West Nile Virus (WNV): One-Health and Eco-Health Global Risks
Simple Summary
Abstract
1. Introduction
2. Epidemiology
3. Viral Life Cycle and Transmission
- Infection of the mosquito after feeding on a viremic bird;
- Viral replication and dissemination within the mosquito body;
- Transmission of the virus from the mosquito to a susceptible vertebrate through another blood meal [90].
4. Geographical Distribution
5. Host Reservoirs
6. Pathogenesis
6.1. Sequence of Cellular Invasion Steps
6.2. Cellular Invasion and Replication—The Keratinocyte and Langerhans Cell Model
6.3. WNV Entry, Replication, Translation, and Viral Particle Assembly
6.4. WNV Entry Routes, Neuroinvasion, and Cellular Lesions
7. Clinical Signs
7.1. In Humans
7.2. In Horses
8. Genetic Susceptibility to Develop Neuroinvasive Forms
9. Immune Responses to WNV
9.1. Birds
9.2. Mosquitos
9.3. Horses
9.4. Humans
10. Clinical Pathology and Imaging
11. Differential Diagnosis
11.1. Herpes Viruses
11.2. Varicella Zoster
11.3. Measles
11.4. Rabies
11.5. Japanese Encephalitis
11.6. Chikungunya Virus
11.7. Dengue Virus
11.8. Zika Virus
11.9. Usutu Virus
12. Laboratory Diagnosis of WNV Infection
12.1. Diagnostic Methods
12.2. Serological Tests
12.3. Molecular Tests
12.4. Testing Strategies
13. Pathology Induced by WNV Infection
14. Mammalia
14.1. Order: Primates
14.1.1. Humans
14.1.2. Non-Human Primates (NHPs)
- (a)
- Natural Infection
- (b)
- Experimental Infection
14.1.3. Other Primates in Wildlife
14.2. Order: Perissodactyla
Equine (Domestic)
14.3. Order: Arctiodactyla
14.3.1. Ruminants (Domestic and Wildlife)
14.3.2. Swine (Domestic and Wildlife)
- (a)
- Natural Infection
- (b)
- Experimental Infection
14.3.3. Cetacea
14.3.4. Marine Mammals
14.3.5. Flying Mammals—Chiroptera
Natural Exposure
14.4. Order: Carnivora (Carnivores and Mesocarnivores; Domestic and Wildlife)
14.4.1. Carnivores—Domestic
- (a)
- Natural Infection
- (b)
- Experimental infection
14.4.2. Carnivores (Wildlife)
Natural Exposure and Natural Infection
14.4.3. Mesocarnivores
Canidae (Coyote—Fox)
Viverridae
Mustelidae
14.4.4. Procyonidae
- (a)
- Natural Exposure
- (b)
- Experimental Infection
14.4.5. Mephitidae
14.4.6. Order: Lagomorpha
Rabbit
14.4.7. Order: Marsupialia
14.4.8. Zoo Animals
- (a)
- USA
- (b)
- Mexico
- (c)
- Spain
- (d)
- Slovenia
- (e)
- France
14.4.9. Order: Rodentia
Squirrel
- (a)
- Natural Infection
- (b)
- Natural Exposure
- (c)
- Experimental Infection
Rat and Mouse
- (a)
- Natural Exposure
- (b)
- Experimental Infection
15. Reptilia
16. Aves
17. Economic Impact and Climate Change
18. WNV Surveillance on the Territory
19. Foundations of the European WND Legislation
- Directive 2002/98/EC (Blood Directive) (DIRECTIVE 2002/98/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 January 2003, setting standards of quality and safety for the collection, testing, processing, storage, and distribution of human blood and blood components and amending Directive 2001/83/EC);
- Directive 2004/23/EC (Tissues and Cells Directive) (DIRECTIVE 2004/23/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage, and distribution of human tissues and cells);
- Directive 2010/45/EU (Organs Directive) (DIRECTIVE 2010/45/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 7 July 2010 on standards of quality and safety of human organs intended for transplantation).
20. Definition of Case and Suspicion of Disease
- clinical symptoms;
- anatomo-pathological lesions;
- positive laboratory test results;
- epidemiological correlation.
- isolation of the pathogen;
- the presence of symptoms or lesions in conjunction with a positive test;
- a positive test result accompanied by epidemiological correlation.
21. International Notification Obligations
22. History and Legislation of WND in Italy: The Italian One-Health Prevention Model
23. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zina, S.M.; Hoarau, G.; Labetoulle, M.; Khairallah, M.; Rousseau, A. Ocular Manifestations of Flavivirus Infections. Pathogens 2023, 12, 1457. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, L.; Loukatou, S.; Sofia, K.; Maroulis, D.; Vlachakis, D. An Updated Evolutionary Study of Flaviviridae NS3 Helicase and NS5 RNA-Dependent RNA Polymerase Reveals Novel Invariable Motifs as Potential Pharmacological Targets. Mol. Biosyst. 2016, 12, 2080–2093. [Google Scholar] [CrossRef]
- Leyssen, P.; De Clercq, E.; Neyts, J. Perspectives for the Treatment of Infections with Flaviviridae. Clin. Microbiol. Rev. 2000, 13, 67–82. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- de Wit, M.M.; Dimas Martins, A.; Delecroix, C.; Heesterbeek, H.; Ten Bosch, Q.A. Mechanistic Models for West Nile Virus Transmission: A Systematic Review of Features, Aims and Parametrization. Proc. Biol. Sci. 2024, 291, 20232432. [Google Scholar] [CrossRef]
- Barrows, N.J.; Campos, R.K.; Liao, K.-C.; Prasanth, K.R.; Soto-Acosta, R.; Yeh, S.-C.; Schott-Lerner, G.; Pompon, J.; Sessions, O.M.; Bradrick, S.S.; et al. Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev. 2018, 118, 4448–4482. [Google Scholar] [CrossRef]
- Barrett, A.D.T. Economic Burden of West Nile Virus in the United States. Am. J. Trop. Med. Hyg. 2014, 90, 389–390. [Google Scholar] [CrossRef]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda. Am. J. Trop. Med. Hyg. 1940, 20, 471–492. [Google Scholar] [CrossRef]
- Karim, S.-U.; Bai, F. Introduction to West Nile Virus. Methods Mol. Biol. Clifton NJ 2023, 2585, 1–7. [Google Scholar] [CrossRef]
- Ludwig, G.V.; Calle, P.P.; Mangiafico, J.A.; Raphael, B.L.; Danner, D.K.; Hile, J.A.; Clippinger, T.L.; Smith, J.F.; Cook, R.A.; McNamara, T. An Outbreak of West Nile Virus in a New York City Captive Wildlife Population. Am. J. Trop. Med. Hyg. 2002, 67, 67–75. [Google Scholar] [CrossRef]
- Nikolay, B. A Review of West Nile and Usutu Virus Co-Circulation in Europe: How Much Do Transmission Cycles Overlap? Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Fall, G.; Di Paola, N.; Faye, M.; Dia, M.; Freire, C.C.d.M.; Loucoubar, C.; Zanotto, P.M.d.A.; Faye, O.; Sall, A.A. Biological and Phylogenetic Characteristics of West African Lineages of West Nile Virus. PLoS Negl. Trop. Dis. 2017, 11, e0006078. [Google Scholar] [CrossRef]
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D.T. Phylogeography of West Nile Virus: From the Cradle of Evolution in Africa to Eurasia, Australia, and the Americas. J. Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef]
- Goldblum, N.; Jasinska-Klingberg, W.; Klingberg, M.A.; Marberg, K.; Sterk, V.V. The Natural History of West Nile Fever. I. Clinical Observations during an Epidemic in Israel. Am. J. Hyg. 1956, 64, 259–269. [Google Scholar] [CrossRef]
- Hurlbut, H.S.; Rizk, F.; Taylor, R.M.; Work, T.H. A Study of the Ecology of West Nile Virus in Egypt. Am. J. Trop. Med. Hyg. 1956, 5, 579–620. [Google Scholar] [CrossRef]
- Bardos, V.; Adamcova, J.; Dedei, S.; Gjini, N.; Rosicky, B.; Simkova, A. Neutralizing Antibodies against Some Neurotropic Viruses Determined in Human Sera in Albania. J. Hyg. Epidemiol. Microbiol. Immunol. 1959, 3, 277–282. [Google Scholar]
- Murgue, B.; Murri, S.; Triki, H.; Deubel, V.; Zeller, H.G. West Nile in the Mediterranean Basin: 1950-2000. Ann. N. Y. Acad. Sci. 2001, 951, 117–126. [Google Scholar] [CrossRef]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile Encephalitis Epidemic in Southeastern Romania. Lancet Lond. Engl. 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile Virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Platonov, A.E.; Shipulin, G.A.; Shipulina, O.Y.; Tyutyunnik, E.N.; Frolochkina, T.I.; Lanciotti, R.S.; Yazyshina, S.; Platonova, O.V.; Obukhov, I.L.; Zhukov, A.N.; et al. Outbreak of West Nile Virus Infection, Volgograd Region, Russia, 1999. Emerg. Infect. Dis. 2001, 7, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. The Outbreak of West Nile Virus Infection in the New York City Area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Metzkor-Cotter, E.; Martin, D.A.; Siegman-Igra, Y.; Korczyn, A.D.; Rosso, R.; Berger, S.A.; Campbell, G.L.; Lanciotti, R.S. West Nile Encephalitis in Israel, 1999: The New York Connection. Emerg. Infect. Dis. 2001, 7, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Ivanics, É.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 Strains of Encephalitic West Nile Virus, Central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Papa, A.; Bakonyi, T.; Xanthopoulou, K.; Vázquez, A.; Tenorio, A.; Nowotny, N. Genetic Characterization of West Nile Virus Lineage 2, Greece, 2010. Emerg. Infect. Dis. 2011, 17, 920–922. [Google Scholar] [CrossRef]
- D’Amore, C.; Grimaldi, P.; Ascione, T.; Conti, V.; Sellitto, C.; Franci, G.; Kafil, S.H.; Pagliano, P. West Nile Virus Diffusion in Temperate Regions and Climate Change. A Systematic Review. Infez. Med. 2022, 31, 20–30. [Google Scholar] [CrossRef]
- Barrett, A.D.T. West Nile in Europe: An Increasing Public Health Problem. J. Travel Med. 2018, 25. [Google Scholar] [CrossRef]
- Walker, B.L.; Naugle, D.E.; Doherty, K.E.; Cornish, T.E. West Nile Virus and Greater Sage-Grouse: Estimating Infection Rate in a Wild Bird Population. Avian Dis. 2007, 51, 691–696. [Google Scholar] [CrossRef]
- Harrigan, R.J.; Thomassen, H.A.; Buermann, W.; Cummings, R.F.; Kahn, M.E.; Smith, T.B. Economic Conditions Predict Prevalence of West Nile Virus. PLoS ONE 2010, 5, e15437. [Google Scholar] [CrossRef]
- Semenza, J.C.; Tran, A.; Espinosa, L.; Sudre, B.; Domanovic, D.; Paz, S. Climate Change Projections of West Nile Virus Infections in Europe: Implications for Blood Safety Practices. Environ. Health Glob. Access Sci. Source 2016, 15 (Suppl. S1), 28. [Google Scholar] [CrossRef]
- Ziegler, U.; Lühken, R.; Keller, M.; Cadar, D.; van der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L.; et al. West Nile Virus Epizootic in Germany, 2018. Antiviral Res. 2019, 162, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; de Azevedo, T.S.; Chiaravalloti-Neto, F. Impact of Climate Change on West Nile Virus Distribution in South America. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-R.; Liu, T.; Gao, X.; Wang, H.-B.; Xiao, J.-H. Impact of Climate Change on the Global Circulation of West Nile Virus and Adaptation Responses: A Scoping Review. Infect. Dis. Poverty 2024, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; LaDeau, S.L.; Marra, P.P. Ecology of West Nile Virus Transmission and Its Impact on Birds in the Western Hemisphere. The Auk 2007, 124, 1121–1136. [Google Scholar] [CrossRef]
- Gómez, A.; Kilpatrick, A.M.; Kramer, L.D.; Dupuis, A.P.; Maffei, J.G.; Goetz, S.J.; Marra, P.P.; Daszak, P.; Aguirre, A.A. Land Use and West Nile Virus Seroprevalence in Wild Mammals. Emerg. Infect. Dis. 2008, 14, 962–965. [Google Scholar] [CrossRef]
- Mrzljak, A.; Dinjar-Kujundzic, P.; Santini, M.; Barbic, L.; Kosuta, I.; Savic, V.; Tabain, I.; Vilibic-Cavlek, T. West Nile Virus: An Emerging Threat in Transplant Population. Vector Borne Zoonotic Dis. Larchmt. N 2020, 20, 613–618. [Google Scholar] [CrossRef]
- Redazione West Nile: che uccelli selvatici monitorare per la sorveglianza del virus? Available online: https://www.izsvenezie.it/west-nile-uccelli-selvatici-monitorare-sorveglianza-virus/ (accessed on 1 March 2025).
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile Virus: Biology, Transmission, and Human Infection. Clin. Microbiol. Rev. 2012, 25, 635–648. [Google Scholar] [CrossRef]
- Koch, R.T.; Erazo, D.; Folly, A.J.; Johnson, N.; Dellicour, S.; Grubaugh, N.D.; Vogels, C.B.F. Genomic Epidemiology of West Nile Virus in Europe. One Health Amst. Neth. 2024, 18, 100664. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. BioMed Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef]
- David, S.; Abraham, A.M. Epidemiological and Clinical Aspects on West Nile Virus, a Globally Emerging Pathogen. Infect. Dis. Lond. Engl. 2016, 48, 571–586. [Google Scholar] [CrossRef]
- Durand, B.; Haskouri, H.; Lowenski, S.; Vachiery, N.; Beck, C.; Lecollinet, S. Seroprevalence of West Nile and Usutu Viruses in Military Working Horses and Dogs, Morocco, 2012: Dog as an Alternative WNV Sentinel Species? Epidemiol. Infect. 2016, 144, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M. Globalization, Land Use and the Invasion of West Nile Virus. Science 2011, 334, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.; Nappo, M.A.; Ferrari, L.; Di Lecce, R.; Guarnieri, C.; Cantoni, A.M.; Corradi, A. Nipah Virus Disease: Epidemiological, Clinical, Diagnostic and Legislative Aspects of This Unpredictable Emerging Zoonosis. Anim. Open Access J. MDPI 2022, 13, 159. [Google Scholar] [CrossRef]
- Nir, Y.; Beemer, A.; Goldwasser, R.A. West Nile Virus Infection in Mice Following Exposure to a Viral Aerosol. Br. J. Exp. Pathol. 1965, 46, 443–449. [Google Scholar]
- Palmieri, C.; Franca, M.; Uzal, F.; Anderson, M.; Barr, B.; Woods, L.; Moore, J.; Woolcock, P.; Shivaprasad, H.L. Pathology and Immunohistochemical Findings of West Nile Virus Infection in Psittaciformes. Vet. Pathol. 2011, 48, 975–984. [Google Scholar] [CrossRef]
- Fiacre, L.; Pagès, N.; Albina, E.; Richardson, J.; Lecollinet, S.; Gonzalez, G. Molecular Determinants of West Nile Virus Virulence and Pathogenesis in Vertebrate and Invertebrate Hosts. Int. J. Mol. Sci. 2020, 21, 9117. [Google Scholar] [CrossRef]
- Paré, J.; Moore, A. West Nile Virus in Horses—What Do You Need to Know to Diagnose the Disease? Can. Vet. J. Rev. Veterinaire Can. 2018, 59, 1119–1120. [Google Scholar]
- Sips, G.J.; Wilschut, J.; Smit, J.M. Neuroinvasive Flavivirus Infections. Rev. Med. Virol. 2012, 22, 69–87. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Barrett, A.D.T.; Deubel, V. The Japanese Encephalitis Serological Group of Flaviviruses: A Brief Introduction to the Group. Curr. Top. Microbiol. Immunol. 2002, 267, 1–10. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kim, C.Y.; Hwang, S.A.; Hassan, A.; Covington, E.; Heydari, K.; Lyerly, M.; Sejvar, J.J.; Hasbun, R.; Prasad, M.; et al. Clinical, Prognostic, and Longitudinal Functional and Neuropsychological Features of West Nile Virus Neuroinvasive Disease in the United States: A Systematic Review and Meta-Analysis. Ann. Neurol. 2025. [Google Scholar] [CrossRef]
- Ahlers, L.R.H.; Goodman, A.G. The Immune Responses of the Animal Hosts of West Nile Virus: A Comparison of Insects, Birds, and Mammals. Front. Cell. Infect. Microbiol. 2018, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Carson, P.J.; Borchardt, S.M.; Custer, B.; Prince, H.E.; Dunn-Williams, J.; Winkelman, V.; Tobler, L.; Biggerstaff, B.J.; Lanciotti, R.; Petersen, L.R.; et al. Neuroinvasive Disease and West Nile Virus Infection, North Dakota, USA, 1999–2008. Emerg. Infect. Dis. 2012, 18, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, N.P.; Staples, J.E.; Lehman, J.A.; Fischer, M. Medical Risk Factors for Severe West Nile Virus Disease, United States, 2008-2010. Am. J. Trop. Med. Hyg. 2012, 87, 179–184. [Google Scholar] [CrossRef]
- Gervais, A.; Rovida, F.; Avanzini, M.A.; Croce, S.; Marchal, A.; Lin, S.-C.; Ferrari, A.; Thorball, C.W.; Constant, O.; Le Voyer, T.; et al. Autoantibodies Neutralizing Type I IFNs Underlie West Nile Virus Encephalitis in ∼40% of Patients. J. Exp. Med. 2023, 220, e20230661. [Google Scholar] [CrossRef]
- Lin, S.-C.; Zhao, F.R.; Janova, H.; Gervais, A.; Rucknagel, S.; Murray, K.O.; Casanova, J.-L.; Diamond, M.S. Blockade of Interferon Signaling Decreases Gut Barrier Integrity and Promotes Severe West Nile Virus Disease. Nat. Commun. 2023, 14, 5973. [Google Scholar] [CrossRef]
- Chirico, F.; Magnavita, N. West Nile Virus Infection in Europe: Need for an Integration of Occupational Health Practice and Public Health Activities. Commentary. Ann. Ist. Super. Sanita 2019, 55, 3–5. [Google Scholar] [CrossRef]
- Nosrat, C.; Altamirano, J.; Anyamba, A.; Caldwell, J.M.; Damoah, R.; Mutuku, F.; Ndenga, B.; LaBeaud, A.D. Impact of Recent Climate Extremes on Mosquito-Borne Disease Transmission in Kenya. PLoS Negl. Trop. Dis. 2021, 15, e0009182. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; Al-Majali, A.M. West Nile Virus Infection in Horses in Jordan: Clinical Cases, Seroprevalence and Risk Factors. Transbound. Emerg. Dis. 2014, 61 (Suppl. S1), 1–6. [Google Scholar] [CrossRef]
- Kramer, L.D.; Ciota, A.T.; Kilpatrick, A.M. Introduction, Spread, and Establishment of West Nile Virus in the Americas. J. Med. Entomol. 2019, 56, 1448–1455. [Google Scholar] [CrossRef]
- Hadfield, J.; Brito, A.F.; Swetnam, D.M.; Vogels, C.B.F.; Tokarz, R.E.; Andersen, K.G.; Smith, R.C.; Bedford, T.; Grubaugh, N.D. Twenty Years of West Nile Virus Spread and Evolution in the Americas Visualized by Nextstrain. PLoS Pathog. 2019, 15, e1008042. [Google Scholar] [CrossRef]
- CDC Current Year Data (2024). Available online: https://www.cdc.gov/west-nile-virus/data-maps/current-year-data.html (accessed on 1 March 2025).
- Epidemiological Update: West Nile Virus Transmission Season in Europe, 2023. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2023-0 (accessed on 1 March 2025).
- Moirano, G.; Richiardi, L.; Calzolari, M.; Merletti, F.; Maule, M. Recent Rapid Changes in the Spatio-Temporal Distribution of West Nile Neuro-Invasive Disease in Italy. Zoonoses Public Health 2020, 67, 54–61. [Google Scholar] [CrossRef]
- Moirano, G.; Gasparrini, A.; Acquaotta, F.; Fratianni, S.; Merletti, F.; Maule, M.; Richiardi, L. West Nile Virus Infection in Northern Italy: Case-Crossover Study on the Short-Term Effect of Climatic Parameters. Environ. Res. 2018, 167, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. Int. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Bellone, R.; Failloux, A.-B. The Role of Temperature in Shaping Mosquito-Borne Viruses Transmission. Front. Microbiol. 2020, 11, 584846. [Google Scholar] [CrossRef]
- Giesen, C.; Herrador, Z.; Fernandez-Martinez, B.; Figuerola, J.; Gangoso, L.; Vazquez, A.; Gómez-Barroso, D. A Systematic Review of Environmental Factors Related to WNV Circulation in European and Mediterranean Countries. One Health Amst. Neth. 2023, 16, 100478. [Google Scholar] [CrossRef]
- Kunkel, K.E.; Novak, R.J.; Lampman, R.L.; Gu, W. Modeling the Impact of Variable Climatic Factors on the Crossover of Culex Restauns and Culex Pipiens (Diptera: culicidae), Vectors of West Nile Virus in Illinois. Am. J. Trop. Med. Hyg. 2006, 74, 168–173. [Google Scholar] [CrossRef]
- Jia, Y.; Moudy, R.M.; Dupuis, A.P.; Ngo, K.A.; Maffei, J.G.; Jerzak, G.V.S.; Franke, M.A.; Kauffman, E.B.; Kramer, L.D. Characterization of a Small Plaque Variant of West Nile Virus Isolated in New York in 2000. Virology 2007, 367, 339–347. [Google Scholar] [CrossRef]
- Watts, M.J.; Sarto I Monteys, V.; Mortyn, P.G.; Kotsila, P. The Rise of West Nile Virus in Southern and Southeastern Europe: A Spatial-Temporal Analysis Investigating the Combined Effects of Climate, Land Use and Economic Changes. One Health Amst. Neth. 2021, 13, 100315. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile Virus: Review of the Literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Sinigaglia, A.; Peta, E.; Riccetti, S.; Barzon, L. New Avenues for Therapeutic Discovery against West Nile Virus. Expert Opin. Drug Discov. 2020, 15, 333–348. [Google Scholar] [CrossRef]
- Wu, B.; Qi, Z.; Qian, X. Recent Advancements in Mosquito-Borne Flavivirus Vaccine Development. Viruses 2023, 15, 813. [Google Scholar] [CrossRef] [PubMed]
- Bellini, R.; Zeller, H.; Van Bortel, W. A Review of the Vector Management Methods to Prevent and Control Outbreaks of West Nile Virus Infection and the Challenge for Europe. Parasit. Vectors 2014, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.-C. Animal and Human Vaccines against West Nile Virus. Pathog. Basel Switz. 2020, 9, 1073. [Google Scholar] [CrossRef]
- Ulbert, S. West Nile Virus Vaccines—Current Situation and Future Directions. Hum. Vaccines Immunother. 2019, 15, 2337–2342. [Google Scholar] [CrossRef]
- Vogels, C.B.; Göertz, G.P.; Pijlman, G.P.; Koenraadt, C.J. Vector Competence of European Mosquitoes for West Nile Virus. Emerg. Microbes Infect. 2017, 6, e96. [Google Scholar] [CrossRef]
- Surveillance, Prevention and Control of West Nile Virus and Usutu Virus Infections in the EU/EEA | EFSA. Available online: https://www.efsa.europa.eu/en/supporting/pub/en-8242 (accessed on 1 March 2025).
- Calistri, P.; Giovannini, A.; Hubalek, Z.; Ionescu, A.; Monaco, F.; Savini, G.; Lelli, R. Epidemiology of West Nile in Europe and in the Mediterranean Basin. Open Virol. J. 2010, 4, 29. [Google Scholar] [CrossRef]
- Mencattelli, G.; Ndione, M.H.D.; Rosà, R.; Marini, G.; Diagne, C.T.; Diagne, M.M.; Fall, G.; Faye, O.; Diallo, M.; Faye, O.; et al. Epidemiology of West Nile Virus in Africa: An Underestimated Threat. PLoS Negl. Trop. Dis. 2022, 16, e0010075. [Google Scholar] [CrossRef]
- Paz, S.; Semenza, J.C. Environmental Drivers of West Nile Fever Epidemiology in Europe and Western Asia—A Review. Int. J. Environ. Res. Public. Health 2013, 10, 3543. [Google Scholar] [CrossRef]
- Simonin, Y. Circulation of West Nile Virus and Usutu Virus in Europe: Overview and Challenges. Viruses 2024, 16, 599. [Google Scholar] [CrossRef]
- Carrasco, L.; Utrilla, M.J.; Fuentes-Romero, B.; Fernandez-Novo, A.; Martin-Maldonado, B. West Nile Virus: An Update Focusing on Southern Europe. Microorganisms 2024, 12, 2623. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Williams, D.T. The Zoonotic Flaviviruses of Southern, South-Eastern and Eastern Asia, and Australasia: The Potential for Emergent Viruses. Zoonoses Public Health 2009, 56, 338–356. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, A.; Jimenez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The Challenge of West Nile Virus in Europe: Knowledge Gaps and Research Priorities. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2015, 20, 21135. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Sanchez-Seco, M.P.; Ruiz, S.; Molero, F.; Hernandez, L.; Moreno, J.; Magallanes, A.; Tejedor, C.G.; Tenorio, A. Putative New Lineage of West Nile Virus, Spain. Emerg. Infect. Dis. 2010, 16, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Capelli, G.; Monaco, F.; Polci, A.; Russo, F.; Di Gennaro, A.; Marini, V.; Teodori, L.; Montarsi, F.; Pinoni, C.; et al. Evidence of West Nile Virus Lineage 2 Circulation in Northern Italy. Vet. Microbiol. 2012, 158, 267–273. [Google Scholar] [CrossRef]
- Klenk, K.; Snow, J.; Morgan, K.; Bowen, R.; Stephens, M.; Foster, F.; Gordy, P.; Beckett, S.; Komar, N.; Gubler, D.; et al. Alligators as West Nile Virus Amplifiers. Emerg. Infect. Dis. 2004, 10, 2150–2155. [Google Scholar] [CrossRef]
- Smith, D.L.; Battle, K.E.; Hay, S.I.; Barker, C.M.; Scott, T.W.; McKenzie, F.E. Ross, Macdonald, and a Theory for the Dynamics and Control of Mosquito-Transmitted Pathogens. PLoS Pathog. 2012, 8, e1002588. [Google Scholar] [CrossRef]
- Girard, Y.A.; Popov, V.; Wen, J.; Han, V.; Higgs, S. Ultrastructural Study of West Nile Virus Pathogenesis in Culex Pipiens Quinquefasciatus (Diptera: culicidae). J. Med. Entomol. 2005, 42, 429–444. [Google Scholar] [CrossRef]
- Dahl, E.; Öborn, L.; Sjöberg, V.; Lundkvist, Å.; Hesson, J.C. Vertical Transmission of Sindbis Virus in Culex Mosquitoes. Viruses 2022, 14, 1915. [Google Scholar] [CrossRef]
- O’Leary, D.R.; Marfin, A.A.; Montgomery, S.P.; Kipp, A.M.; Lehman, J.A.; Biggerstaff, B.J.; Elko, V.L.; Collins, P.D.; Jones, J.E.; Campbell, G.L. The Epidemic of West Nile Virus in the United States, 2002. Vector Borne Zoonotic Dis. Larchmt. N 2004, 4, 61–70. [Google Scholar] [CrossRef]
- Flores, F.S.; Zanluca, C.; Guglielmone, A.A.; dos Santos, C.N.D.; Labruna, M.B.; Diaz, A. Vector Competence for West Nile Virus and St. Louis Encephalitis Virus (Flavivirus) of Three Tick Species of the Genus Amblyomma (Acari: ixodidae). Am. J. Trop. Med. Hyg. 2019, 100, 1230–1235. [Google Scholar] [CrossRef]
- Anderson, J.F.; Main, A.J.; Andreadis, T.G.; Wikel, S.K.; Vossbrinck, C.R. Transstadial Transfer of West Nile Virus by Three Species of Ixodid Ticks (Acari: ixodidae). J. Med. Entomol. 2003, 40, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Răileanu, C.; Tauchmann, O.; Vasić, A.; Neumann, U.; Tews, B.A.; Silaghi, C. Transstadial Transmission and Replication Kinetics of West Nile Virus Lineage 1 in Laboratory Reared Ixodes Ricinus Ticks. Pathogens 2020, 9, 780. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.M.; Ceianu, C.; Nicolescu, G.; Karabatsos, N.; Lanciotti, R.; Vladimirescu, A.; Laiv, L.; Ungureanu, A.; Romanca, C.; Tsai, T.F. Entomologic and Avian Investigations of an Epidemic of West Nile Fever in Romania in 1996, with Serologic and Molecular Characterization of a Virus Isolate from Mosquitoes. Am. J. Trop. Med. Hyg. 1999, 61, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Sudre, B.; Paz, S.; Rossi, M.; Desbrosse, A.; Chevalier, V.; Semenza, J.C. Environmental Predictors of West Nile Fever Risk in Europe. Int. J. Health Geogr. 2014, 13, 26. [Google Scholar] [CrossRef]
- Ferraccioli, F.; Riccetti, N.; Fasano, A.; Mourelatos, S.; Kioutsioukis, I.; Stilianakis, N.I. Effects of Climatic and Environmental Factors on Mosquito Population Inferred from West Nile Virus Surveillance in Greece. Sci. Rep. 2023, 13, 18803. [Google Scholar] [CrossRef]
- Factsheet about West Nile Virus Infection. Available online: https://www.ecdc.europa.eu/en/west-nile-fever/facts (accessed on 1 March 2025).
- Austin, R.J.; Whiting, T.L.; Anderson, R.A.; Drebot, M.A. An Outbreak of West Nile Virus-Associated Disease in Domestic Geese (Anser anser domesticus) upon Initial Introduction to a Geographic Region, with Evidence of Bird to Bird Transmission. Can. Vet. J. 2004, 45, 117. [Google Scholar]
- Constant, O.; Bollore, K.; Clé, M.; Barthelemy, J.; Foulongne, V.; Chenet, B.; Gomis, D.; Virolle, L.; Gutierrez, S.; Desmetz, C.; et al. Evidence of Exposure to USUV and WNV in Zoo Animals in France. Pathog. Basel Switz. 2020, 9, 1005. [Google Scholar] [CrossRef]
- Kvapil, P.; Račnik, J.; Kastelic, M.; Bártová, E.; Korva, M.; Jelovšek, M.; Avšič-Županc, T. A Sentinel Serological Study in Selected Zoo Animals to Assess Early Detection of West Nile and Usutu Virus Circulation in Slovenia. Viruses 2021, 13, 626. [Google Scholar] [CrossRef]
- Ma, K.; An, A.; Vp, B.; Ze, G. [Experimental Evidence for Infection of Culex Pipiens L. Mosquitoes by West Nile Fever Virus from Rana Ridibunda Pallas and Its Transmission by Bites]. Med. Parazitol. 1986, 76–78. [Google Scholar]
- Saiz, J.-C.; Martín-Acebes, M.A.; Blázquez, A.B.; Escribano-Romero, E.; Poderoso, T.; Jiménez de Oya, N. Pathogenicity and Virulence of West Nile Virus Revisited Eight Decades after Its First Isolation. Virulence 2021, 12, 1145–1173. [Google Scholar] [CrossRef]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Jacobson, E.R.; Ginn, P.E.; Troutman, J.M.; Farina, L.; Stark, L.; Klenk, K.; Burkhalter, K.L.; Komar, N. West Nile Virus Infection in Farmed American Alligators (Alligator mississippiensis) in Florida. J. Wildl. Dis. 2005, 41, 96–106. [Google Scholar] [CrossRef] [PubMed]
- DeCarlo, C.; Omar, A.H.; Haroun, M.I.; Bigler, L.; Bin Rais, M.N.; Abu, J.; Omar, A.R.; Mohammed, H.O. Potential Reservoir and Associated Factors for West Nile Virus in Three Distinct Climatological Zones. Vector-Borne Zoonotic Dis. 2017, 17, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Root, J.J.; Bentler, K.T.; Nemeth, N.M.; Gidlewski, T.; Spraker, T.R.; Franklin, A.B. Experimental Infection of Raccoons (Procyon lotor) with West Nile Virus. Am. J. Trop. Med. Hyg. 2010, 83, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Bischof, R.; Rogers, D.G. Serologic Survey of Select Infectious Diseases in Coyotes and Raccoons in Nebraska. J. Wildl. Dis. 2005, 41, 787–791. [Google Scholar] [CrossRef]
- Blitvich, B.J.; Juarez, L.I.; Tucker, B.J.; Rowley, W.A.; Platt, K.B. Antibodies to West Nile Virus in Raccoons and Other Wild Peridomestic Mammals in Iowa. J. Wildl. Dis. 2009, 45, 1163–1168. [Google Scholar] [CrossRef]
- St. Leger, J.; Wu, G.; Anderson, M.; Dalton, L.; Nilson, E.; Wang, D. West Nile Virus Infection in Killer Whale, Texas, USA, 2007. Emerg. Infect. Dis. 2011, 17, 1531–1533. [Google Scholar] [CrossRef]
- Byas, A.D.; Ebel, G.D. Comparative Pathology of West Nile Virus in Humans and Non-Human Animals. Pathogens 2020, 9, 48. [Google Scholar] [CrossRef]
- Hussmann, K.L.; Samuel, M.A.; Kim, K.S.; Diamond, M.S.; Fredericksen, B.L. Differential Replication of Pathogenic and Nonpathogenic Strains of West Nile Virus within Astrocytes. J. Virol. 2013, 87, 2814–2822. [Google Scholar] [CrossRef]
- Styer, L.M.; Lim, P.-Y.; Louie, K.L.; Albright, R.G.; Kramer, L.D.; Bernard, K.A. Mosquito Saliva Causes Enhancement of West Nile Virus Infection in Mice. J. Virol. 2011, 85, 1517–1527. [Google Scholar] [CrossRef]
- Zimmerman, M.G.; Bowen, J.R.; McDonald, C.E.; Pulendran, B.; Suthar, M.S. West Nile Virus Infection Blocks Inflammatory Response and T Cell Costimulatory Capacity of Human Monocyte-Derived Dendritic Cells. J. Virol. 2019, 93, e00664-19. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.-Y.; Behr, M.J.; Chadwick, C.M.; Shi, P.-Y.; Bernard, K.A. Keratinocytes Are Cell Targets of West Nile Virus in Vivo. J. Virol. 2011, 85, 5197–5201. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.J.; Osvath, S.R.; Hall, R.A.; King, N.J.C.; Sedger, L.M. Regulation of Antigen Processing and Presentation Molecules in West Nile Virus-Infected Human Skin Fibroblasts. Virology 2004, 324, 286–296. [Google Scholar] [CrossRef]
- Schneider, B.S.; Soong, L.; Girard, Y.A.; Campbell, G.; Mason, P.; Higgs, S. Potentiation of West Nile Encephalitis by Mosquito Feeding. Viral Immunol. 2006, 19, 74–82. [Google Scholar] [CrossRef]
- Garcia, M.; Alout, H.; Diop, F.; Damour, A.; Bengue, M.; Weill, M.; Missé, D.; Lévêque, N.; Bodet, C. Innate Immune Response of Primary Human Keratinocytes to West Nile Virus Infection and Its Modulation by Mosquito Saliva. Front. Cell. Infect. Microbiol. 2018, 8, 387. [Google Scholar] [CrossRef]
- Appler, K.K.; Brown, A.N.; Stewart, B.S.; Behr, M.J.; Demarest, V.L.; Wong, S.J.; Bernard, K.A. Persistence of West Nile Virus in the Central Nervous System and Periphery of Mice. PloS One 2010, 5, e10649. [Google Scholar] [CrossRef]
- Lim, P.-Y.; Louie, K.L.; Styer, L.M.; Shi, P.-Y.; Bernard, K.A. Viral Pathogenesis in Mice Is Similar for West Nile Virus Derived from Mosquito and Mammalian Cells. Virology 2010, 400, 93–103. [Google Scholar] [CrossRef]
- Ci, Y.; Shi, L. Compartmentalized Replication Organelle of Flavivirus at the ER and the Factors Involved. Cell. Mol. Life Sci. CMLS 2021, 78, 4939–4954. [Google Scholar] [CrossRef]
- Martin, M.-F.; Nisole, S. West Nile Virus Restriction in Mosquito and Human Cells: A Virus under Confinement. Vaccines 2020, 8, 256. [Google Scholar] [CrossRef]
- Bai, F.; Thompson, E.A.; Vig, P.J.S.; Leis, A.A. Current Understanding of West Nile Virus Clinical Manifestations, Immune Responses, Neuroinvasion, and Immunotherapeutic Implications. Pathogens 2019, 8, 193. [Google Scholar] [CrossRef]
- Saxena, V.; Xie, G.; Li, B.; Farris, T.; Welte, T.; Gong, B.; Boor, P.; Wu, P.; Tang, S.-J.; Tesh, R.; et al. A Hamster-Derived West Nile Virus Isolate Induces Persistent Renal Infection in Mice. PLoS Negl. Trop. Dis. 2013, 7, e2275. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.A.; Morrey, J.D.; Diamond, M.S. Caspase 3-Dependent Cell Death of Neurons Contributes to the Pathogenesis of West Nile Virus Encephalitis. J. Virol. 2007, 81, 2614–2623. [Google Scholar] [CrossRef]
- Shrestha, B.; Gottlieb, D.; Diamond, M.S. Infection and Injury of Neurons by West Nile Encephalitis Virus. J. Virol. 2003, 77, 13203–13213. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; Blázquez, A.-B.; Saiz, J.-C. Reconciling West Nile Virus with the Autophagic Pathway. Autophagy 2015, 11, 861–864. [Google Scholar] [CrossRef]
- Agliani, G.; Giglia, G.; Marshall, E.M.; Gröne, A.; Rockx, B.H.G.; van den Brand, J.M.A. Pathological Features of West Nile and Usutu Virus Natural Infections in Wild and Domestic Animals and in Humans: A Comparative Review. One Health Amst. Neth. 2023, 16, 100525. [Google Scholar] [CrossRef]
- Lim, S.M.; van den Ham, H.-J.; Oduber, M.; Martina, E.; Zaaraoui-Boutahar, F.; Roose, J.M.; van IJcken, W.F.J.; Osterhaus, A.D.M.E.; Andeweg, A.C.; Koraka, P.; et al. Transcriptomic Analyses Reveal Differential Gene Expression of Immune and Cell Death Pathways in the Brains of Mice Infected with West Nile Virus and Chikungunya Virus. Front. Microbiol. 2017, 8, 1556. [Google Scholar] [CrossRef]
- Diniz, J.A.P.; Da Rosa, A.P.A.T.; Guzman, H.; Xu, F.; Xiao, S.-Y.; Popov, V.L.; Vasconcelos, P.F.C.; Tesh, R.B. West Nile Virus Infection of Primary Mouse Neuronal and Neuroglial Cells: The Role of Astrocytes in Chronic Infection. Am. J. Trop. Med. Hyg. 2006, 75, 691–696. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J. West Nile Fever--a Reemerging Mosquito-Borne Viral Disease in Europe. Emerg. Infect. Dis. 1999, 5, 643–650. [Google Scholar] [CrossRef]
- Bunning, M.L.; Bowen, R.A.; Cropp, C.B.; Sullivan, K.G.; Davis, B.S.; Komar, N.; Godsey, M.S.; Baker, D.; Hettler, D.L.; Holmes, D.A.; et al. Experimental Infection of Horses with West Nile Virus. Emerg. Infect. Dis. 2002, 8, 380–386. [Google Scholar] [CrossRef]
- Zou, S.; Foster, G.A.; Dodd, R.Y.; Petersen, L.R.; Stramer, S.L. West Nile Fever Characteristics among Viremic Persons Identified through Blood Donor Screening. J. Infect. Dis. 2010, 202, 1354–1361. [Google Scholar] [CrossRef]
- Busch, M.P.; Wright, D.J.; Custer, B.; Tobler, L.H.; Stramer, S.L.; Kleinman, S.H.; Prince, H.E.; Bianco, C.; Foster, G.; Petersen, L.R.; et al. West Nile Virus Infections Projected from Blood Donor Screening Data, United States, 2003. Emerg. Infect. Dis. 2006, 12, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Center for Food Security and Public Health, Iowa State University, College of Veterinary Medicine, Ames, Iowa, USA. West Nile Fever. 2003. Available online: https://www.nj.gov/agriculture/divisions/ah/diseases/westnile.html (accessed on 1 March 2025).
- Jiménez de Oya, N.; Escribano-Romero, E.; Blázquez, A.-B.; Martín-Acebes, M.A.; Saiz, J.-C. Current Progress of Avian Vaccines Against West Nile Virus. Vaccines 2019, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.; Tillman, G.; Kraut, M.A.; Chiang, H.-S.; Strain, J.F.; Li, Y.; Agrawal, A.G.; Jester, P.; Gnann, J.W.; Whitley, R.J.; et al. West Nile Virus Neuroinvasive Disease: Neurological Manifestations and Prospective Longitudinal Outcomes. BMC Infect. Dis. 2014, 14, 248. [Google Scholar] [CrossRef]
- Rousseau, A.; Haigh, O.; Ksiaa, I.; Khairallah, M.; Labetoulle, M. Ocular Manifestations of West Nile Virus. Vaccines 2020, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Nakawah, M.O. West Nile Virus Encephalomyelitis in an Immunocompromised Patient. Radiol. Case Rep. 2023, 18, 4499–4506. [Google Scholar] [CrossRef]
- Leblond, A.; Hendrikx, P.; Sabatier, P. West Nile Virus Outbreak Detection Using Syndromic Monitoring in Horses. Vector Borne Zoonotic Dis. Larchmt. N 2007, 7, 403–410. [Google Scholar] [CrossRef]
- Snook, C.S.; Hyman, S.S.; Del Piero, F.; Palmer, J.E.; Ostlund, E.N.; Barr, B.S.; Desrochers, A.M.; Reilly, L.K. West Nile Virus Encephalomyelitis in Eight Horses. J. Am. Vet. Med. Assoc. 2001, 218, 1576–1579. [Google Scholar] [CrossRef]
- Porter, M.B.; Long, M.T.; Getman, L.M.; Giguère, S.; MacKay, R.J.; Lester, G.D.; Alleman, A.R.; Wamsley, H.L.; Franklin, R.P.; Jacks, S.; et al. West Nile Virus Encephalomyelitis in Horses: 46 Cases (2001). J. Am. Vet. Med. Assoc. 2003, 222, 1241–1247. [Google Scholar] [CrossRef]
- Kutasi, O.; Bakonyi, T.; Lecollinet, S.; Biksi, I.; Ferenczi, E.; Bahuon, C.; Sardi, S.; Zientara, S.; Szenci, O. Equine Encephalomyelitis Outbreak Caused by a Genetic Lineage 2 West Nile Virus in Hungary. J. Vet. Intern. Med. 2011, 25, 586–591. [Google Scholar] [CrossRef]
- Salazar, P.; Traub-Dargatz, J.L.; Morley, P.S.; Wilmot, D.D.; Steffen, D.J.; Cunningham, W.E.; Salman, M.D. Outcome of Equids with Clinical Signs of West Nile Virus Infection and Factors Associated with Death. J. Am. Vet. Med. Assoc. 2004, 225, 267–274. [Google Scholar] [CrossRef]
- Fulton, C.D.M.; Beasley, D.W.C.; Bente, D.A.; Dineley, K.T. Long-Term, West Nile Virus-Induced Neurological Changes: A Comparison of Patients and Rodent Models. Brain Behav. Immun. Health 2020, 7, 100105. [Google Scholar] [CrossRef] [PubMed]
- Fehér, O.E.; Fehérvári, P.; Tolnai, C.H.; Forgách, P.; Malik, P.; Jerzsele, Á.; Wagenhoffer, Z.; Szenci, O.; Korbacska-Kutasi, O. Epidemiology and Clinical Manifestation of West Nile Virus Infections of Equines in Hungary, 2007-2020. Viruses 2022, 14, 2551. [Google Scholar] [CrossRef] [PubMed]
- Loeb, M. Genetic Susceptibility to West Nile Virus and Dengue. Public Health Genomics 2013, 16, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Naveed, A.; Eertink, L.G.; Wang, D.; Li, F. Lessons Learned from West Nile Virus Infection:Vaccinations in Equines and Their Implications for One Health Approaches. Viruses 2024, 16, 781. [Google Scholar] [CrossRef]
- Stejskalova, K.; Cvanova, M.; Oppelt, J.; Janova, E.; Horecky, C.; Horecka, E.; Knoll, A.; Leblond, A.; Horin, P. Genetic Susceptibility to West Nile Virus Infection in Camargue Horses. Res. Vet. Sci. 2019, 124, 284–292. [Google Scholar] [CrossRef]
- Woods, M.W.; Kelly, J.N.; Hattlmann, C.J.; Tong, J.G.K.; Xu, L.S.; Coleman, M.D.; Quest, G.R.; Smiley, J.R.; Barr, S.D. Human HERC5 Restricts an Early Stage of HIV-1 Assembly by a Mechanism Correlating with the ISGylation of Gag. Retrovirology 2011, 8, 95. [Google Scholar] [CrossRef]
- Tang, Y.; Zhong, G.; Zhu, L.; Liu, X.; Shan, Y.; Feng, H.; Bu, Z.; Chen, H.; Wang, C. Herc5 Attenuates Influenza A Virus by Catalyzing ISGylation of Viral NS1 Protein. J. Immunol. Baltim. Md 1950 2010, 184, 5777–5790. [Google Scholar] [CrossRef]
- Loeb, M.; Eskandarian, S.; Rupp, M.; Fishman, N.; Gasink, L.; Patterson, J.; Bramson, J.; Hudson, T.J.; Lemire, M. Genetic Variants and Susceptibility to Neurological Complications Following West Nile Virus Infection. J. Infect. Dis. 2011, 204, 1031–1037. [Google Scholar] [CrossRef]
- Pérez-Ramírez, E.; Llorente, F.; Jiménez-Clavero, M.Á. Experimental Infections of Wild Birds with West Nile Virus. Viruses 2014, 6, 752–781. [Google Scholar] [CrossRef]
- Diamond, M.S.; Sitati, E.M.; Friend, L.D.; Higgs, S.; Shrestha, B.; Engle, M. A Critical Role for Induced IgM in the Protection against West Nile Virus Infection. J. Exp. Med. 2003, 198, 1853–1862. [Google Scholar] [CrossRef]
- Diamond, M.S.; Shrestha, B.; Marri, A.; Mahan, D.; Engle, M. B Cells and Antibody Play Critical Roles in the Immediate Defense of Disseminated Infection by West Nile Encephalitis Virus. J. Virol. 2003, 77, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Gamino, V.; Höfle, U. Pathology and Tissue Tropism of Natural West Nile Virus Infection in Birds: A Review. Vet. Res. 2013, 44, 39. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, R.G.; Kang, S.; Simões, M.L.; Angleró-Rodríguez, Y.I.; Dimopoulos, G. Mosquito Gut Antiparasitic and Antiviral Immunity. Dev. Comp. Immunol. 2016, 64, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Prince, B.C.; Walsh, E.; Torres, T.Z.B.; Rückert, C. Recognition of Arboviruses by the Mosquito Immune System. Biomolecules 2023, 13, 1159. [Google Scholar] [CrossRef]
- Samuel, G.H.; Adelman, Z.N.; Myles, K.M. Antiviral Immunity and Virus-Mediated Antagonism in Disease Vector Mosquitoes. Trends Microbiol. 2018, 26, 447–461. [Google Scholar] [CrossRef]
- Suthar, M.S.; Diamond, M.S.; Gale, M. West Nile Virus Infection and Immunity. Nat. Rev. Microbiol. 2013, 11, 115–128. [Google Scholar] [CrossRef]
- Anderson, C.; Baha, H.; Boghdeh, N.; Barrera, M.; Alem, F.; Narayanan, A. Interactions of Equine Viruses with the Host Kinase Machinery and Implications for One Health and Human Disease. Viruses 2023, 15, 1163. [Google Scholar] [CrossRef]
- Khatibzadeh, S.M.; Gold, C.B.; Keggan, A.E.; Perkins, G.A.; Glaser, A.L.; Dubovi, E.J.; Wagner, B. West Nile Virus-Specific Immunoglobulin Isotype Responses in Vaccinated and Infected Horses. Am. J. Vet. Res. 2015, 76, 92–100. [Google Scholar] [CrossRef]
- Trobaugh, D.; Green, S. Of Mice and Men: Protective and Pathogenic Immune Responses to West Nile Virus Infection. Curr. Trop. Med. Rep. 2015, 2, 41–48. [Google Scholar] [CrossRef]
- Qian, F.; Goel, G.; Meng, H.; Wang, X.; You, F.; Devine, L.; Raddassi, K.; Garcia, M.N.; Murray, K.O.; Bolen, C.R.; et al. Systems Immunology Reveals Markers of Susceptibility to West Nile Virus Infection. Clin. Vaccine Immunol. CVI 2015, 22, 6–16. [Google Scholar] [CrossRef]
- Lee, H.-J.; Zhao, Y.; Fleming, I.; Mehta, S.; Wang, X.; Wyk, B.V.; Ronca, S.E.; Kang, H.; Chou, C.-H.; Fatou, B.; et al. Early Cellular and Molecular Signatures Correlate with Severity of West Nile Virus Infection. iScience 2023, 26, 108387. [Google Scholar] [CrossRef] [PubMed]
- Behari, J.; Yadav, K.; Khare, P.; Kumar, B.; Kushwaha, A.K. Recent Insights on Pattern Recognition Receptors and the Interplay of Innate Immune Responses against West Nile Virus Infection. Virology 2024, 600, 110267. [Google Scholar] [CrossRef] [PubMed]
- Harioudh, M.K.; Perez, J.; Chong, Z.; Nair, S.; So, L.; McCormick, K.D.; Ghosh, A.; Shao, L.; Srivastava, R.; Soveg, F.; et al. Oligoadenylate Synthetase 1 Displays Dual Antiviral Mechanisms in Driving Translational Shutdown and Protecting Interferon Production. Immunity 2024, 57, 446–461.e7. [Google Scholar] [CrossRef]
- Kovats, S.; Turner, S.; Simmons, A.; Powe, T.; Chakravarty, E.; Alberola-Ila, J. West Nile Virus-Infected Human Dendritic Cells Fail to Fully Activate Invariant Natural Killer T Cells. Clin. Exp. Immunol. 2016, 186, 214–226. [Google Scholar] [CrossRef]
- Slonchak, A.; Clarke, B.; Mackenzie, J.; Amarilla, A.A.; Setoh, Y.X.; Khromykh, A.A. West Nile Virus Infection and Interferon Alpha Treatment Alter the Spectrum and the Levels of Coding and Noncoding Host RNAs Secreted in Extracellular Vesicles. BMC Genomics 2019, 20, 474. [Google Scholar] [CrossRef]
- Martínez-Rojas, P.P.; Monroy-Martínez, V.; Ruiz-Ordaz, B.H. Role of Extracellular Vesicles in the Pathogenesis of Mosquito-Borne Flaviviruses That Impact Public Health. J. Biomed. Sci. 2025, 32, 4. [Google Scholar] [CrossRef]
- Pavesi, A.; Tiecco, G.; Rossi, L.; Sforza, A.; Ciccarone, A.; Compostella, F.; Lovatti, S.; Tomasoni, L.R.; Castelli, F.; Quiros-Roldan, E. Inflammatory Response Associated with West Nile Neuroinvasive Disease: A Systematic Review. Viruses 2024, 16, 383. [Google Scholar] [CrossRef]
- Cunha, B.A.; Minnaganti, V.; Johnson, D.H.; Klein, N.C. Profound and Prolonged Lymphocytopenia with West Nile Encephalitis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 31, 1116–1117. [Google Scholar] [CrossRef]
- Chowers, M.Y.; Lang, R.; Nassar, F.; Ben-David, D.; Giladi, M.; Rubinshtein, E.; Itzhaki, A.; Mishal, J.; Siegman-Igra, Y.; Kitzes, R.; et al. Clinical Characteristics of the West Nile Fever Outbreak, Israel, 2000. Emerg. Infect. Dis. 2001, 7, 675–678. [Google Scholar] [CrossRef]
- Weiss, D.; Carr, D.; Kellachan, J.; Tan, C.; Phillips, M.; Bresnitz, E.; Layton, M. West Nile Virus Outbreak Response Working Group Clinical Findings of West Nile Virus Infection in Hospitalized Patients, New York and New Jersey, 2000. Emerg. Infect. Dis. 2001, 7, 654–658. [Google Scholar] [CrossRef]
- Burton, J.M.; Kern, R.Z.; Halliday, W.; Mikulis, D.; Brunton, J.; Fearon, M.; Pepperell, C.; Jaigobin, C. Neurological Manifestations of West Nile Virus Infection. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2004, 31, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J.; Marfin, A.A. Manifestations of West Nile Neuroinvasive Disease. Rev. Med. Virol. 2006, 16, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J.; Haddad, M.B.; Tierney, B.C.; Campbell, G.L.; Marfin, A.A.; Van Gerpen, J.A.; Fleischauer, A.; Leis, A.A.; Stokic, D.S.; Petersen, L.R. Neurologic Manifestations and Outcome of West Nile Virus Infection. JAMA 2003, 290, 511–515. [Google Scholar] [CrossRef]
- Giordano, D.; Draves, K.E.; Young, L.B.; Roe, K.; Bryan, M.A.; Dresch, C.; Richner, J.M.; Diamond, M.S.; Gale, M.; Clark, E.A. Protection of Mice Deficient in Mature B Cells from West Nile Virus Infection by Passive and Active Immunization. PLoS Pathog. 2017, 13, e1006743. [Google Scholar] [CrossRef]
- Silverman, R.H. Viral Encounters with 2′,5′-Oligoadenylate Synthetase and RNase L during the Interferon Antiviral Response. J. Virol. 2007, 81, 12720–12729. [Google Scholar] [CrossRef]
- Tyler, K.L.; Pape, J.; Goody, R.J.; Corkill, M.; Kleinschmidt-DeMasters, B.K. CSF Findings in 250 Patients with Serologically Confirmed West Nile Virus Meningitis and Encephalitis. Neurology 2006, 66, 361–365. [Google Scholar] [CrossRef]
- Jaijakul, S.; Salazar, L.; Wootton, S.H.; Aguilera, E.; Hasbun, R. The Clinical Significance of Neutrophilic Pleocytosis in Cerebrospinal Fluid in Patients with Viral Central Nervous System Infections. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2017, 59, 77–81. [Google Scholar] [CrossRef]
- Moreno-Reina, C.; Martínez-Moya, M.; Piñero-González de la Peña, P.; Caro-Domínguez, P. Neuroinvasive Disease Due to West Nile Virus: Clinical and Imaging Findings Associated with a Re-Emerging Pathogen. Radiologia 2022, 64, 473–483. [Google Scholar] [CrossRef]
- Li, J.; Loeb, J.A.; Shy, M.E.; Shah, A.K.; Tselis, A.C.; Kupski, W.J.; Lewis, R.A. Asymmetric Flaccid Paralysis: A Neuromuscular Presentation of West Nile Virus Infection. Ann. Neurol. 2003, 53, 703–710. [Google Scholar] [CrossRef]
- Jeha, L.E.; Sila, C.A.; Lederman, R.J.; Prayson, R.A.; Isada, C.M.; Gordon, S.M. West Nile Virus Infection: A New Acute Paralytic Illness. Neurology 2003, 61, 55–59. [Google Scholar] [CrossRef]
- Brilla, R.; Block, M.; Geremia, G.; Wichter, M. Clinical and Neuroradiologic Features of 39 Consecutive Cases of West Nile Virus Meningoencephalitis. J. Neurol. Sci. 2004, 220, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Safriel, Y.; Sohi, J.; Llave, A.; Weathers, S. West Nile Virus Infection: MR Imaging Findings in the Nervous System. AJNR Am. J. Neuroradiol. 2005, 26, 289–297. [Google Scholar] [PubMed]
- Petropoulou, K.A.; Gordon, S.M.; Prayson, R.A.; Ruggierri, P.M. West Nile Virus Meningoencephalitis: MR Imaging Findings. AJNR Am. J. Neuroradiol. 2005, 26, 1986–1995. [Google Scholar] [PubMed]
- Rodriguez, A.J.; Westmoreland, B.F. Electroencephalographic Characteristics of Patients Infected with West Nile Virus. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2007, 24, 386–389. [Google Scholar] [CrossRef]
- Gandelman-Marton, R.; Kimiagar, I.; Itzhaki, A.; Klein, C.; Theitler, J.; Rabey, J.M. Electroencephalography Findings in Adult Patients with West Nile Virus-Associated Meningitis and Meningoencephalitis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2003, 37, 1573–1578. [Google Scholar] [CrossRef]
- Sejvar, J.J. Clinical Manifestations and Outcomes of West Nile Virus Infection. Viruses 2014, 6, 606–623. [Google Scholar] [CrossRef]
- Bielefeldt-Ohmann, H.; Bosco-Lauth, A.; Hartwig, A.-E.; Uddin, M.J.; Barcelon, J.; Suen, W.W.; Wang, W.; Hall, R.A.; Bowen, R.A. Characterization of Non-Lethal West Nile Virus (WNV) Infection in Horses: Subclinical Pathology and Innate Immune Response. Microb. Pathog. 2017, 103, 71–79. [Google Scholar] [CrossRef]
- Delcambre, G.H.; Liu, J.; Streit, W.J.; Shaw, G.P.J.; Vallario, K.; Herrington, J.; Wenzlow, N.; Barr, K.L.; Long, M.T. Phenotypic Characterisation of Cell Populations in the Brains of Horses Experimentally Infected with West Nile Virus. Equine Vet. J. 2017, 49, 815–820. [Google Scholar] [CrossRef]
- Weese, J.S.; Baird, J.D.; DeLay, J.; Kenney, D.G.; Staempfli, H.R.; Viel, L.; Parent, J.; Smith-Maxie, L.; Poma, R. West Nile Virus Encephalomyelitis in Horses in Ontario: 28 Cases. Can. Vet. J. 2003, 44, 469–473. [Google Scholar]
- Schwarz, E.R.; Long, M.T. Comparison of West Nile Virus Disease in Humans and Horses: Exploiting Similarities for Enhancing Syndromic Surveillance. Viruses 2023, 15, 1230. [Google Scholar] [CrossRef]
- Bai, F.; Kong, K.-F.; Dai, J.; Qian, F.; Zhang, L.; Brown, C.R.; Fikrig, E.; Montgometry, R.R. A Paradoxical Role for Neutrophils in the Pathogenesis of West Nile Virus. J. Infect. Dis. 2010, 202, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Bertram, F.-M.; Thompson, P.N.; Venter, M. Epidemiology and Clinical Presentation of West Nile Virus Infection in Horses in South Africa, 2016-2017. Pathog. Basel Switz. 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Feyer, S.; Bartenschlager, F.; Bertram, C.A.; Ziegler, U.; Fast, C.; Klopfleisch, R.; Müller, K. Clinical, Pathological and Virological Aspects of Fatal West Nile Virus Infections in Ten Free-Ranging Goshawks (Accipiter gentilis) in Germany. Transbound. Emerg. Dis. 2021, 68, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; Griem, J.; Gummery, A.; Marsh, L.; Defres, S.; Bhojak, M.; Das, K.; Easton, A.; Solomon, T.; Kopelman, M.; et al. Neuropsychological and Psychiatric Outcomes in Encephalitis: A Multi-Centre Case-Control Study. PloS One 2020, 15, e0230436. [Google Scholar] [CrossRef]
- Eckerström, M.; Nilsson, S.; Zetterberg, H.; Blennow, K.; Grahn, A. Cognitive Impairment without Altered Levels of Cerebrospinal Fluid Biomarkers in Patients with Encephalitis Caused by Varicella-Zoster Virus: A Pilot Study. Sci. Rep. 2020, 10, 22400. [Google Scholar] [CrossRef]
- Petrić, D. Dengue Virus Disease from Origin to Outbreak; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Hills, S.L.; Netravathi, M.; Solomon, T. Japanese Encephalitis among Adults: A Review. Am. J. Trop. Med. Hyg. 2023, 108, 860–864. [Google Scholar] [CrossRef]
- Sarkari, N.B.S.; Thacker, A.K.; Barthwal, S.P.; Mishra, V.K.; Prapann, S.; Srivastava, D.; Sarkari, M. Japanese Encephalitis (JE) Part II: 14 Years’ Follow-up of Survivors. J. Neurol. 2012, 259, 58–69. [Google Scholar] [CrossRef]
- Srichawla, B.S.; Manan, M.R.; Kipkorir, V.; Dhali, A.; Diebel, S.; Sawant, T.; Zia, S.; Carrion-Alvarez, D.; Suteja, R.C.; Nurani, K.; et al. Neuroinvasion of Emerging and Re-Emerging Arboviruses: A Scoping Review. SAGE Open Med. 2024, 12, 20503121241229847. [Google Scholar] [CrossRef]
- Frasca, F.; Sorrentino, L.; Fracella, M.; D’Auria, A.; Coratti, E.; Maddaloni, L.; Bugani, G.; Gentile, M.; Pierangeli, A.; d’Ettorre, G.; et al. An Update on the Entomology, Virology, Pathogenesis, and Epidemiology Status of West Nile and Dengue Viruses in Europe (2018–2023). Trop. Med. Infect. Dis. 2024, 9, 166. [Google Scholar] [CrossRef]
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile Virus in Europe: Emergence, Epidemiology, Diagnosis, Treatment, and Prevention. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2013, 19, 699–704. [Google Scholar] [CrossRef]
- Girl, P.; Euringer, K.; Coroian, M.; Mihalca, A.D.; Borde, J.P.; Dobler, G. Comparison of Five Serological Methods for the Detection of West Nile Virus Antibodies. Viruses 2024, 16, 788. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, A.; Lorusso, A.; Casaccia, C.; Conte, A.; Monaco, F.; Savini, G. Serum Neutralization Assay Can Efficiently Replace Plaque Reduction Neutralization Test for Detection and Quantitation of West Nile Virus Antibodies in Human and Animal Serum Samples. Clin. Vaccine Immunol. CVI 2014, 21, 1460–1462. [Google Scholar] [CrossRef] [PubMed]
- Vista, F.E.S.; Tantengco, O.A.G.; Dispo, M.D.; Opiso, D.M.S.; Badua, C.L.D.C.; Gerardo, J.P.Z.; Perez, J.R.M.; Baldo, K.A.T.; Chao, D.-Y.; Dalmacio, L.M.M. Trends in ELISA-Based Flavivirus IgG Serosurveys: A Systematic Review. Trop. Med. Infect. Dis. 2023, 8, 224. [Google Scholar] [CrossRef]
- Dauphin, G.; Zientara, S. West Nile Virus: Recent Trends in Diagnosis and Vaccine Development. Vaccine 2007, 25, 5563–5576. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Laboratory Biosafety Manual, 4th Edition. Available online: https://www.who.int/publications/i/item/9789240011311 (accessed on 1 March 2025).
- Eloit, M. (Ed.) West Nile Fever, Chapter 3 1.26. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 13th ed.; Organisation for Animal Health: Paris, France, 2024. [Google Scholar]
- Sewgobind, S.; McCracken, F.; Schilling, M. JMM Profile: West Nile Virus. J. Med. Microbiol. 2023, 72. [Google Scholar] [CrossRef]
- Ronca, S.E.; Ruff, J.C.; Murray, K.O. A 20-Year Historical Review of West Nile Virus since Its Initial Emergence in North America: Has West Nile Virus Become a Neglected Tropical Disease? PLoS Negl. Trop. Dis. 2021, 15, e0009190. [Google Scholar] [CrossRef]
- Agamanolis, D.P.; Leslie, M.J.; Caveny, E.A.; Guarner, J.; Shieh, W.-J.; Zaki, S.R. Neuropathological Findings in West Nile Virus Encephalitis: A Case Report. Ann. Neurol. 2003, 54, 547–551. [Google Scholar] [CrossRef]
- Kelley, T.W.; Prayson, R.A.; Ruiz, A.I.; Isada, C.M.; Gordon, S.M. The Neuropathology of West Nile Virus Meningoencephalitis. A Report of Two Cases and Review of the Literature. Am. J. Clin. Pathol. 2003, 119, 749–753. [Google Scholar] [CrossRef]
- Bouffard, J.P.; Riudavets, M.A.; Holman, R.; Rushing, E.J. Neuropathology of the Brain and Spinal Cord in Human West Nile Virus Infection. Clin. Neuropathol. 2004, 23, 59–61. [Google Scholar]
- Shieh, W.J.; Guarner, J.; Layton, M.; Fine, A.; Miller, J.; Nash, D.; Campbell, G.L.; Roehrig, J.T.; Gubler, D.J.; Zaki, S.R. The Role of Pathology in an Investigation of an Outbreak of West Nile Encephalitis in New York, 1999. Emerg. Infect. Dis. 2000, 6, 370–372. [Google Scholar] [CrossRef]
- Burt, F.J.; Grobbelaar, A.A.; Leman, P.A.; Anthony, F.S.; Gibson, G.V.F.; Swanepoel, R. Phylogenetic Relationships of Southern African West Nile Virus Isolates. Emerg. Infect. Dis. 2002, 8, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Ølberg, R.-A.; Barker, I.K.; Crawshaw, G.J.; Bertelsen, M.F.; Drebot, M.A.; Andonova, M. West Nile Virus Encephalitis in a Barbary Macaque (Macaca sylvanus). Emerg. Infect. Dis. 2004, 10, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.N.; Cameron, A.I.; Morales, P.R.; Burnside, W.M. West Nile Virus Seroprevalence in an Outdoor Nonhuman Primate Breeding Colony in South Florida. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2021, 60, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ratterree, M.S.; da Rosa, A.P.A.T.; Bohm, R.P.; Cogswell, F.B.; Phillippi, K.M.; Caillouet, K.; Schwanberger, S.; Shope, R.E.; Tesh, R.B. West Nile Virus Infection in Nonhuman Primate Breeding Colony, Concurrent with Human Epidemic, Southern Louisiana. Emerg. Infect. Dis. 2003, 9, 1388–1394. [Google Scholar] [CrossRef]
- Verstrepen, B.E.; Fagrouch, Z.; van Heteren, M.; Buitendijk, H.; Haaksma, T.; Beenhakker, N.; Palù, G.; Richner, J.M.; Diamond, M.S.; Bogers, W.M.; et al. Experimental Infection of Rhesus Macaques and Common Marmosets with a European Strain of West Nile Virus. PLoS Negl. Trop. Dis. 2014, 8, e2797. [Google Scholar] [CrossRef]
- Chaves, A.; Piche-Ovares, M.; Ibarra-Cerdeña, C.N.; Corrales-Aguilar, E.; Suzán, G.; Moreira-Soto, A.; Gutiérrez-Espeleta, G.A. Serosurvey of Nonhuman Primates in Costa Rica at the Human-Wildlife Interface Reveals High Exposure to Flaviviruses. Insects 2021, 12, 554. [Google Scholar] [CrossRef]
- Rodhain, F.; Clerc, Y.; Albignac, R.; Ricklin, B.; Ranaivosata, J.; Coulanges, P. Arboviruses and Lemurs in Madagascar: A Preliminary Note. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 227–231. [Google Scholar] [CrossRef]
- Sondgeroth, K.; Blitvich, B.; Blair, C.; Terwee, J.; Junge, R.; Sauther, M.; VandeWoude, S. Assessing Flavivirus, Lentivirus, and Herpesvirus Exposure in Free-Ranging Ring-Tailed Lemurs in Southwestern Madagascar. J. Wildl. Dis. 2007, 43, 40–47. [Google Scholar] [CrossRef]
- Rodhain, F.; Petter, J.J.; Albignac, R.; Coulanges, P.; Hannoun, C. Arboviruses and Lemurs in Madagascar: Experimental Infection of Lemur Fulvus with Yellow Fever and West Nile Viruses. Am. J. Trop. Med. Hyg. 1985, 34, 816–822. [Google Scholar] [CrossRef]
- Morales, M.A.; Barrandeguy, M.; Fabbri, C.; Garcia, J.B.; Vissani, A.; Trono, K.; Gutierrez, G.; Pigretti, S.; Menchaca, H.; Garrido, N.; et al. West Nile Virus Isolation from Equines in Argentina, 2006. Emerg. Infect. Dis. 2006, 12, 1559–1561. [Google Scholar] [CrossRef]
- Ward, M.P.; Schuermann, J.A.; Highfield, L.D.; Murray, K.O. Characteristics of an Outbreak of West Nile Virus Encephalomyelitis in a Previously Uninfected Population of Horses. Vet. Microbiol. 2006, 118, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Venter, M.; Pretorius, M.; Fuller, J.A.; Botha, E.; Rakgotho, M.; Stivaktas, V.; Weyer, C.; Romito, M.; Williams, J. West Nile Virus Lineage 2 in Horses and Other Animals with Neurologic Disease, South Africa, 2008–2015. Emerg. Infect. Dis. 2017, 23, 2060–2064. [Google Scholar] [CrossRef]
- Cantile, C.; Del Piero, F.; Di Guardo, G.; Arispici, M. Pathologic and Immunohistochemical Findings in Naturally Occuring West Nile Virus Infection in Horses. Vet. Pathol. 2001, 38, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Tber, A.A. West Nile Fever in Horses in Morocco. Bull OIE 1996, 108, 867–869. [Google Scholar]
- Toplu, N.; Oğuzoğlu, T.Ç.; Ural, K.; Albayrak, H.; Ozan, E.; Ertürk, A.; Epikmen, E.T. West Nile Virus Infection in Horses: Detection by Immunohistochemistry, In Situ Hybridization, and ELISA. Vet. Pathol. 2015, 52, 1073–1076. [Google Scholar] [CrossRef]
- Venter, M.; Steyl, J.; Human, S.; Weyer, J.; Zaayman, D.; Blumberg, L.; Leman, P.A.; Paweska, J.; Swanepoel, R. Transmission of West Nile Virus during Horse Autopsy. Emerg. Infect. Dis. 2010, 16, 573–575. [Google Scholar] [CrossRef]
- Dunkel, B.; Del Piero, F.; Wotman, K.L.; Johns, I.C.; Beech, J.; Wilkins, P.A. Encephalomyelitis from West Nile Flavivirus in 3 Alpacas. J. Vet. Intern. Med. 2004, 18, 365–367. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Bildfell, R.J.; Gardner-Graff, K.K.; Baker, R.J.; Delay, J.P.; Mattson, D.E. West Nile Virus Infection in Two Alpacas. J. Am. Vet. Med. Assoc. 2004, 225, 921–924, 880. [Google Scholar] [CrossRef]
- Palmer, M.V.; Stoffregen, W.C.; Rogers, D.G.; Hamir, A.N.; Richt, J.A.; Pedersen, D.D.; Waters, W.R. West Nile Virus Infection in Reindeer (Rangifer tarandus). J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc 2004, 16, 219–222. [Google Scholar] [CrossRef]
- West Nile Virus and Kunjin Virus Disease. Available online: https://www.health.vic.gov.au/infectious-diseases/west-nile-virus-and-west-nile-virus-kunjin-disease (accessed on 1 March 2025).
- Spradbrow, P.B.; Clark, L. Experimental Infection of Calves with a Group B Arbovirus (Kunjin virus). Aust. Vet. J. 1966, 42, 65–69. [Google Scholar] [CrossRef]
- Rimoldi, G.; Mete, A.; Adaska, J.M.; Anderson, M.L.; Symmes, K.P.; Diab, S. West Nile Virus Infection in Sheep. Vet. Pathol. 2017, 54, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Kecskeméti, S.; Bajmócy, E.; Bacsadi, A.; Kiss, I.; Bakonyi, T. Encephalitis Due to West Nile Virus in a Sheep. Vet. Rec. 2007, 161, 568–569. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, M.; Yoon, K.-J.; Schwartz, K.; Berkland, L. West Nile Virus Meningoencephalitis in a Suri Alpaca and Suffolk Ewe. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc 2004, 16, 64–66. [Google Scholar] [CrossRef]
- Tyler, J.W.; Turnquist, S.E.; David, A.T.; Kleiboeker, S.B.; Middleton, J.R. West Nile Virus Encephalomyelitis in a Sheep. J. Vet. Intern. Med. 2003, 17, 242–244. [Google Scholar] [CrossRef]
- Miller, D.L.; Radi, Z.A.; Baldwin, C.; Ingram, D. Fatal West Nile Virus Infection in a White-Tailed Deer (Odocoileus virginianus). J. Wildl. Dis. 2005, 41, 246–249. [Google Scholar] [CrossRef]
- Escribano-Romero, E.; Lupulović, D.; Merino-Ramos, T.; Blázquez, A.-B.; Lazić, G.; Lazić, S.; Saiz, J.-C.; Petrović, T. West Nile Virus Serosurveillance in Pigs, Wild Boars, and Roe Deer in Serbia. Vet. Microbiol. 2015, 176, 365–369. [Google Scholar] [CrossRef]
- Petruccelli, A.; Zottola, T.; Ferrara, G.; Iovane, V.; Di Russo, C.; Pagnini, U.; Montagnaro, S. West Nile Virus and Related Flavivirus in European Wild Boar (Sus scrofa), Latium Region, Italy: A Retrospective Study. Anim. Open Access J. MDPI 2020, 10, 494. [Google Scholar] [CrossRef]
- Gutiérrez-Guzmán, A.-V.; Vicente, J.; Sobrino, R.; Perez-Ramírez, E.; Llorente, F.; Höfle, U. Antibodies to West Nile Virus and Related Flaviviruses in Wild Boar, Red Foxes and Other Mesomammals from Spain. Vet. Microbiol. 2012, 159, 291–297. [Google Scholar] [CrossRef]
- Ilkal, M.A.; Prasanna, Y.; Jacob, P.G.; Geevarghese, G.; Banerjee, K. Experimental Studies on the Susceptibility of Domestic Pigs to West Nile Virus Followed by Japanese Encephalitis Virus Infection and Vice Versa. Acta Virol. 1994, 38, 157–161. [Google Scholar]
- Teehee, M.L.; Bunning, M.L.; Stevens, S.; Bowen, R.A. Experimental Infection of Pigs with West Nile Virus. Arch. Virol. 2005, 150, 1249–1256. [Google Scholar] [CrossRef]
- Sierra, E.; Sánchez, S.; Saliki, J.T.; Blas-Machado, U.; Arbelo, M.; Zucca, D.; Fernández, A. Retrospective Study of Etiologic Agents Associated with Nonsuppurative Meningoencephalitis in Stranded Cetaceans in the Canary Islands. J. Clin. Microbiol. 2014, 52, 2390–2397. [Google Scholar] [CrossRef]
- Del Piero, F.; Stremme, D.W.; Habecker, P.L.; Cantile, C. West Nile Flavivirus Polioencephalomyelitis in a Harbor Seal (Phoca vitulina). Vet. Pathol. 2006, 43, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Veterinary Information Network®, Inc.—VIN. Available online: https://www.vin.com/vin/ (accessed on 1 March 2025).
- Pilipski, J.D.; Pilipskl, L.M.; Risley, L.S. West Nile Virus Antibodies in Bats from New Jersey and New York. J. Wildl. Dis. 2004, 40, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Lanciotti, R.; Bowen, R.; Langevin, S.; Bunning, M. Detection of West Nile Virus in Oral and Cloacal Swabs Collected from Bird Carcasses. Emerg. Infect. Dis. 2002, 8, 741–742. [Google Scholar] [CrossRef]
- Lichtensteiger, C.A.; Heinz-Taheny, K.; Osborne, T.S.; Novak, R.J.; Lewis, B.A.; Firth, M.L. West Nile Virus Encephalitis and Myocarditis in Wolf and Dog. Emerg. Infect. Dis. 2003, 9, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Austgen, L.E.; Bowen, R.A.; Bunning, M.L.; Davis, B.S.; Mitchell, C.J.; Chang, G.-J.J. Experimental Infection of Cats and Dogs with West Nile Virus. Emerg. Infect. Dis. 2004, 10, 82–88. [Google Scholar] [CrossRef]
- Egberink, H.; Addie, D.D.; Boucraut-Baralon, C.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Horzinek, M.C.; Hosie, M.J.; Marsilio, F.; Lloret, A.; et al. West Nile Virus Infection in Cats: ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2015, 17, 617–619. [Google Scholar] [CrossRef]
- Madić, J.; Huber, D.; Lugović, B. Serologic Survey for Selected Viral and Rickettsial Agents of Brown Bears (Ursus arctos) in Croatia. J. Wildl. Dis. 1993, 29, 572–576. [Google Scholar] [CrossRef]
- Farajollahi, A.; Panella, N.A.; Carr, P.; Crans, W.; Burguess, K.; Komar, N. Serologic Evidence of West Nile Virus Infection in Black Bears (Ursus americanus) from New Jersey. J. Wildl. Dis. 2003, 39, 894–896. [Google Scholar] [CrossRef]
- West Nile Virus Exposure in Black Bears of Northeastern Wisconsin—KATZ—2007—The Journal of Wildlife Management—Wiley Online Library. Available online: https://wildlife.onlinelibrary.wiley.com/doi/10.2193/2005-708 (accessed on 1 March 2025).
- Vitásková, E.; Molnár, L.; Holko, I.; Supuka, P.; Černíková, L.; Bártová, E.; Sedlák, K. Serologic Survey of Selected Viral Pathogens in Free-Ranging Eurasian Brown Bears (Ursus arctos arctos) from Slovakia. J. Wildl. Dis. 2019, 55, 499–503. [Google Scholar] [CrossRef]
- Dutton, C.J.; Quinnell, M.; Lindsay, R.; DeLay, J.; Barker, I.K. Paraparesis in a Polar Bear (Ursus maritimus) Associated with West Nile Virus Infection. J. Zoo Wildl. Med. Off. Publ. Am. Assoc. Zoo Vet. 2009, 40, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Schuler, K.; Claymore, M.; Schnitzler, H.; Dubovi, E.; Rocke, T.; Perry, M.J.; Bowman, D.; Abbott, R.C. Sentinel coyote pathogen survey to assess declining black-footed ferret (Mustela nigripes) population in south dakota, usa. J. Wildl. Dis. 2021, 57, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Suen, W.W.; Uddin, M.J.; Wang, W.; Brown, V.; Adney, D.R.; Broad, N.; Prow, N.A.; Bowen, R.A.; Hall, R.A.; Bielefeldt-Ohmann, H. Experimental West Nile Virus Infection in Rabbits: An Alternative Model for Studying Induction of Disease and Virus Control. Pathog. Basel Switz. 2015, 4, 529–558. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.; Harmon, J.R.; Lash, R.R.; Weiss, S.; Langevin, S.; Savage, H.M.; Godsey, M.S.; Burkhalter, K.; Root, J.J.; Gidlewski, T.; et al. West Nile Virus Isolated from a Virginia Opossum (Didelphis virginiana) in Northwestern Missouri, USA, 2012. J. Wildl. Dis. 2014, 50, 976–978. [Google Scholar] [CrossRef]
- Lamglait, B.; Lair, S. Fatal West Nile Virus Infection in a Virginia Opossum (Didelphis virginiana) with Pulmonary Lepidic-Predominant Adenocarcinoma. J. Wildl. Dis. 2019, 55, 990–994. [Google Scholar] [CrossRef]
- Gasse, H. Nomina Anatómica Veterinaria. International Committee on Veterinary Gross Anatomic Nomenclature, 6th ed.; Chairman Committee: Hannover, Germany, 2017. [Google Scholar]
- Calle, P.P.; Ludwig, G.V.; Smith, J.F.; Raphael, B.L.; Clippinger, T.L.; Rush, E.M.; McNamara, T.; Manduca, R.; Linn, M.; Turell, M.J.; et al. Clinical Aspects of West Nile Virus Infection in a Zoological Collection. VIN.com 2015. [Google Scholar]
- Farfán-Ale, J.A.; Blitvich, B.J.; Marlenee, N.L.; Loroño-Pino, M.A.; Puerto-Manzano, F.; García-Rejón, J.E.; Rosado-Paredes, E.P.; Flores-Flores, L.F.; Ortega-Salazar, A.; Chávez-Medina, J.; et al. Antibodies to West Nile Virus in Asymptomatic Mammals, Birds, and Reptiles in the Yucatan Peninsula of Mexico. Am. J. Trop. Med. Hyg. 2006, 74, 908–914. [Google Scholar] [CrossRef]
- Puerto, F.I.; Hidalgo-Martinez, A.C.; Garcia-Rejon, J.E.; Lorono-Pino, M.A.; Farfan-Ale, J.A. Antibodies Against West Nile Virus in Zoo Animals and Employers From, Tabasco and Yucatan Mexico. Int. J. Infect. Dis. 2008, 12, e125. [Google Scholar] [CrossRef]
- Caballero-Gómez, J.; Cano-Terriza, D.; Lecollinet, S.; Carbonell, M.D.; Martínez-Valverde, R.; Martínez-Nevado, E.; García-Párraga, D.; Lowenski, S.; García-Bocanegra, I. Evidence of Exposure to Zoonotic Flaviviruses in Zoo Mammals in Spain and Their Potential Role as Sentinel Species. Vet. Microbiol. 2020, 247, 108763. [Google Scholar] [CrossRef]
- Tiawsirisup, S.; Blitvich, B.J.; Tucker, B.J.; Halbur, P.G.; Bartholomay, L.C.; Rowley, W.A.; Platt, K.B. Susceptibility of Fox Squirrels (Sciurus niger) to West Nile Virus by Oral Exposure. Vector Borne Zoonotic Dis. Larchmt. N 2010, 10, 207–209. [Google Scholar] [CrossRef]
- Kiupel, M.; Simmons, H.A.; Fitzgerald, S.D.; Wise, A.; Sikarskie, J.G.; Cooley, T.M.; Hollamby, S.R.; Maes, R. West Nile Virus Infection in Eastern Fox Squirrels (Sciurus niger). Vet. Pathol. 2003, 40, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Heinz-Taheny, K.M.; Andrews, J.J.; Kinsel, M.J.; Pessier, A.P.; Pinkerton, M.E.; Lemberger, K.Y.; Novak, R.J.; Dizikes, G.J.; Edwards, E.; Komar, N. West Nile Virus Infection in Free-Ranging Squirrels in Illinois. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc 2004, 16, 186–190. [Google Scholar] [CrossRef]
- Padgett, K.A.; Reisen, W.K.; Kahl-Purcell, N.; Fang, Y.; Cahoon-Young, B.; Carney, R.; Anderson, N.; Zucca, L.; Woods, L.; Husted, S.; et al. West Nile Virus Infection in Tree Squirrels (Rodentia: sciuridae) in California, 2004-2005. Am. J. Trop. Med. Hyg. 2007, 76, 810–813. [Google Scholar] [CrossRef]
- Root, J.J.; Hall, J.S.; McLean, R.G.; Marlenee, N.L.; Beaty, B.J.; Gansowski, J.; Clark, L. Serologic Evidence of Exposure of Wild Mammals to Flaviviruses in the Central and Eastern United States. Am. J. Trop. Med. Hyg. 2005, 72, 622–630. [Google Scholar] [CrossRef]
- Root, J.J.; Oesterle, P.T.; Sullivan, H.J.; Hall, J.S.; Marlenee, N.L.; McLean, R.G.; Montenieri, J.A.; Clark, L. Fox Squirrel (Sciurus niger) Associations with West Nile Virus. Am. J. Trop. Med. Hyg. 2007, 76, 782–784. [Google Scholar] [CrossRef]
- Root, J.J. West Nile Virus Associations in Wild Mammals: A Synthesis. Arch. Virol. 2013, 158, 735–752. [Google Scholar] [CrossRef]
- Root, J.J.; Oesterle, P.T.; Nemeth, N.M.; Klenk, K.; Gould, D.H.; McLean, R.G.; Clark, L.; Hall, J.S. Experimental Infection of Fox Squirrels (Sciurus niger) with West Nile Virus. Am. J. Trop. Med. Hyg. 2006, 75, 697–701. [Google Scholar] [CrossRef]
- Dietrich, G.; Montenieri, J.A.; Panella, N.A.; Langevin, S.; Lasater, S.E.; Klenk, K.; Kile, J.C.; Komar, N. Serologic Evidence of West Nile Virus Infection in Free-Ranging Mammals, Slidell, Louisiana, 2002. Vector Borne Zoonotic Dis. Larchmt. N 2005, 5, 288–292. [Google Scholar] [CrossRef]
- Garcia-Tapia, D.; Hassett, D.E.; Mitchell, W.J.; Johnson, G.C.; Kleiboeker, S.B. West Nile Virus Encephalitis: Sequential Histopathological and Immunological Events in a Murine Model of Infection. J. Neurovirol. 2007, 13, 130–138. [Google Scholar] [CrossRef]
- Klenk, K.; Komar, N. Poor Replication of West Nile Virus (New York 1999 Strain) in Three Reptilian and One Amphibian Species. Am. J. Trop. Med. Hyg. 2003, 69, 260–262. [Google Scholar] [CrossRef]
- Steinman, A.; Banet-Noach, C.; Simanov, L.; Grinfeld, N.; Aizenberg, Z.; Levi, O.; Lahav, D.; Malkinson, M.; Perk, S.; Shpigel, N.Y. Experimental Infection of Common Garter Snakes (Thamnophis sirtalis) with West Nile Virus. Vector Borne Zoonotic Dis. Larchmt. N 2006, 6, 361–368. [Google Scholar] [CrossRef]
- Reisen, W.K.; Brault, A.C.; Martinez, V.M.; Fang, Y.; Simmons, K.; Garcia, S.; Omi-Olsen, E.; Lane, R.S. Ability of Transstadially Infected Ixodes Pacificus (Acari: ixodidae) to Transmit West Nile Virus to Song Sparrows or Western Fence Lizards. J. Med. Entomol. 2007, 44, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.R.; Hughes, D.F.; Meshaka, W.E.; Coleman, C.; Henning, J.D. Wild Snakes Harbor West Nile Virus. One Health Amst. Neth. 2016, 2, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Marschang, R.E. Viruses Infecting Reptiles. Viruses 2011, 3, 2087–2126. [Google Scholar] [CrossRef] [PubMed]
- Simulundu, E.; Ndashe, K.; Chambaro, H.M.; Squarre, D.; Reilly, P.M.; Chitanga, S.; Changula, K.; Mukubesa, A.N.; Ndebe, J.; Tembo, J.; et al. West Nile Virus in Farmed Crocodiles, Zambia, 2019. Emerg. Infect. Dis. 2020, 26, 811–814. [Google Scholar] [CrossRef]
- Isberg, S.R.; Moran, J.L.; De Araujo, R.; Elliott, N.; Davis, S.S.; Melville, L. First Evidence of Kunjin Strain of West Nile Virus Associated with Saltwater Crocodile (Crocodylus porosus) Skin Lesions. Aust. Vet. J. 2019, 97, 390–393. [Google Scholar] [CrossRef]
- Nevarez, J.G.; Mitchell, M.A.; Morgan, T.; Roy, A.; Johnson, A. Association of West Nile Virus with Lymphohistiocytic Proliferative Cutaneous Lesions in American Alligators (Alligator mississippiensis) Detected by RT-PCR. J. Zoo Wildl. Med. Off. Publ. Am. Assoc. Zoo Vet. 2008, 39, 562–566. [Google Scholar] [CrossRef]
- Giglia, G.; Mencattelli, G.; Lepri, E.; Agliani, G.; Gobbi, M.; Gröne, A.; van den Brand, J.M.A.; Savini, G.; Mandara, M.T. West Nile Virus and Usutu Virus: A Post-Mortem Monitoring Study in Wild Birds from Rescue Centers, Central Italy. Viruses 2022, 14, 1994. [Google Scholar] [CrossRef]
- Stockman, J.; Hawkins, M.G.; Burns, R.E.; Fang, Y.; Brault, A.C.; Lowenstine, L.J. West Nile Virus Infection in a Green-Winged Macaw (Ara chloropterus). Avian Dis. 2010, 54, 164–169. [Google Scholar] [CrossRef]
- Zhang, Z.; Wilson, F.; Read, R.; Pace, L.; Zhang, S. Detection and Characterization of Naturally Acquired West Nile Virus Infection in a Female Wild Turkey. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. Inc. 2006, 18, 204–208. [Google Scholar] [CrossRef]
- Nemeth, N.; Gould, D.; Bowen, R.; Komar, N. Natural and Experimental West Nile Virus Infection in Five Raptor Species. J. Wildl. Dis. 2006, 42, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.E.; Mead, D.G.; Allison, A.B.; Stallknecht, D.E.; Howerth, E.W. Pathology and Epidemiology of Natural West Nile Viral Infection of Raptors in Georgia. J. Wildl. Dis. 2007, 43, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Pauli, A.M.; Cruz-Martinez, L.A.; Ponder, J.B.; Redig, P.T.; Glaser, A.L.; Klauss, G.; Schoster, J.V.; Wünschmann, A. Ophthalmologic and Oculopathologic Findings in Red-Tailed Hawks and Cooper’s Hawks with Naturally Acquired West Nile Virus Infection. J. Am. Vet. Med. Assoc. 2007, 231, 1240–1248. [Google Scholar] [CrossRef]
- Study: West Nile Virus Cost Equine Industries in Colorado, Nebraska Millions in 2002|American Veterinary Medical Association. Available online: https://www.avma.org/javma-news/2003-06-15/study-west-nile-virus-cost-equine-industries-colorado-nebraska-millions-2002 (accessed on 1 March 2025).
- Zohrabian, A.; Meltzer, M.I.; Ratard, R.; Billah, K.; Molinari, N.A.; Roy, K.; Scott, R.D.; Petersen, L.R. West Nile Virus Economic Impact, Louisiana, 2002. Emerg. Infect. Dis. 2004, 10, 1736–1744. [Google Scholar] [CrossRef]
- Ndiva Mongoh, M.; Hearne, R.; Dyer, N.W.; Khaitsa, M.L. The Economic Impact of West Nile Virus Infection in Horses in the North Dakota Equine Industry in 2002. Trop. Anim. Health Prod. 2008, 40, 69–76. [Google Scholar] [CrossRef]
- Barber, L.M.; Schleier, J.J.; Peterson, R.K.D. Economic Cost Analysis of West Nile Virus Outbreak, Sacramento County, California, USA, 2005. Emerg. Infect. Dis. 2010, 16, 480–486. [Google Scholar] [CrossRef]
- Galvan, R.; Dean, A.; Rene, A.; Bae, S.; Singh, K.P. An Analytical Study of the Perceptions, Prevention Srategies, Treatment and Economic Impact of Equine West Nile Virus. Tex. Public Health Assoc. J. 2005, 57, 3. [Google Scholar]
- Kolimenakis, A.; Bithas, K.; Richardson, C.; Latinopoulos, D.; Baka, A.; Vakali, A.; Hadjichristodoulou, C.; Mourelatos, S.; Kalaitzopoulou, S.; Gewehr, S.; et al. Economic Appraisal of the Public Control and Prevention Strategy against the 2010 West Nile Virus Outbreak in Central Macedonia, Greece. Public Health 2016, 131, 63–70. [Google Scholar] [CrossRef]
- Humblet, M.-F.; Vandeputte, S.; Fecher-Bourgeois, F.; Léonard, P.; Gosset, C.; Balenghien, T.; Durand, B.; Saegerman, C. Estimating the Economic Impact of a Possible Equine and Human Epidemic of West Nile Virus Infection in Belgium. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2016, 21, 30309. [Google Scholar] [CrossRef]
- Paternoster, G.; Babo Martins, S.; Mattivi, A.; Cagarelli, R.; Angelini, P.; Bellini, R.; Santi, A.; Galletti, G.; Pupella, S.; Marano, G.; et al. Economics of One Health: Costs and Benefits of Integrated West Nile Virus Surveillance in Emilia-Romagna. PLoS ONE 2017, 12, e0188156. [Google Scholar] [CrossRef]
- Shing, E.; Wang, J.; Nelder, M.P.; Parpia, C.; Gubbay, J.B.; Loeb, M.; Kristjanson, E.; Marchand-Austin, A.; Moore, S.; Russell, C.; et al. The Direct Healthcare Costs Attributable to West Nile Virus Illness in Ontario, Canada: A Population-Based Cohort Study Using Laboratory and Health Administrative Data. BMC Infect. Dis. 2019, 19, 1059. [Google Scholar] [CrossRef] [PubMed]
- Chilakam, N.; Lakshminarayanan, V.; Keremutt, S.; Rajendran, A.; Thunga, G.; Poojari, P.G.; Rashid, M.; Mukherjee, N.; Bhattacharya, P.; John, D. Economic Burden of Mosquito-Borne Diseases in Low- and Middle-Income Countries: Protocol for a Systematic Review. JMIR Res. Protoc. 2023, 12, e50985. [Google Scholar] [CrossRef] [PubMed]
- Belova, A.; Mills, D.; Hall, R.; Juliana, A.S.; Crimmins, A.; Barker, C.; Jones, R. Impacts of Increasing Temperature on the Future Incidence of West Nile Neuroinvasive Disease in the United States. Am. J. Clim. Change 2017, 6, 166–216. [Google Scholar] [CrossRef]
- Erazo, D.; Grant, L.; Ghisbain, G.; Marini, G.; Colón-González, F.J.; Wint, W.; Rizzoli, A.; Van Bortel, W.; Vogels, C.B.F.; Grubaugh, N.D.; et al. Contribution of Climate Change to the Spatial Expansion of West Nile Virus in Europe. Nat. Commun. 2024, 15, 1196. [Google Scholar] [CrossRef]
- Ministero della Salute (Italian Ministry of Health). Linee Guida Applicative e Procedura per la Programmazione e lo Svolgimento delle Attività Veterinarie di Prevenzione Sorveglianza e Controllo dei Virus WEST NILE e USUTU (Application Guidelines and Procedure for Planning and Carrying out Veterinary Activities for the Prevention, Surveillance and Control of WEST NILE and USUTU Viruses). 2025. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=3515 (accessed on 1 March 2025).
- Istituto Superiore di Sanità (National Institute of Health, Italy)—EpiCentro. Epidemiology for Public Health. Available online: https://www.epicentro.iss.it/en (accessed on 1 March 2025).
- Istituto Superiore di Sanità (National Institute of Health, Italy)—EpiCentro. Epidemiology for Public Health. West Nile Fever. Surveillance of West Nile and Usutu Virus Infections in Humans. Summer–Autumn 2022. Available online: https://www.epicentro.iss.it/en/west-nile-fever/surveillance (accessed on 1 March 2025).
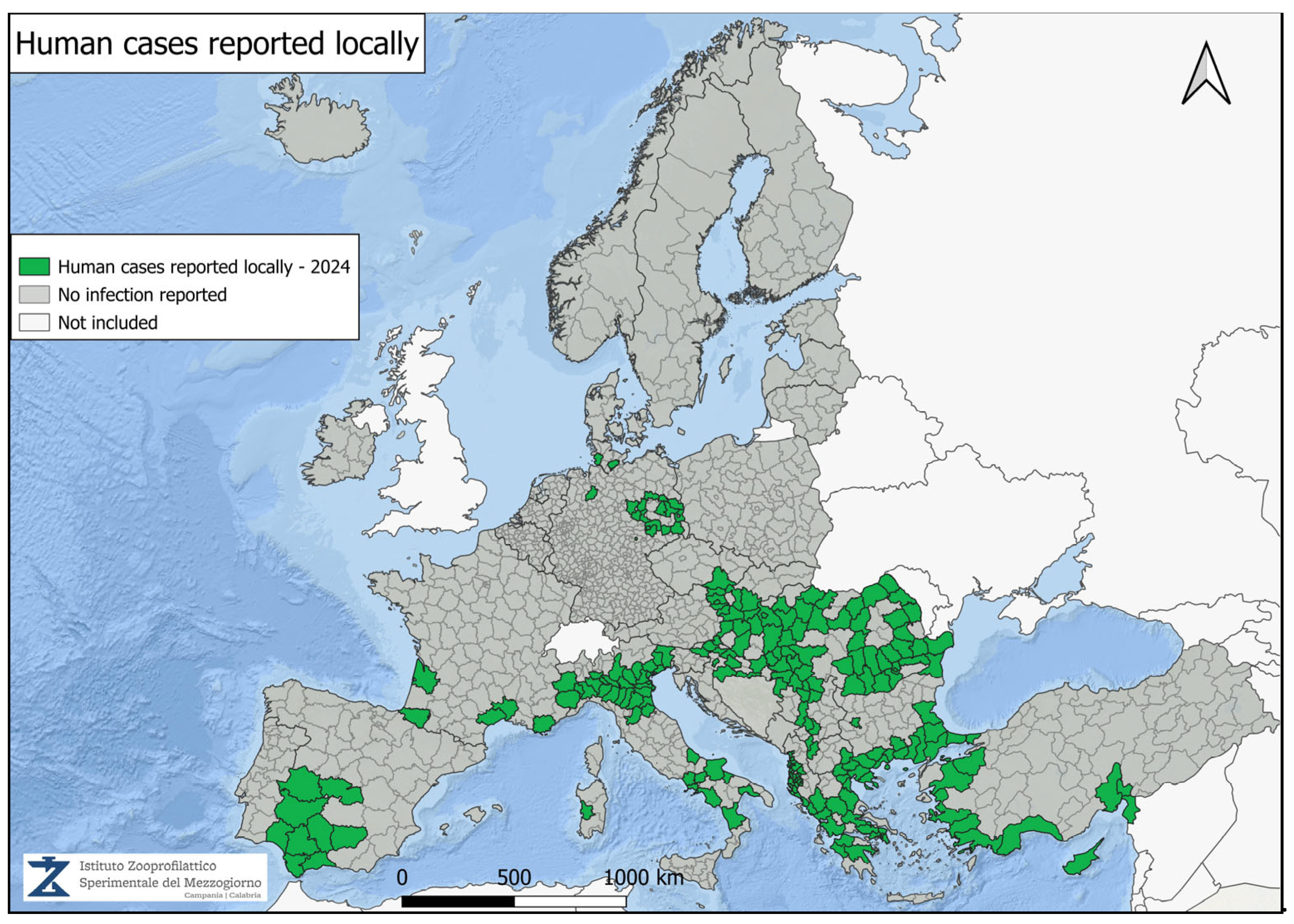
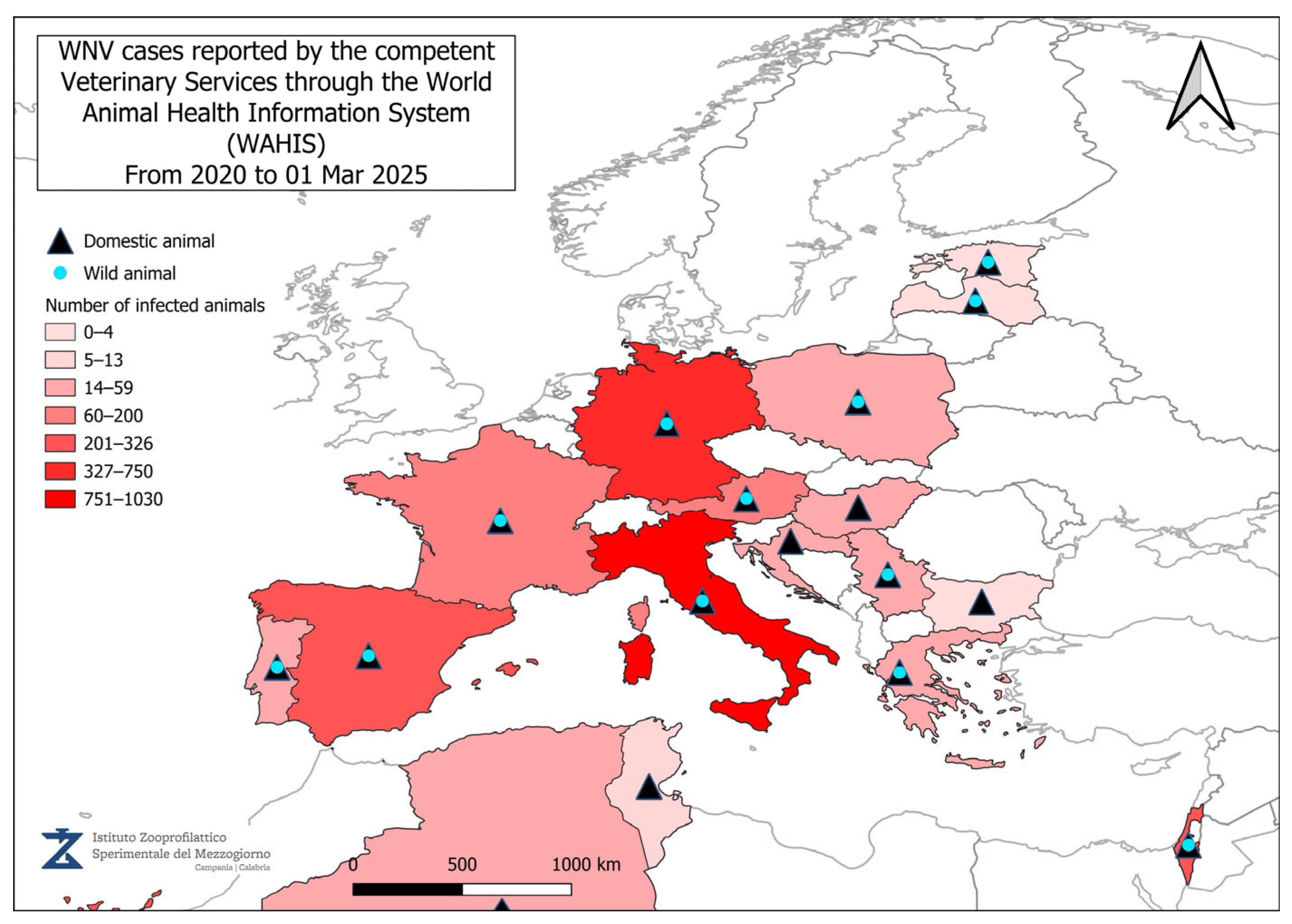
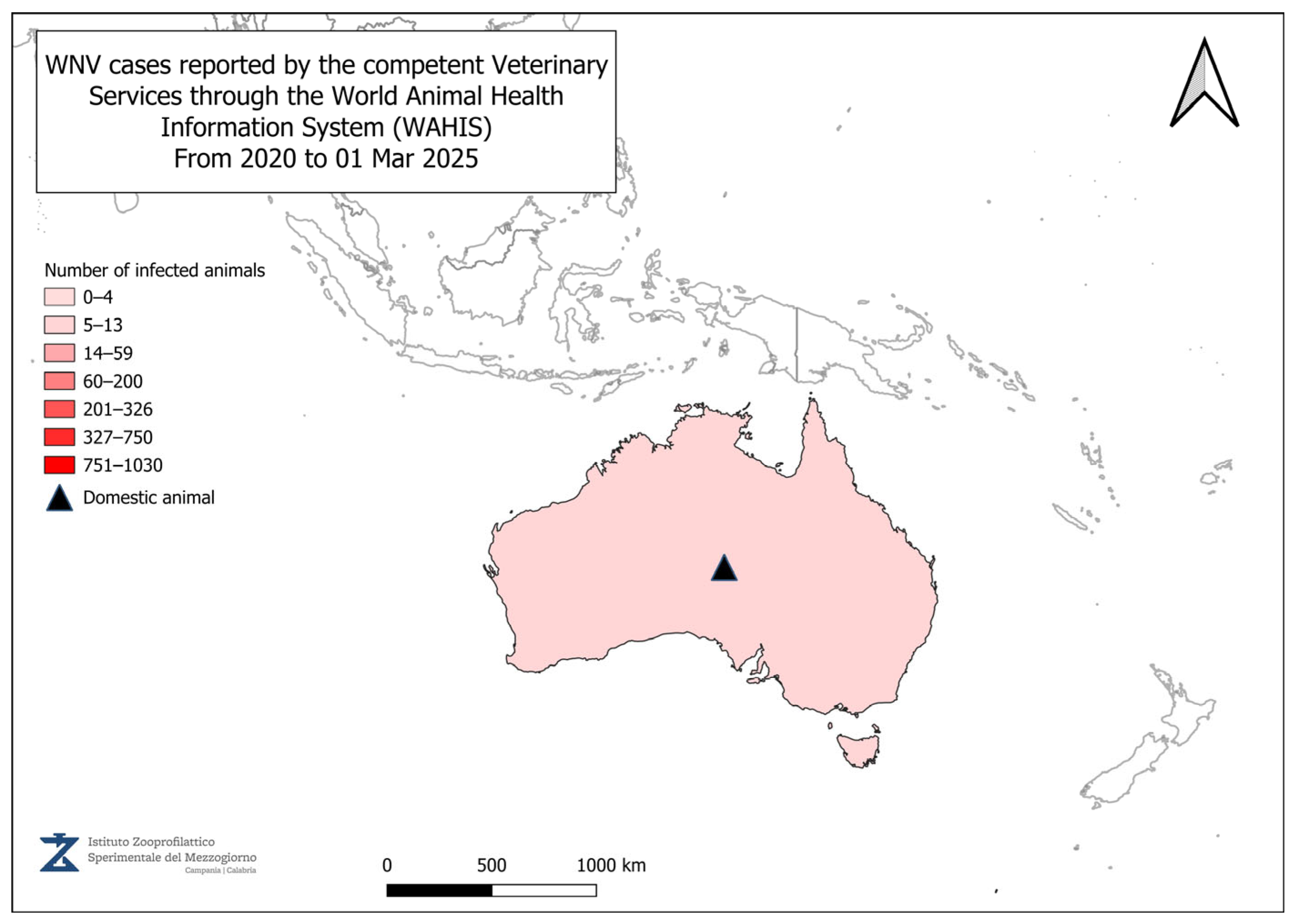

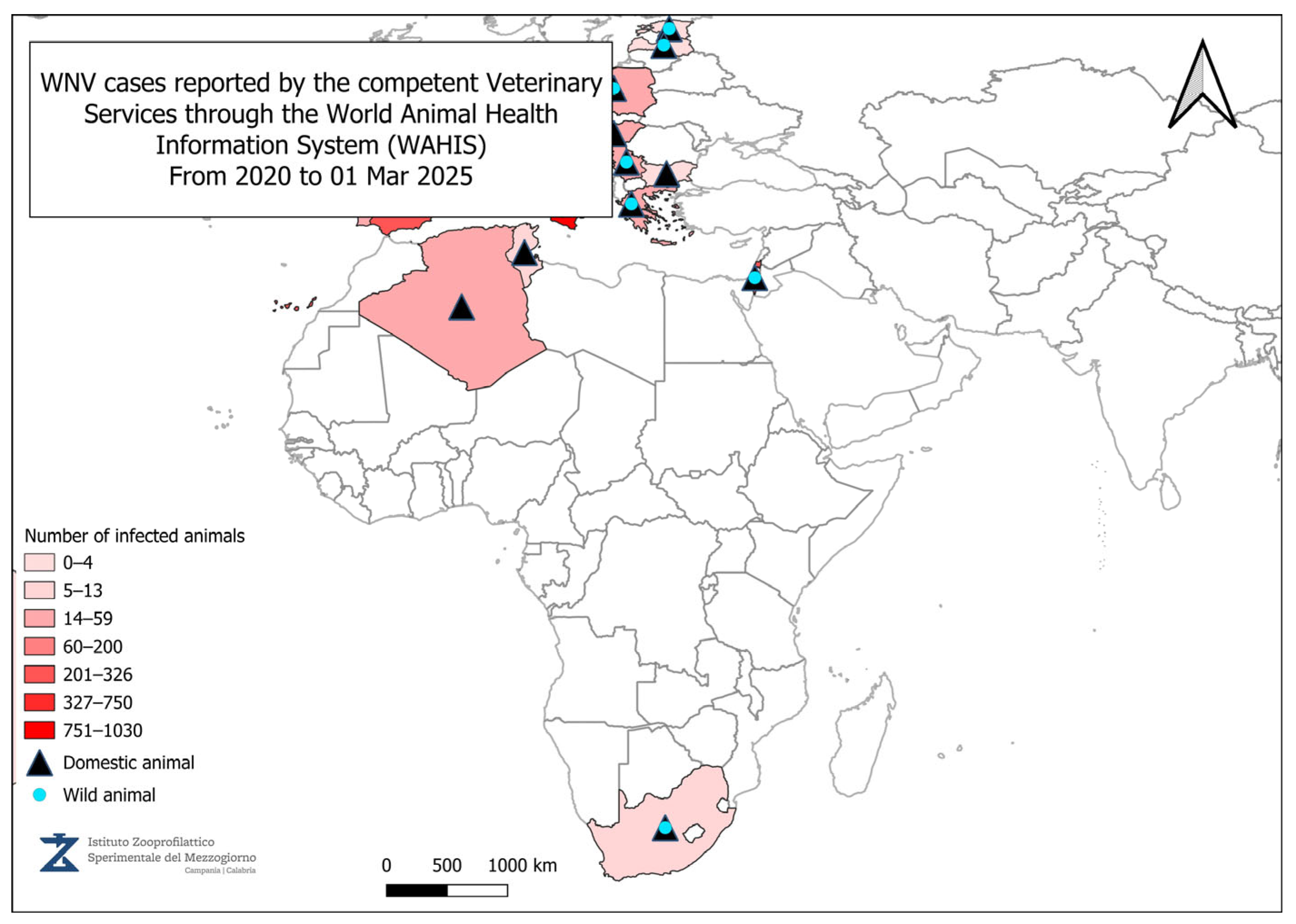
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, L.; Nappo, M.A.; Frontoso, R.; Perrotta, M.G.; Di Lecce, R.; Guarnieri, C.; Ferrari, L.; Corradi, A. West Nile Virus (WNV): One-Health and Eco-Health Global Risks. Vet. Sci. 2025, 12, 288. https://doi.org/10.3390/vetsci12030288
Bruno L, Nappo MA, Frontoso R, Perrotta MG, Di Lecce R, Guarnieri C, Ferrari L, Corradi A. West Nile Virus (WNV): One-Health and Eco-Health Global Risks. Veterinary Sciences. 2025; 12(3):288. https://doi.org/10.3390/vetsci12030288
Chicago/Turabian StyleBruno, Luigi, Maria Anna Nappo, Raffaele Frontoso, Maria Gabriella Perrotta, Rosanna Di Lecce, Chiara Guarnieri, Luca Ferrari, and Attilio Corradi. 2025. "West Nile Virus (WNV): One-Health and Eco-Health Global Risks" Veterinary Sciences 12, no. 3: 288. https://doi.org/10.3390/vetsci12030288
APA StyleBruno, L., Nappo, M. A., Frontoso, R., Perrotta, M. G., Di Lecce, R., Guarnieri, C., Ferrari, L., & Corradi, A. (2025). West Nile Virus (WNV): One-Health and Eco-Health Global Risks. Veterinary Sciences, 12(3), 288. https://doi.org/10.3390/vetsci12030288







