Effect of Continuous Lipopolysaccharide Induction on Oxidative Stress and Heart Injury in Weaned Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Serum Cardiac Injury Indicator Assay
2.3. Assessment of Antioxidant Level
2.4. Histomorphological Observations of Heart
2.5. RNA Extraction and Real-Time Quantitative PCR
2.6. Statistical Analysis
3. Results
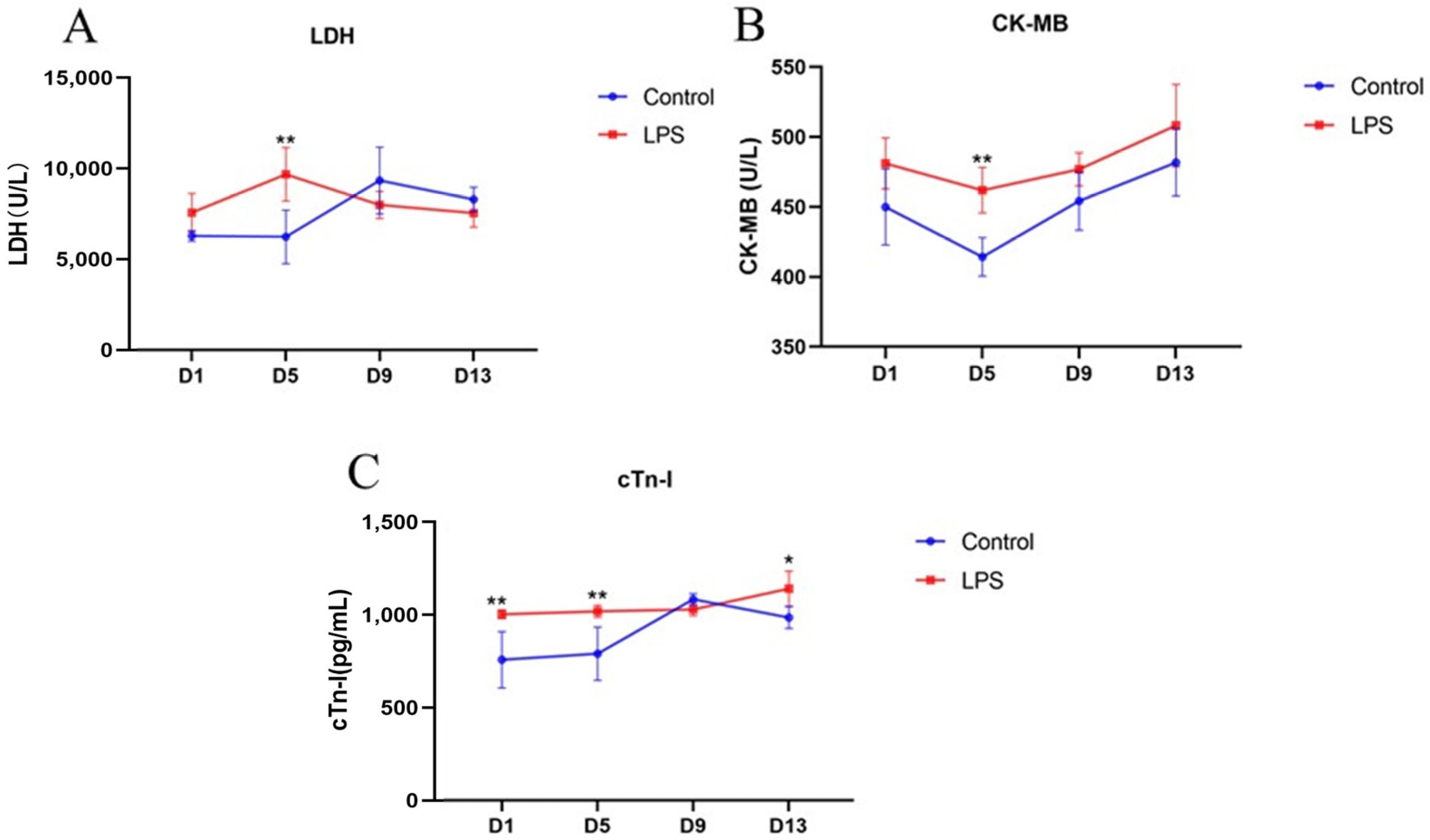
3.1. Changes in Serum Biochemical Indices
3.2. Changes in Cardiac Antioxidant Levels
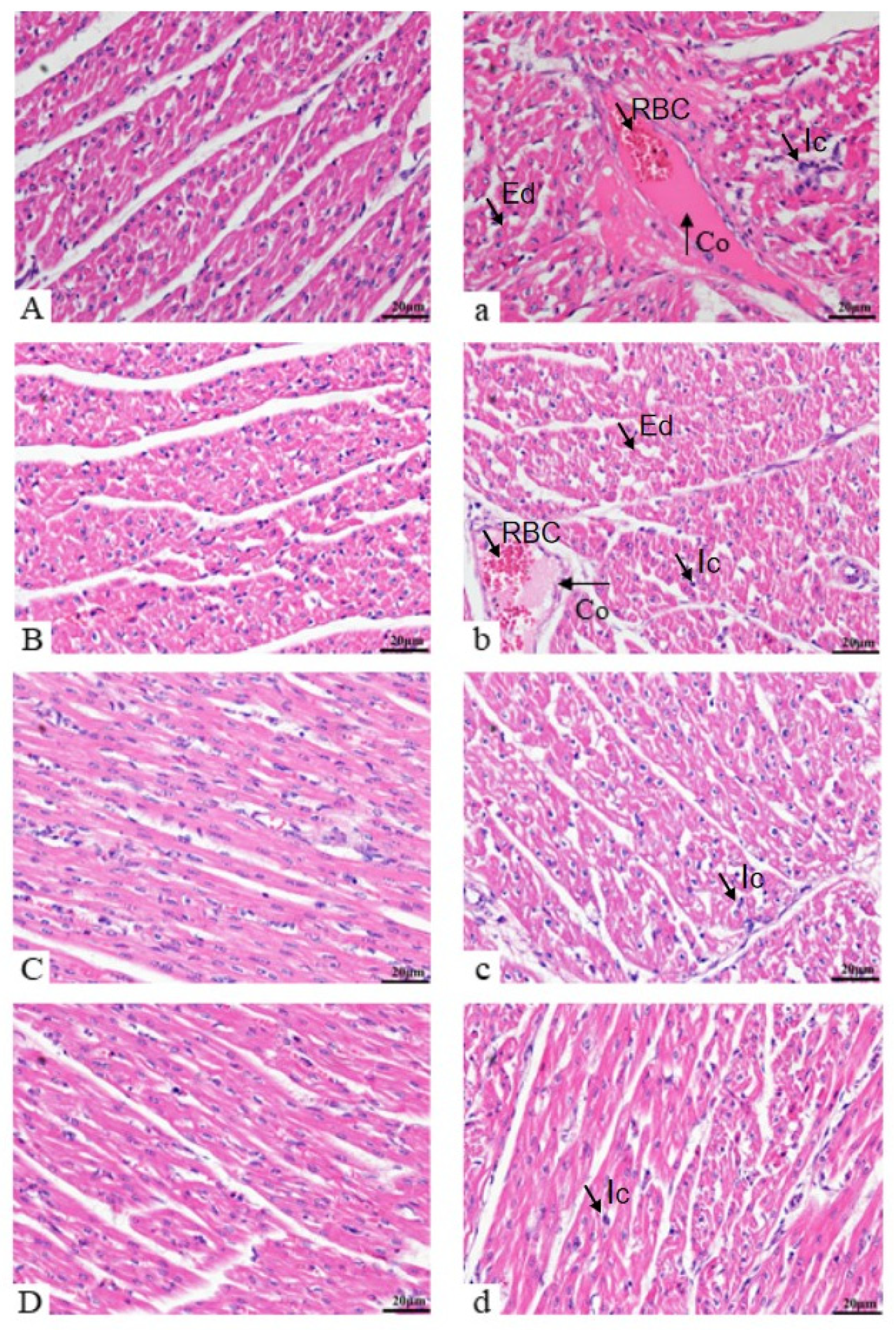
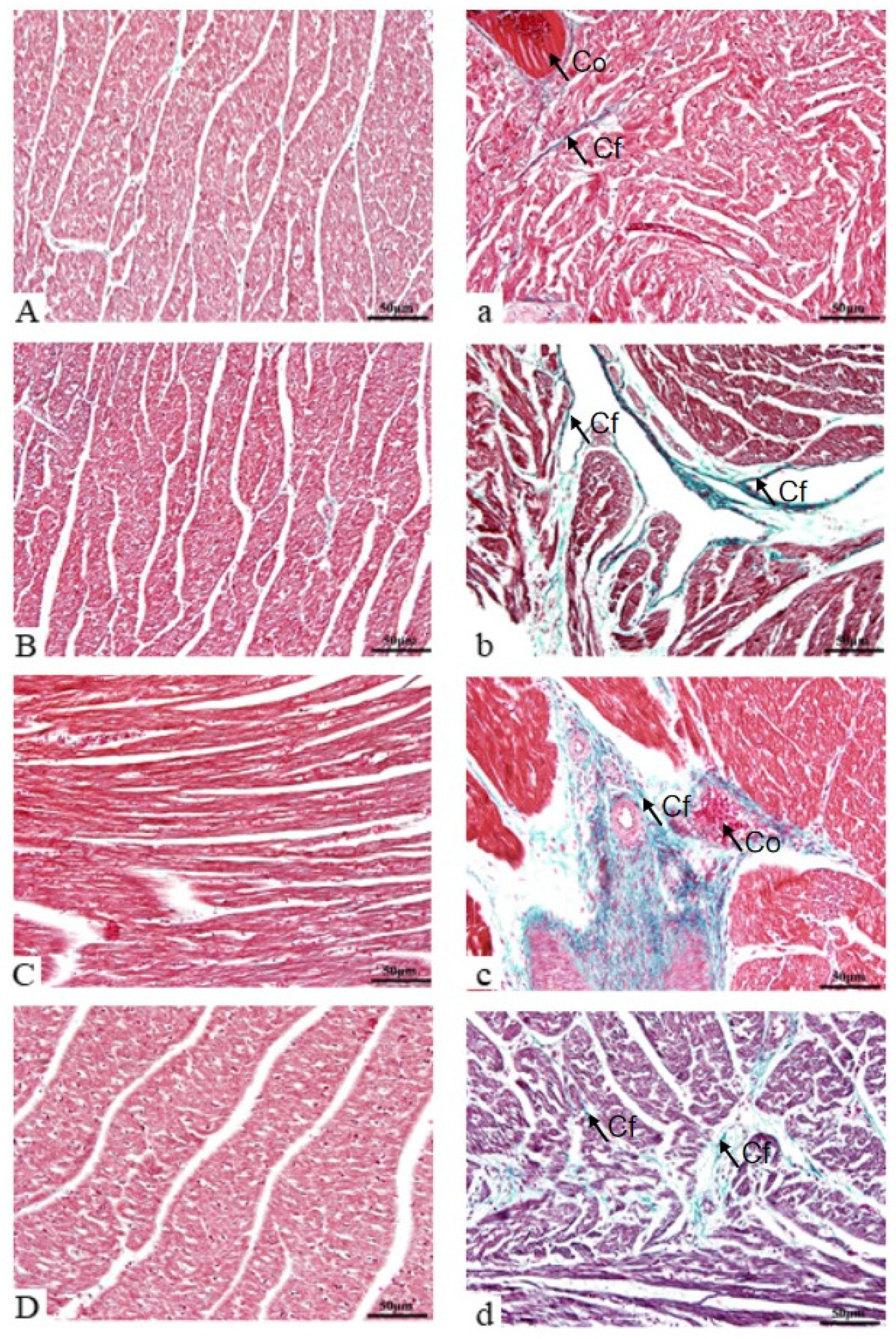
3.3. Histomorphological Observations of Heart
3.3.1. Histology Assessment
3.3.2. Cardiac Fiber Evaluation
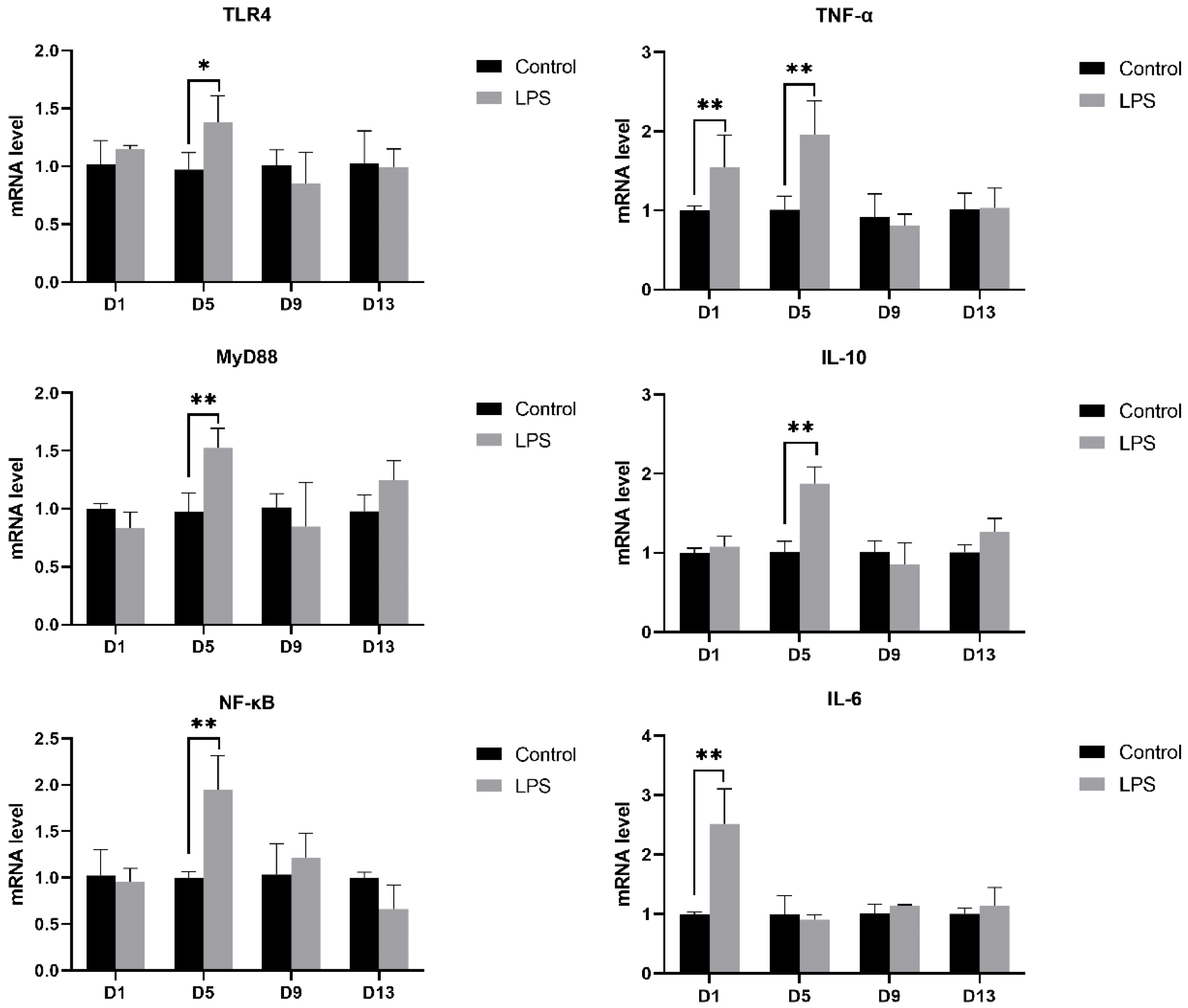
3.4. Gene mRNA Expression in the Heart
3.4.1. TLR4 Pathway
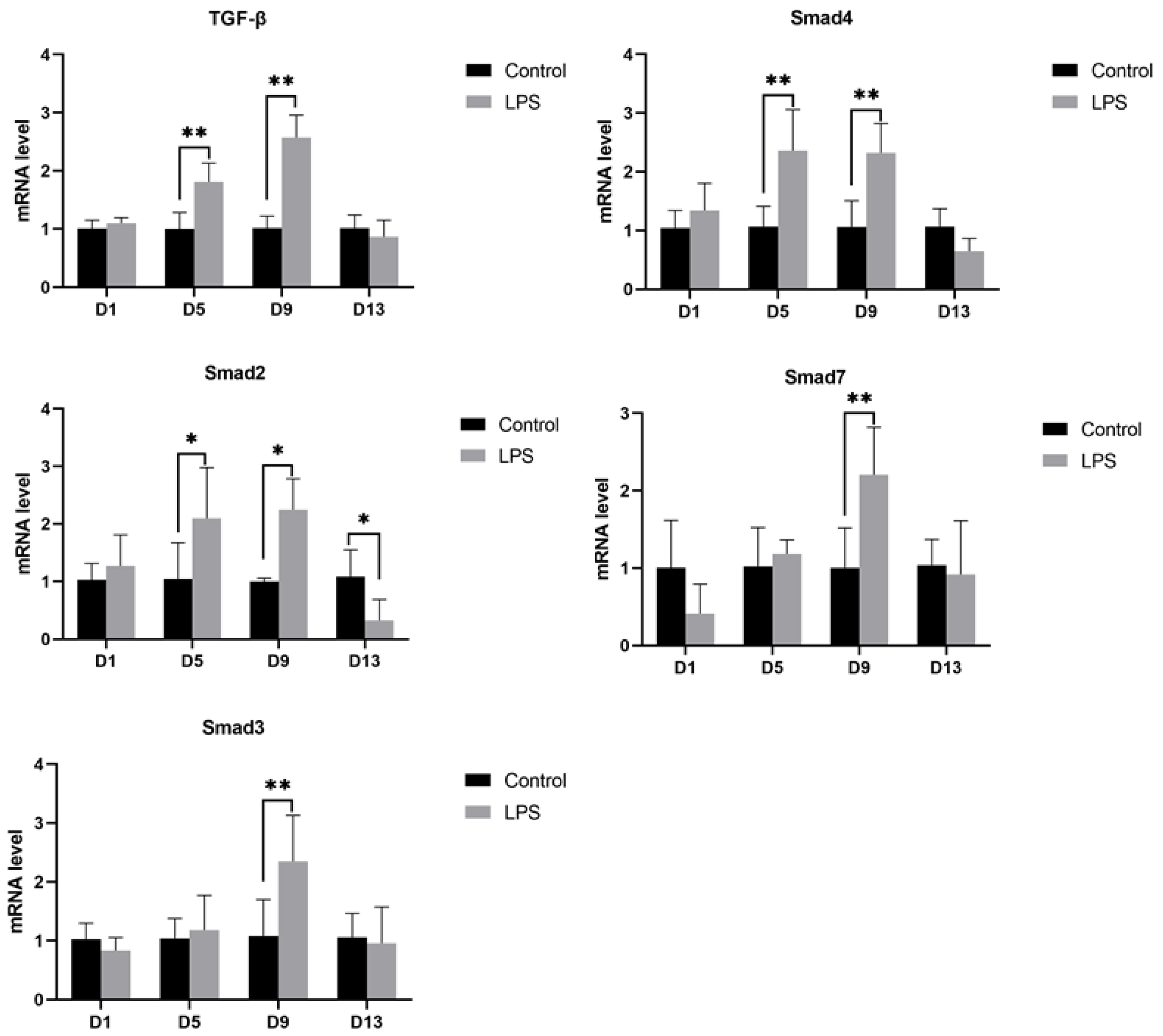
3.4.2. TGF-β Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Novais, A.; Martel-Kennes, Y.; Roy, C.; Deschene, K.; Beaulieu, S.; Bergeron, N.; Laforest, J.-P.; Lessard, M.; Matte, J.J.; Lapointe, J. Tissue-specific profiling reveals modulation of cellular and mitochondrial oxidative stress in normal-and low-birthweight piglets throughout the peri-weaning period. Animal 2020, 14, 1014–1024. [Google Scholar] [PubMed]
- Frangogiannis, N.G.; Lindsey, M.L.; Michael, L.H.; Youker, K.A.; Bressler, R.B.; Mendoza, L.H.; Spengler, R.N.; Smith, C.W.; Entman, M.L. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 1998, 98, 699–710. [Google Scholar] [CrossRef]
- Gwechenberger, M.; Mendoza, L.H.; Youker, K.A.; Frangogiannis, N.G.; Smith, C.W.; Michael, L.H.; Entman, M.L. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 1999, 99, 546–551. [Google Scholar]
- Wu, L.; Ong, S.; Talor, M.V.; Barin, J.G.; Baldeviano, G.C.; Kass, D.A.; Bedja, D.; Zhang, H.; Sheikh, A.; Margolick, J.B.; et al. Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J. Exp. Med. 2014, 211, 1449–1464. [Google Scholar] [PubMed]
- Anzai, A.; Choi, J.L.; He, S.; Fenn, A.M.; Nairz, M.; Rattik, S.; McAlpine, C.S.; Mindur, J.E.; Chan, C.T.; Iwamoto, Y.; et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J. Exp. Med. 2017, 214, 3293–3310. [Google Scholar]
- Hilgendorf, I.; Gerhardt, L.M.; Tan, T.C.; Winter, C.; Holderried, T.A.W.; Chousterman, B.G.; Iwamoto, Y.; Liao, R.; Zirlik, A.; Scherer-Crosbie, M.; et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res. 2014, 114, 1611–1622. [Google Scholar]
- Epelman, S.; Lavine, K.J.; Beaudin, A.E.; Sojka, D.K.; Carrero, J.A.; Calderon, B.; Brija, T.; Gautier, E.L.; Ivanov, S.; Satpathy, A.T.; et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014, 40, 91–104. [Google Scholar]
- Ramos, G.C.; Van Den Berg, A.; Nunes-Silva, V.; Weirather, J.; Peters, L.; Burkard, M.; Friedrich, M.; Pinnecker, J.; Abeßer, M.; Heinze, K.G.; et al. Myocardial aging as a T-cell-mediated phenomenon. Proc. Natl. Acad. Sci. USA 2017, 114, E2420–E2429. [Google Scholar]
- Horckmans, M.; Bianchini, M.; Santovito, D.; Megens, R.T.A.; Springael, J.Y.; Negri, I.; Vacca, M.; Eusanio, M.D.; Moschetta, A.; Weber, C.; et al. Pericardial adipose tissue regulates granulopoiesis, fibrosis, and cardiac function after myocardial infarction. Circulation 2018, 137, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; Ten Dijke, P. TGF-β signaling in control of cardiovascular function. Cold Spring Harb. Perspect. Biol. 2018, 10, a022210. [Google Scholar] [CrossRef]
- Brown, R.D.; Ambler, S.K.; Mitchell, M.D.; Long, c.s. The cardiac fibroblast: Therapeutic target in myocardial remodeling and failure. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 657–687. [Google Scholar] [CrossRef]
- Rakhshandeh, A.; De Lange, C. Evaluation of chronic immune system stimulation models in growing pigs. Animal 2012, 6, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Haudek, S.B.; Koroglu, T.; Tsen, M.F.; Bryant, D.D.; White, D.J.; Kusewitt, D.F.; Horton, J.W.; Giroir, B.P. IRAK1 deletion disrupts cardiac Toll/IL-1 signaling and protects against contractile dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H597–H606. [Google Scholar] [CrossRef]
- Nagatomo, Y.; Tang, W.H. Intersections between microbiome and heart failure: Revisiting the gut hypothesis. J. Card. Fail. 2015, 21, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Tian, R.; Chen, Q.; Wang, L.; Gu, Q.; Zhao, H.; Huang, C.; Liu, Y.; Li, J.; Yang, X.; et al. MiR-330-5p inhibits NLRP3 inflammasome-mediated myocardial ischaemia-reperfusion injury by targeting TIM3. Cardiovasc. Drugs Ther. 2021, 35, 691–705. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.J.; Saini, H.K.; Turan, B.; Liu, P.P.; Dhalla, N.S. Pentoxifylline attenuates cardiac dysfunction and reduces TNF-alpha level in ischemic-reperfused heart. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H832–H839. [Google Scholar] [CrossRef]
- Meldrum, D.R.; Dinarello, C.A.; Shames, B.D.; Cleveland, J.C., Jr.; Cain, B.S.; Banerjee, A.; Meng, X.; Harken, A.H. Ischemic preconditioning decreases postischemic myocardial tumor necrosis factor-alpha production, potential ultimate effector mechanism of preconditioning. Circulation 1998, 98 (Suppl. S19), II214–II218; discussion II218–II219. [Google Scholar]
- Churchill, E.N.; Murriel, C.L.; Chen, C.H.; Mochly-Rosen, D.; Szweda, L.I. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ. Res. 2005, 97, 78–85. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. Methods Mol. Biol. 2014, 1180, 31–43. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar]
- Zhou, Y.X.; Hu, X.; Zhong, S.; Yu, W.; Wang, J.; Zhu, W.; Yang, T.; Zhao, G.; Jiang, Y.; Li, Y. Effects of continuous LPS induction on oxidative stress and liver injury in weaned piglets. Vet. Sci. 2022, 10, 22. [Google Scholar] [CrossRef]
- Epelman, S.; Liu, P.P.; Mann, D.L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015, 15, 117–129. [Google Scholar] [PubMed]
- Missov, E.; Calzolari, C. Elevated cardiac troponin I in some patients with severe congestive heart failure. J. Mol. Cell. Cardiol. 1995, 27, A405. [Google Scholar]
- Zhang, D.Z.; Jia, M.Y.; Wei, H.Y.; Yao, M.; Jiang, L. Systematic review and meta-analysis of the interventional effects of resveratrol in a rat model of myocardial ischemia-reperfusion injury. Front. Pharmacol. 2024, 15, 1301502. [Google Scholar]
- Sheafor, B.A. Metabolic enzyme activities across an altitudinal gradient: An examination of pikas (genus Ochotona). J. Exp. Biol. 2003, 206 Pt 7, 1241–1249. [Google Scholar] [PubMed]
- Hawkins, R.C.; Tan, H.L. Comparison of the diagnostic utility of CK, CK-MB (activity and mass), troponin T and troponin I in patients with suspected acute myocardial infarction. Singap. Med. J. 1999, 40, 680–684. [Google Scholar]
- Biesiadecki, B.J.; Westfall, M.V. Troponin I modulation of cardiac performance: Plasticity in the survival switch. Arch. Biochem. Biophys. 2019, 664, 9–14. [Google Scholar]
- Kobayashi, T.; Solaro, R.J. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu. Rev. Physiol. 2005, 67, 39–67. [Google Scholar] [CrossRef]
- Zare, M.F.R.; Rakhshan, K.; Aboutaleb, N.; Nikbakht, F.; Naderi, N.; Bakhshesh, M.; Azizi, Y. Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sci. 2019, 232, 116623. [Google Scholar] [CrossRef] [PubMed]
- Bodor, G.S. Biochemical markers of myocardial damage. eJIFCC 2016, 27, 95. [Google Scholar] [PubMed]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Ducatelle, R.; Goossens, E.; De Meyer, F.; Eeckhaut, V.; Antonissen, G.; Haesebrouck, F.; Van Immerseel, F. Biomarkers for monitoring intestinal health in poultry: Present status and future perspectives. Vet. Res. 2018, 49, 43. [Google Scholar]
- Bi, S.; Qu, Y.; Shao, J.; Zhang, J.; Li, W.; Zhang, L.; Ni, J.; Cao, L. Ginsenoside Rg3 ameliorates stress of broiler chicks induced by escherichia coli lipopolysaccharide. Front. Vet. Sci. 2022, 9, 878018. [Google Scholar]
- Sampath, V. Bacterial endotoxin-lipopolysaccharide; structure, function and its role in immunity in vertebrates and invertebrates. Agric. Nat. Resour. 2018, 52, 115–120. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar]
- Hu, W.; He, Z.; Du, L.; Zhang, L.; Li, J.; Ma, Y.; Bi, S. Biomarkers of oxidative stress in broiler chickens attacked by lipopolysaccharide: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2023, 266, 115606. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar]
- Xing, Y.Y.; Zheng, Y.K.; Yang, S.; Zhang, L.H.; Guo, S.W.; Shi, L.L.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Artemisia ordosica polysaccharide alleviated lipopolysaccharide-induced oxidative stress of broilers via Nrf2/Keap1 and TLR4/NF-κB pathway. Ecotoxicol. Environ. Saf. 2021, 223, 112566. [Google Scholar]
- Zheng, X.C.; Wu, Q.J.; Song, Z.H.; Zhang, H.; Zhang, J.F.; Zhang, L.L.; Zhang, T.Y.; Wang, C.; Wang, T. Effects of oridonin on growth performance and oxidative stress in broilers challenged with lipopolysaccharide. Poult. Sci. 2016, 95, 2281–2289. [Google Scholar]
- Zhang, X.H.; Zhao, P.; Kuai, J.K.; Chang, C.; Yuan, Q. Spermidine attenuates lipopolysaccharide-induced myocardial injury in mice by inhibiting apoptosis, ROS production and ferroptosis. J. South. Med. Univ. 2024, 1, 166–172. [Google Scholar]
- Wang, Y.; Liu, P.; Shi, J.Y.; Wang, C.; Wang, C.; Hou, J.Q.; Qiu, X.P.; Zhao, H.B. Effects of different doses of chaihujia-longgu-muli decoction on cardiac function, behavior and expression of inflammatory factors in rats with myocardial infarction and anxiety. World J. Integr. Tradit. Chin. West. Med. 2023, 4, 692–697+704. [Google Scholar]
- Zhuang, J.; Chen, L.; Li, G.; Xia, L.; Wu, S.; Leng, J.; Tao, X.; Hong, J.; Wu, Y.; Wang, S.; et al. RCAN1 deficiency aggravates sepsis-induced cardiac remodeling and dysfunction by accelerating mitochondrial pathological fission. Inflamm. Res. 2022, 71, 1589–1602. [Google Scholar] [PubMed]
- Poltorak, A.; Smirnova, I.; He, X.; Liu, M.Y.; Huffel, C.V.; McNally, O.; Birdwell, D.; Alejos, E.; Silva, M.; Du, X.; et al. Genetic and physical mapping of the LPS locus: Identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol. Dis. 1998, 24, 340–355. [Google Scholar] [CrossRef]
- Rathi, S.S.; Xu, Y.J.; Dhalla, N.S. Mechanism of cardioprotective action of TNF-alpha in the isolated rat heart. Exp. Clin. Cardiol. 2002, 7, 146–150. [Google Scholar]
- Sack, M. Tumor necrosis factor-alpha in cardiovascular biology and the potential role for antitumor necrosis factor-alpha therapy in heart disease. Pharmacol. Ther. 2002, 94, 123–135. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, B.; Fang, J.; Qin, T.; Dai, C.; Shuai, W.; Huang, H. Ferrostatin-1 alleviates lipopolysaccharide-induced cardiac dysfunction. Bioengineered 2021, 12, 9367–9376. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, J.; Zhang, Z.; Turdi, S.; Han, X.; Liu, Q.; Hu, H.; Ye, H.; Dong, M.; Duan, Y.; et al. Cardiac-specific overexpression of catalase attenuates lipopolysaccharide-induced cardiac anomalies through reconciliation of autophagy and ferroptosis. Life Sci. 2023, 328, 121821. [Google Scholar] [CrossRef]
- Jung, M.; Ma, Y.; Iyer, R.P.; Deleon-Pennell, K.Y.; Yabluchanskiy, A.; Garrett, M.R.; Lindsey, M.L. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic. Res. Cardiol. 2017, 112, 33. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, K.; Ohdan, H.; Tokita, D.; Shishida, M.; Tanaka, Y.; Irei, T.; Asahara, T. Induction of endotoxin tolerance inhibits alloimmune responses. Transpl. Immunol. 2006, 16, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.C.; Fujiwara, K.; Lehoux, S. ECM remodeling in hypertensive heart disease. J. Clin. Investig. 2007, 117, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting cardiac cellular composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor–β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Tsutsui, H.; Shiomi, T.; Matsusaka, H.; Matsushima, S.; Wen, J.; Kubota, T.; Takeshita, A. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc. Res. 2004, 64, 526–535. [Google Scholar] [CrossRef]
- Rainer, P.P.; Hao, S.; Vanhoutte, D.; Lee, D.I.; Koitabashi, N.; Molkentin, J.D.; Kass, D.A. Cardiomyocyte-specific transforming growth factor β suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ. Res. 2014, 114, 1246–1257. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.G. TGF-β signaling from receptors to smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef]
- Itoh, S.; Ten Dijke, P. Negative regulation of TGF-β receptor/Smad signal transduction. Curr. Opin. Cell Biol. 2007, 19, 176–184. [Google Scholar] [PubMed]
- Xu, Z.M.; Yang, K.; Yang, M.; Li, X.Q.; Li, X.P.; Liao, S.S.; Peng, P. Pyrrolidine dithiocarbamate ameliorates myocardial fibrosis in lipopolysaccharide-induced septic rats by inhibiting Toll-like receptor 4/nuclear factor-κB signaling pathway. Chin. Med. 2023, 3, 405–409. [Google Scholar]
| Gene | Primer Sequence (5′ → 3′) | Product Size (bp) | GenBank No. |
|---|---|---|---|
| GAPDH | F: TGACATCAAGAAGGTGGTGAAG | 159 | NM_001206359.1 |
| R: TTGACGAAGTGGTCGTTGAG | |||
| TLR4 | F: CTCCGGGTCACTTCTGTTCA | 143 | NM_001293317.1 |
| R: TAATGTTAGGAACCACCTGCAC | |||
| MyD88 | F: TGCCTTCATCTGCTACTGC | 81 | NM_001099923.1 |
| R: AGCCGATAGTTGGTCTGTTC | |||
| NF-κB | F: TGCCAGACACAGATGACCG | 195 | NM_001114281.1 |
| R: ATGGCGTAAAGGGATAGGGC | |||
| IL-6 | F: ACCTGCTTGATGAGAATCACC | 97 | NM_214399.1 |
| R: CCTCGACATTTCCCTTATTGCT | |||
| TNF-α | F: CCAATGGCAGAGTGGGTATG | 116 | NM_214022.1 |
| R: TGAAGAGGACCTGGGAGTAG | |||
| IL-10 | F: GAAGACGTAATGCCGAAGGC | 121 | NM_214041.1 |
| R: AGGGCAGAAATTGATGACAGC | |||
| TGFB1 | F: AAAGCGGCAACCAAATCTATGA | 206 | NM_214015.2 |
| R: GCTGAGGTAGCGCCAGGAAT | |||
| SMAD2 | F: TGTGTTACCATACCAAGGTCCC | 100 | NM_001256148.1 |
| R: GATCAGGCCAGCGCCATAAT | |||
| SMAD3 | F: ACGACTACAGCCATTCCATCC | 110 | NM_214137.1 |
| R: CTCTCCATCTTCACTCAGGTAGC | |||
| SMAD4 | F: CAGGACAGCACAGAATGGATT | 110 | NM_214072.1 |
| R: GGTGAGGCAAATTAGGTGGGTATG | |||
| SMAD7 | F: TACTGGGAGGAGAAGACGAGAGTG | 241 | NM_001244175.1 |
| R: TGGCTGACTTGATGAAGATGGG |
| Items | Group | D1 | D5 | D9 | D13 |
|---|---|---|---|---|---|
| CAT | control | 4.98 ± 0.63 | 5.40 ± 0.30 | 6.70 ± 1.37 | 5.06 ± 0.56 |
| (U/mL) | LPS | 9.04 ± 0.85 ** | 8.40 ± 1.46 * | 6.56 ± 1.42 | 5.61 ± 0.86 |
| MDA | control | 0.779 ± 0.217 | 0.898 ± 0.268 | 0.791 ± 0.268 | 0.647 ± 0.117 |
| (nmol/mL) | LPS | 0.838 ± 0.261 | 1.061 ± 0.575 | 0.891 ± 0.124 | 0.501 ± 0.044 |
| SOD | control | 42.22 ± 3.63 | 45.18 ± 2.88 | 46.05 ± 6 | 46.77 ± 0.37 |
| (U/mL) | LPS | 51.28 ± 2.32 ** | 53.36 ± 2.42 * | 43.01 ± 5.24 | 43.7 ± 0.98 |
| T-AOC | control | 0.355 ± 0.041 | 0.327 ± 0.04 | 0.272 ± 0.006 | 0.298 ± 0.027 |
| mM | LPS | 0.285 ± 0.027 ## | 0.269 ± 0.023 # | 0.287 ± 0.13 | 0.290 ± 0.026 |
| GSH-Px | control | 64.91 ± 5.86 | 75.42 ± 8.14 | 60.51 ± 5.53 | 64.31 ± 3.61 |
| (U/mL) | LPS | 70.93 ± 17.38 | 56.47 ± 5.89 # | 56.30 ± 5.61 | 66.64 ± 11.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhao, G.; Liu, J.; Hu, X.; Yu, W.; Wang, J.; Zhong, S.; Zhu, W.; Yang, T.; Zhou, Y.; et al. Effect of Continuous Lipopolysaccharide Induction on Oxidative Stress and Heart Injury in Weaned Piglets. Vet. Sci. 2025, 12, 330. https://doi.org/10.3390/vetsci12040330
Li J, Zhao G, Liu J, Hu X, Yu W, Wang J, Zhong S, Zhu W, Yang T, Zhou Y, et al. Effect of Continuous Lipopolysaccharide Induction on Oxidative Stress and Heart Injury in Weaned Piglets. Veterinary Sciences. 2025; 12(4):330. https://doi.org/10.3390/vetsci12040330
Chicago/Turabian StyleLi, Jinyan, Guotong Zhao, Jin Liu, Xiaofen Hu, Wanting Yu, Jue Wang, Shengwei Zhong, Wenlu Zhu, Tingyu Yang, Yunxiao Zhou, and et al. 2025. "Effect of Continuous Lipopolysaccharide Induction on Oxidative Stress and Heart Injury in Weaned Piglets" Veterinary Sciences 12, no. 4: 330. https://doi.org/10.3390/vetsci12040330
APA StyleLi, J., Zhao, G., Liu, J., Hu, X., Yu, W., Wang, J., Zhong, S., Zhu, W., Yang, T., Zhou, Y., Jiang, Y., Bai, L., Tu, M., Yang, Q., & Li, Y. (2025). Effect of Continuous Lipopolysaccharide Induction on Oxidative Stress and Heart Injury in Weaned Piglets. Veterinary Sciences, 12(4), 330. https://doi.org/10.3390/vetsci12040330





