Heuvel, J.; Hoffmann, J.E.; de Marco, A.; Peleg, Y.; et al. A concise guide to choosing suitable gene expression systems for recombinant protein production. STAR Protoc. 2023, 4, 102572. [Google Scholar] [CrossRef]
- Gräslund, S.; Nordlund, P.; Weigelt, J.; Hallberg, B.M.; Bray, J.; Gileadi, O.; Knapp, S.; Oppermann, U.; Arrowsmith, C.; Hui, R.; et al. Protein production and purification. Nat. Methods 2008, 5, 135–146. [Google Scholar] [CrossRef]
- Delic, M.; Valli, M.; Graf, A.B.; Pfeffer, M.; Mattanovich, D.; Gasser, B. The secretory pathway: Exploring yeast diversity. FEMS Microbiol. Rev. 2013, 37, 872–914. [Google Scholar] [CrossRef] [PubMed]
- De Wachter, C.; Van Landuyt, L.; Callewaert, N. Engineering of Yeast Glycoprotein Expression. Adv. Biochem. Eng. Biotechnol. 2021, 175, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Errey, J.C.; Fiez-Vandal, C. Production of membrane proteins in industry: The example of GPCRs. Protein Expr. Purif. 2020, 169, 105569. [Google Scholar] [CrossRef]
- Gupta, K.; Tölzer, C.; Sari-Ak, D.; Fitzgerald, D.J.; Schaffitzel, C.; Berger, I. MultiBac: Baculovirus-Mediated Multigene DNA Cargo Delivery in Insect and Mammalian Cells. Viruses 2019, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Tomiya, N.; Narang, S.; Lee, Y.C.; Betenbaugh, M.J. Comparing N-glycan processing in mammalian cell lines to native and engineered lepidopteran insect cell lines. Glycoconj. J. 2004, 21, 343–360. [Google Scholar] [CrossRef]
- Ma, J.K.C.; Hiatt, A.; Hein, M.; Vine, N.D.; Wang, F.; Stabila, P.; van Dolleweerd, C. Generation and assembly of secretory antibodies in plants. Science 1995, 268, 716–719. [Google Scholar] [CrossRef]
- Cabanes-Macheteau, M.; Fitchette-Lainé, A.C.; Loutelier-Bourhis, C.; Lange, C.; Vine, N.D.; Ma, J.K.; Lerouge, P.; Faye, L. N-Glycosylation of a mouse IgG expressed in transgenic tobacco plants. Glycobiology 1999, 9, 365–372. [Google Scholar] [CrossRef]
- Lerouge, P.; Cabanes-Macheteau, M.; Rayon, C.; AC, F.-L.; Gomord, V.; Faye, L. N-Glycoprotein biosynthesis in plants: Recent developments and future trends. Plant Mol. Biol. 1998, 38, 31. [Google Scholar] [CrossRef]
- Basile, G.; Peticca, M. Recombinant Protein Expression in Leishmania tarentolae. Mol. Biotechnol. 2009, 43, 273–278. [Google Scholar] [CrossRef]
- Lai, J.Y.; Klatt, S.; Lim, T.S. Potential application of Leishmania tarentolae as an alternative platform for antibody expression. Crit. Rev. Biotechnol. 2019, 39, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.G.; Lee, G.M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Appl. Microbiol. Biotechnol. 2012, 93, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Biopharmaceutical benchmarks 2014. Nat. Biotechnol. 2014, 32, 992–1000. [Google Scholar] [CrossRef]
- Dias, J.A.; Cohen, B.D.; Lindau-Shepard, B.; Nechamen, C.A.; Peterson, A.J.; Schmidt, A. Molecular, structural, and cellular biology of follitropin and follitropin receptor. Vitam. Horm. 2002, 64, 249–322. [Google Scholar] [CrossRef] [PubMed]
- Steelman, S.L.; Pohley, F.M. Assay of the follicle stimulating hormone based on the augmentation with human chorionic gonadotropin. Endocrinology 1953, 53, 604–616. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef]
- Heffner, K.M.; Wang, Q.; Hizal, D.B.; Can, Ö.; Betenbaugh, M.J. Glycoengineering of Mammalian Expression Systems on a Cellular Level. Adv. Biochem. Eng. Biotechnol. 2021, 175, 37–69. [Google Scholar] [CrossRef]
- Wehrman, M.E.; Fike, K.E.; Kojima, F.N.; Bergfeld, E.G.; Cupp, A.S.; Mariscal, V.; Sanchez, T.; Kinder, J.E. Development of persistent ovarian follicles during synchronization of estrus influences the superovulatory response to FSH treatment in cattle. Theriogenology 1996, 45, 593–610. [Google Scholar] [CrossRef]
- Khodadadi, A.; Niasari-Naslaji, A.; Nikjou, D.; Mohammadi, B. Superovulation of high-producing Holstein lactating dairy cows with human recombinant FSH and hMG. Theriogenology 2022, 191, 239–244. [Google Scholar] [CrossRef]
- Gutiérrez-Reinoso, M.A.; Arreseigor, C.J.; Driedger, B.; Cabezas, I.; Hugues, F.; Parra, N.C.; Sánchez, O.; Toledo, J.R.; Garcia-Herreros, M. Effects of recombinant FSH (bscrFSH) and pituitary FSH (FSH-p) on embryo production in superovulated dairy heifers inseminated with unsorted and sex-sorted semen. Anim. Reprod. Sci. 2023, 252, 107226. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Hackbart, K.S.; Bender, R.W.; Baez, G.M.; Dresch, A.R.; Guenther, J.N.; Souza, A.H.; Fricke, P.M. Use of a single injection of long-acting recombinant bovine FSH to superovulate Holstein heifers: A preliminary study. Theriogenology 2014, 82, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, N.; Martinez, M. A single administration of a long-acting recombinant ovine FSH (roFSH) for cattle superovulation. Theriogenology 2020, 154, 66–72. [Google Scholar] [CrossRef]
- Fares, F.A.; Suganuma, N.; Nishimori, K.; LaPolt, P.S.; Hsueh, A.J.; Boime, I. Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin beta subunit to the follitropin beta subunit. Proc. Natl. Acad. Sci. USA 1992, 89, 4304–4308. [Google Scholar] [CrossRef]
- Viana, J.H.M.; Moura, R.M.; Martins, L.P.; Figueiredo, R.A.; Siqueira, L.G.B.; Fernandes, C.A.C. Superovulating cattle with corifollitropin-alpha, a long-acting recombinant human FSH (rhFSH): Dose-response, half-life, effects on the ovaries, and embryo outcomes. Theriogenology 2024, 226, 302–307. [Google Scholar] [CrossRef]
- Howles, C.M. Genetic engineering of human FSH (Gonal-F®). Hum. Reprod. Update 1996, 2, 172. [Google Scholar] [CrossRef]
- Olijve, W.; de Boer, W.; Mulders, J.W.M.; van Wezenbeek, P.M. Molecular biology and biochemistry of human recombinant follicle stimulating hormone (Puregon). Mol. Hum. Reprod. 1996, 2, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Greve, T.; Callesen, H.; Hyttel, P. Endocrine profiles and egg quality in the superovulated cow. Nord. Veterinaermedicin 1983, 35, 408–421. [Google Scholar] [CrossRef]
- Dieleman, S.J.; Bevers, M.M.; Vos, P.L.A.M.; Deloos, F. PMSG/anti-PMSG in cattle: A simple and efficient superovulatory treatment? Theriogenology 1993, 39, 25–41. [Google Scholar] [CrossRef]
- González, A.; Wang, H.; Carruthers, T.D.; Murphy, B.D.; Mapletoft, R.J. Increased ovulation rates in PMSG-stimulated beef heifers treated with a monoclonal PMSG antibody. Theriogenology 1994, 41, 1631–1642. [Google Scholar] [CrossRef]
- Lin, Z.L.; Ni, H.M.; Liu, Y.H.; Sheng, X.H.; Cui, X.S.; Kim, N.H.; Guo, Y. Effect of anti-PMSG on distribution of estrogen receptor alpha and progesterone receptor in mouse ovary, oviduct and uterus. Zygote 2015, 23, 695–703. [Google Scholar] [CrossRef]
- Hesser, M.W.; Morris, J.C.; Gibbons, J.R. Advances in Recombinant Gonadotropin Production for Use in Bovine Superovulation. Reprod. Domest. Anim. 2011, 46, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Baruselli, P.S.; de Abreu, L.; Catussi, B.L.C.; Oliveira, A.; Rebeis, L.M.; Gricio, E.A.; Albertini, S.; Sales, J.N.S.; Rodrigues, C.A. Use of new recombinant proteins for ovarian stimulation in ruminants. Anim. Reprod. 2023, 20, e20230092. [Google Scholar] [CrossRef] [PubMed]
| Expression System | Advantages | Disadvantages |
|---|---|---|
| E. coli | High expression efficiency, low cost, and simple operational procedures | Lack of post-translational modifications and control endotoxin levels |
| Yeast | The cost-effectiveness and ease of purification | The glycosylation differs from that of mammalian proteins |
| Insect cell | The high expression efficiency and production of relatively complex proteins | The glycosylation differs from that of mammalian proteins, higher costs, more complex operational procedures, and longer production cycles |
| Mammalian cell | Complex post-translational modifications | Higher costs, complex operational procedures, and longer production cycles |
| Species | Molecular Design | Expression System | Expression Level | In Vitro Bioassay | In Vivo Bioassay | Half-Life | Reference |
|---|---|---|---|---|---|---|---|
| ovine | α and β non-covalently linked | CHO cell | 0.10–0.16 pg/cell/day | Activity in the testis radioreceptor assay and in vitro Sertoli cell bioassay | NR | NR | [61] |
| porcine | α and β non-covalently linked | Insect cell | NR | Estradiol production in Sertoli cell assay; in vitro bioactivity of 1.11 units/mg | Mouse uterine weight assay and ovulation; ovarian and uterine weights in rat | NR | [62] |
| porcine | α and β non-covalently linked | Yeast | 10 mg/L | cAMP production in CHO cells; progesterone production in Y1 cells | NR | NR | [63] |
| bovine | α and β non-covalently linked | Insect cell | 1–5 mg/L | Y1 cell-rounding assay; Y1 cell cAMP assay; cAMP in Sertoli cell assay; GVBD of bovine oocytes; in vitro bioactivity of 6–23 IU/ml | NR | NR | [64] |
| porcine | α and β non-covalently linked | Insect cell | NR | Stimulation of ovarian tPA enzyme activity | Induces ovulation in rats | NR | [65] |
| porcine | α and β non-covalently linked | Insect cell | 1 mg/L | Progesterone in granulosa cells; GVBD of porcine oocytes | NR | NR | [66] |
| bovine | β and α fused via overlap PCR | Plant cell | 3% of the total soluble proteins | cAMP production in CHO cells; in vitro bioactivity of 850 IU/mg | Superovulatory of mice | NR | [67] |
| ovine | β and α linked by a two amino acid linker | Yeast | 0.1 mg/L | cAMP production in CHO cells | NR | NR | [48] |
| porcine | α and HSA-β non-covalently linked | Yeast | 40.8 mg/L | cAMP production in HEK293 cells | NR | NR | [68] |
| bovine | α and β linked by a flexible spacer peptide | CHO cell | NR | nr | Superovulation and pharmacokinetics in cattle | circulating half-life higher than 48 h | [70] |
| bovine | β and α linked by a 33-amino acid spacer peptide | CHO cell | 30 pg/cell/day | NR | Superovulation in cattle | NR | [2] |
| bovine | β and α linked by CTP linker | Leishmania tarentolae | 2.95 ± 0.14 mg/L | NR | In vivo bioactivity of 56.3 IU/mg in SP bioassay | NR | [71] |
| bovine | α and β non-covalently linked | CHO cell | 0.2 pg/cell/day | NR | Pharmacokinetics in rat; in vivo bioactivity of 4200 IU/mg in SP bioassay | 12.80 ± 0.03 h | [69] |
| bovine | α and β-mGMOP non-covalently linked | CHO cell | 1.3 pg/cell/day | NR | Pharmacokinetics in rat; in vivo bioactivity of 10,000 IU/mg in SP bioassay | 14 ± 2 h | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Luo, H. Development of Recombinant Follicle-Stimulating Hormone for the Superovulation of Cattle: A Review. Vet. Sci. 2025, 12, 264. https://doi.org/10.3390/vetsci12030264
Zhang J, Luo H. Development of Recombinant Follicle-Stimulating Hormone for the Superovulation of Cattle: A Review. Veterinary Sciences. 2025; 12(3):264. https://doi.org/10.3390/vetsci12030264
Chicago/Turabian StyleZhang, Jiawei, and Haoshu Luo. 2025. "Development of Recombinant Follicle-Stimulating Hormone for the Superovulation of Cattle: A Review" Veterinary Sciences 12, no. 3: 264. https://doi.org/10.3390/vetsci12030264
APA StyleZhang, J., & Luo, H. (2025). Development of Recombinant Follicle-Stimulating Hormone for the Superovulation of Cattle: A Review. Veterinary Sciences, 12(3), 264. https://doi.org/10.3390/vetsci12030264







