Associations Between Thoracic Ultrasound Chute-Side Evaluations and 60-Day Outcomes in Feedyard Cattle at Time of First Treatment for Respiratory Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Enrollment Criteria
2.2. Pulse Oximetry
2.3. Pulmonary Auscultation Score
2.4. Targeted Thoracic Point-of-Care Ultrasound
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.1.1. Did Not Finish Model
3.1.2. First Treatment Failure Model
3.2. Logistic Regression
3.2.1. Did Not Finish Model
3.2.2. First Treatment Failure Model
4. Discussion
4.1. Associations with First Treatment Failure and Did Not Finish Outcomes
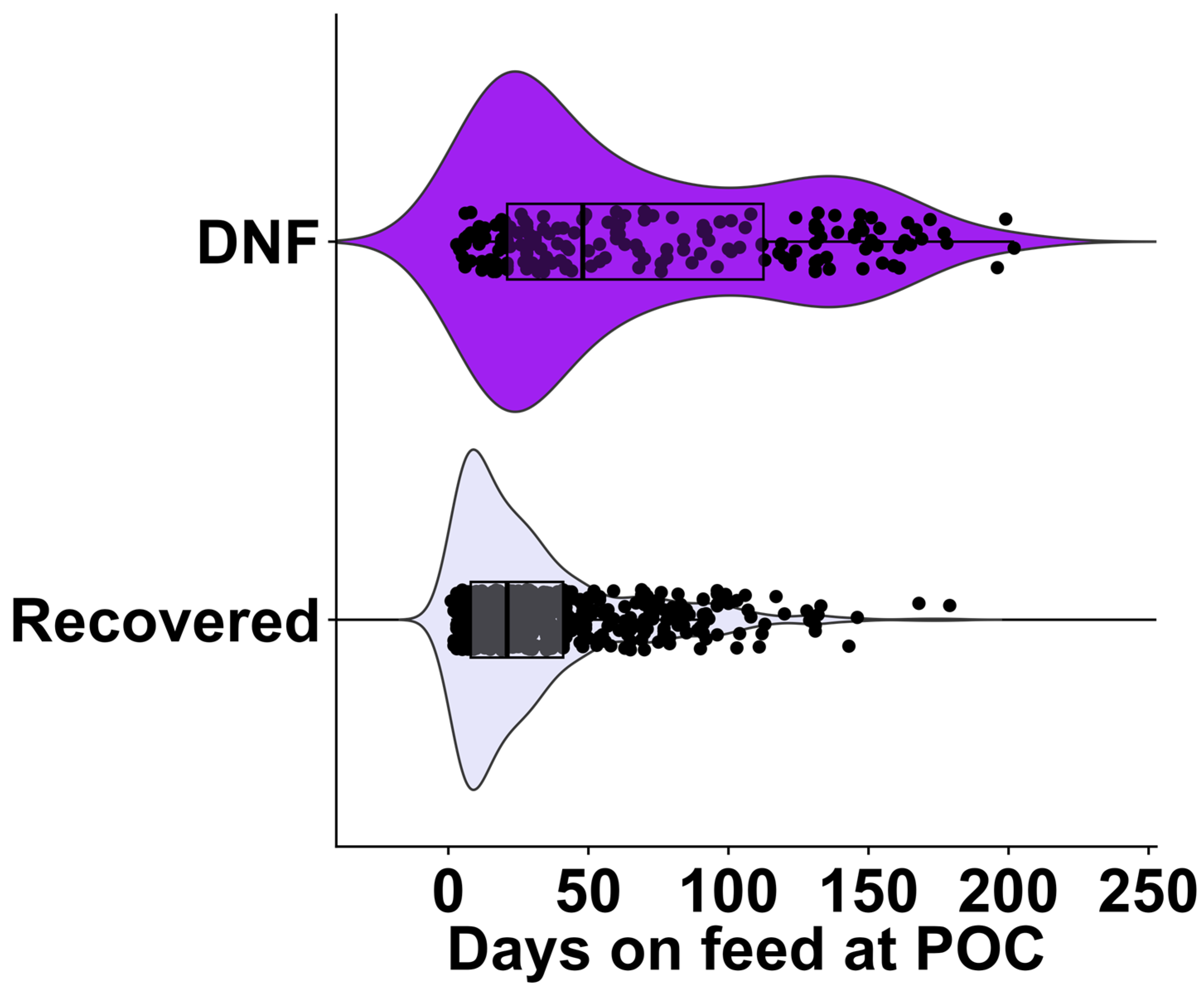
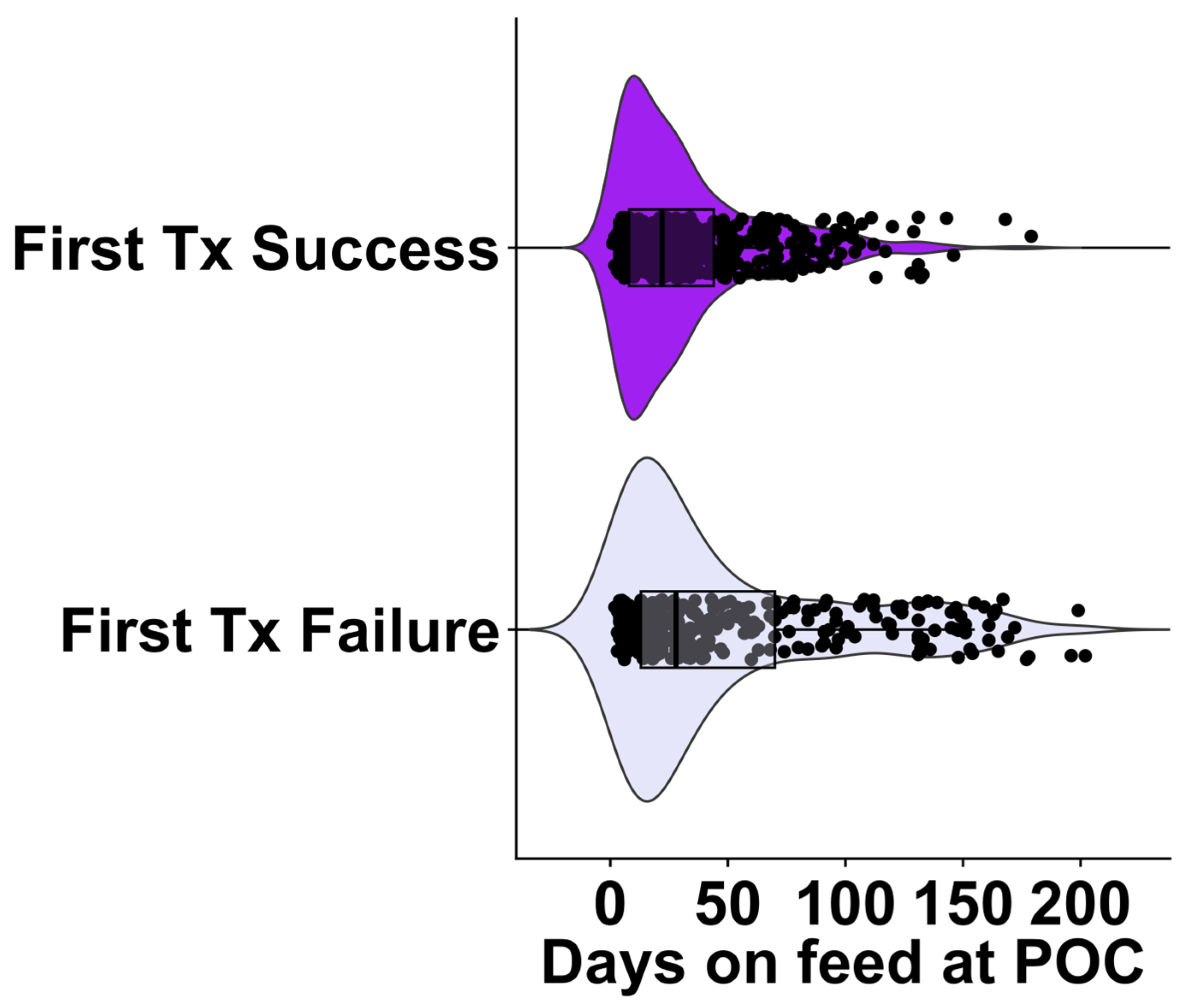
4.2. Days on Feed and Bodyweight as Prognostic Indicators
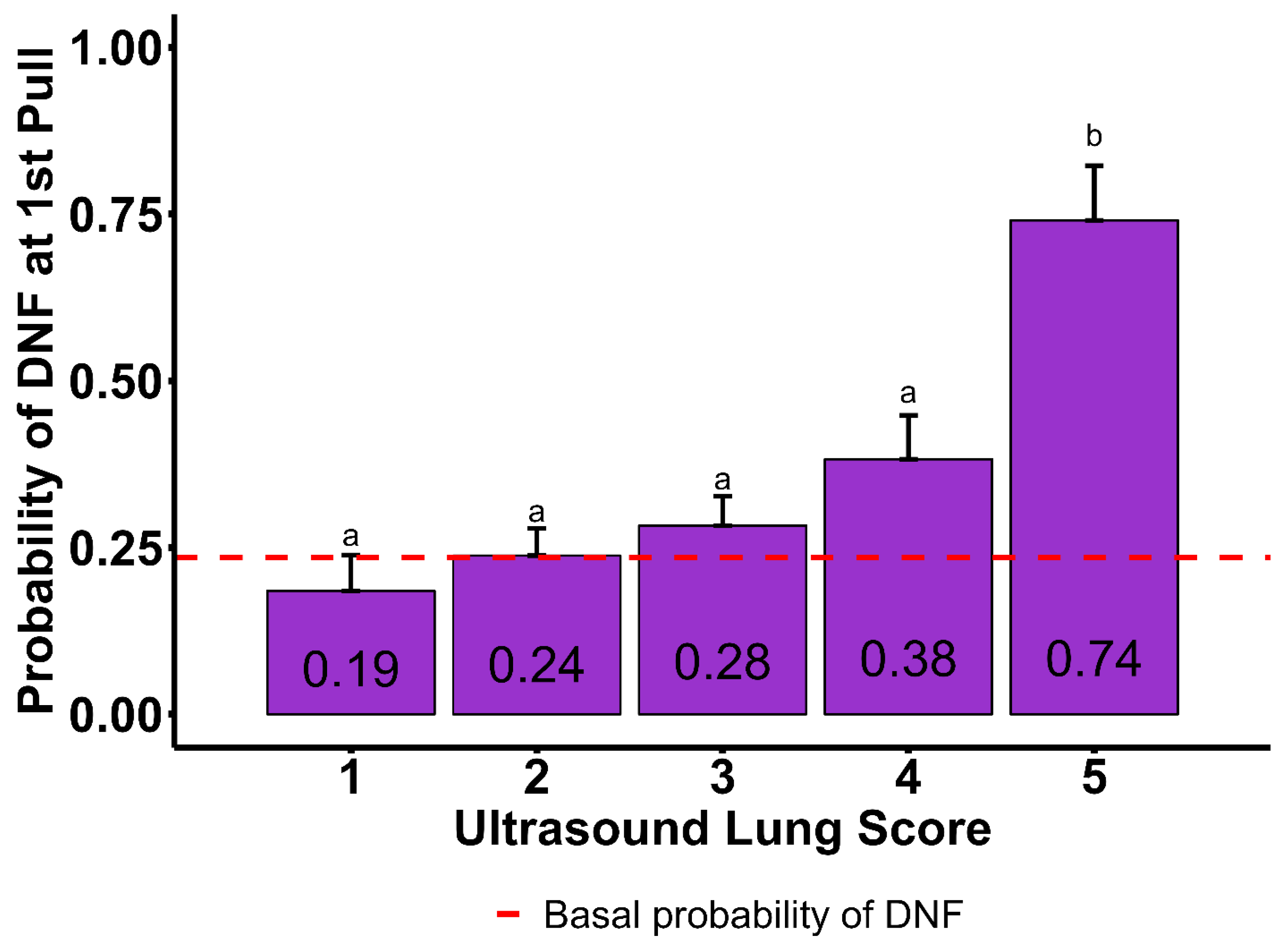
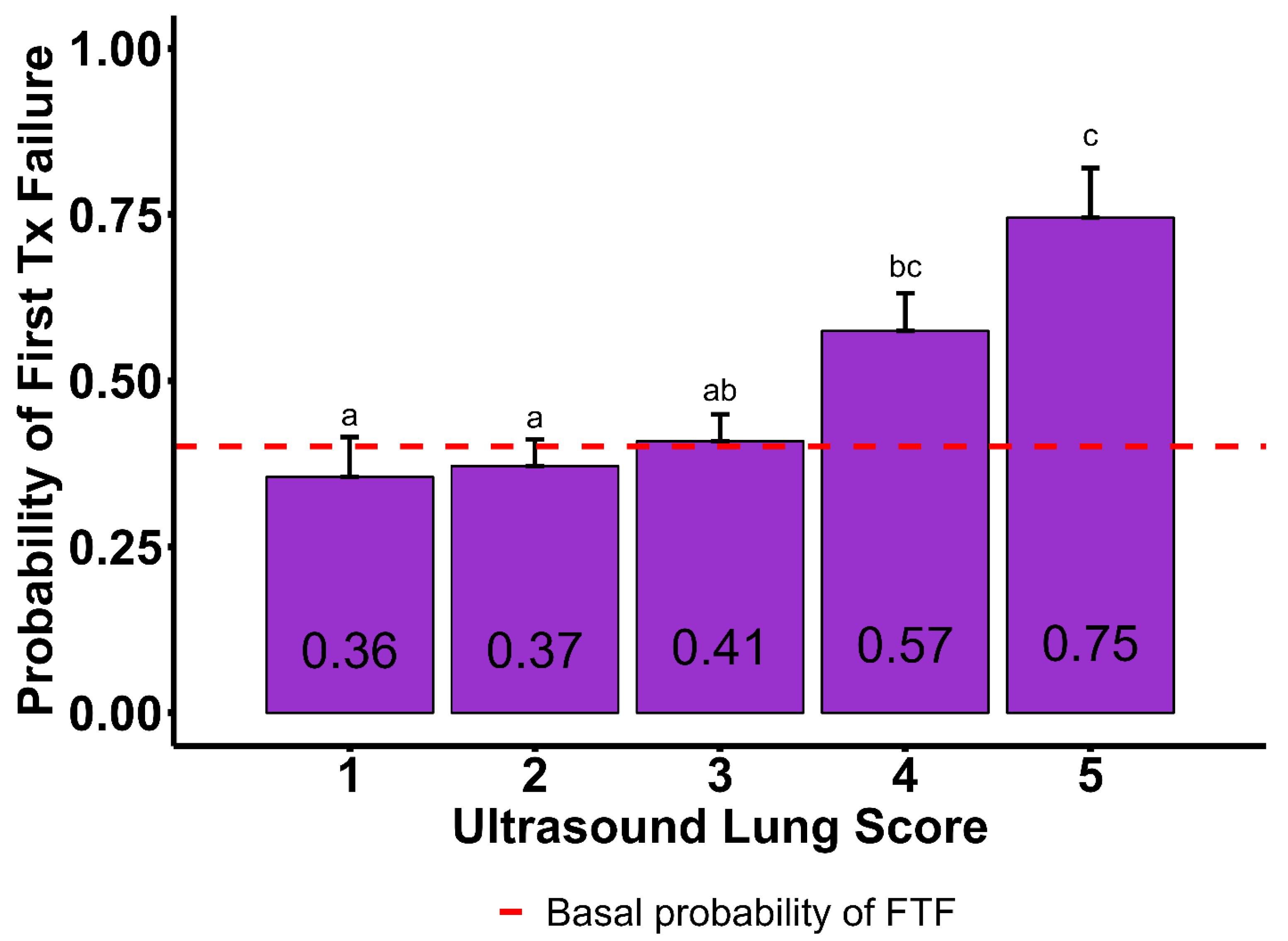
4.3. Ultrasound Lung Score, B-Line Count, and Moth Sign
4.4. Pulse Oximetry and Pulmonary Auscultation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| POC | Point of care |
| FTF | First treatment failure |
| FTS | First treatment success |
| DNF | Did not finish |
| BRD | Bovine respiratory disease |
| DOF | Days on feed |
| BW | Bodyweight |
| ULS | Ultrasound lung score |
| SPO2 | Saturation of peripheral oxygen (blood oxygen saturation) |
| PPM | Pulse per minute |
| TT-POCUS | Target thoracic ultrasonography |
| PAS | Pulmonary auscultation score |
| ICS | Intercostal space |
| VIF | Variance inflation factor |
| SD | Standard deviation |
References
- White, B.J.; Larson, B.L. Impact of bovine respiratory disease in U.S. beef cattle. Anim. Health Res. Rev. 2020, 21, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Booker, C.W. Bovine respiratory disease treatment failure: Definition and impact. Anim. Health Res. Rev. 2020, 21, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.J.; Tait, R.G.; Busby, W.D.; Reecy, J.M. An evaluation of bovine respiratory disease complex in feedlot cattle: Impact on performance and carcass traits using treatment records and lung lesion scores. J. Anim. Sci. 2009, 87, 1821–1827. [Google Scholar] [CrossRef]
- Blakebrough-Hall, C.; McMeniman, J.P.; González, L.A. An evaluation of the economic effects of bovine respiratory disease on animal performance, carcass traits, and economic outcomes in feedlot cattle defined using four BRD diagnosis methods. J. Anim. Sci. 2020, 98, skaa005. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; del Carmen Ferreras, M.; Giráldez, F.J.; Benavides, J.; Pérez, V. Production Significance of Bovine Respiratory Disease Lesions in Slaughtered Beef Cattle. Animals 2020, 10, 1770. [Google Scholar] [CrossRef]
- Cusack, P.M.V.; McMeniman, N.; Lean, I.J. The medicine and epidemiology of bovine respiratory disease in feedlots. Aust. Vet. J. 2003, 81, 480–487. [Google Scholar] [CrossRef]
- Smith, R.A.; Step, D.L.; Woolums, A.R. Bovine respiratory disease: Looking back and looking forward, what do we see? Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 239–251. [Google Scholar] [CrossRef]
- Griffin, D. The monster we don’t see: Subclinical BRD in beef cattle. Anim. Health Res. Rev. 2014, 15, 138–141. [Google Scholar] [CrossRef]
- Johnson, B.T.; Amrine, D.A.; Larson, R.L.; White, B.J. Association of feedlot disease treatments on the probability of heart disease syndrome in U.S. feedlot cattle. Bov. Pract. 2022, 56, 1–12. [Google Scholar] [CrossRef]
- Sundman, E.R.; Dewell, G.A.; Dewell, R.D.; Johnson, A.K.; Thomson, D.U.; Millman, S.T. The welfare of ill and injured feedlot cattle: A review of the literature and implications for managing feedlot hospital and chronic pens. Front. Vet. Sci. 2024, 11, 1398116. [Google Scholar] [CrossRef]
- Baruch, J.; Cernicchiaro, N.; Cull, C.A.; Lechtenberg, K.F.; Nickell, J.S.; Renter, D.G. Performance of multiple diagnostic methods in assessing the progression of bovine respiratory disease in calves challenged with infectious bovine rhinotracheitis virus and Mannheimia haemolytica. J. Anim. Sci. 2019, 97, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Uystepruyst, C.H.; Coghe, J.; Bureau, F.; Lekeux, P. Evaluation of accuracy of pulse oximetry in newborn calves. Vet. J. 2000, 159, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Coghe, J.; Uystepruyst, C.; Bureau, F.; Lekeux, P. Non-invasive assessment of arterial haemoglobin oxygen saturation in cattle by pulse oximetry. Vet. Rec. 1999, 145, 666–669. [Google Scholar]
- Wilkins, P.A.; Lascola, K.M.; Woolums, A.R.; Bedenice, D.; Giguère, S.; Boyle, A.G.; Dunkel, B.; Williams, K.J.; Landolt, G.A.; Austin, S.M.; et al. Diseases of the respiratory system. In Large Animal Internal Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 515–701.e42. [Google Scholar] [CrossRef]
- DeDonder, K.; Thomson, D.U.; Loneragan, G.H.; Noffsinger, T.; Taylor, W.; Apley, M.D. Lung auscultation and rectal temperature as a predictor of lung lesions and bovine respiratory disease treatment outcome in feedyard cattle. Bov. Pract. 2010, 44, 146–153. [Google Scholar] [CrossRef]
- Buczinski, S.; Pardon, B. Bovine Respiratory Disease Diagnosis: What progress has been made in clinical diagnosis? Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 399–423. [Google Scholar] [CrossRef] [PubMed]
- Buczinski, S.; Forté, G.; Bélanger, A.M. Short communication: Ultrasonographic assessment of the thorax as a fast technique to assess pulmonary lesions in dairy calves with bovine respiratory disease. J. Dairy. Sci. 2013, 96, 4523–4528. [Google Scholar] [CrossRef]
- Buczinski, S.; Forté, G.; Francoz, D.; Bélanger, A.-M. Comparison of thoracic auscultation, clinical score, and ultrasonography as indicators of bovine respiratory disease in Preweaned Dairy Calves. J. Vet. Intern. Med. 2014, 28, 234–242. [Google Scholar] [CrossRef]
- Mang, A.V.; Buczinski, S.; Booker, C.W.; Timsit, E. Evaluation of a computer-aided lung auscultation system for diagnosis of bovine respiratory disease in feedlot cattle. J. Vet. Intern. Med. 2015, 29, 1112–1116. [Google Scholar] [CrossRef]
- Timsit, E.; Tison, N.; Booker, C.W.; Buczinski, S. Association of lung lesions measured by thoracic ultrasonography at first diagnosis of bronchopneumonia with relapse rate and growth performance in feedlot cattle. J. Vet. Intern. Med. 2019, 33, 1540–1546. [Google Scholar] [CrossRef]
- Rademacher, R.D.; Buczinski, S.; Tripp, H.M.; Edmonds, M.D.; Johnson, E.G. Systematic thoracic ultrasonography in acute bovine respiratory disease of feedlot steers: Impact of lung consolidation on diagnosis and prognosis in a case-control study. Bov. Pract. 2014, 48, 1–10. [Google Scholar] [CrossRef]
- Ollivett, T.L. BRD treatment failure: Clinical and pathologic considerations. Anim. Health Res. Rev. 2020, 21, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Wolfger, B.; Timsit, E.; White, B.J.; Orsel, K. A systematic review of bovine respiratory disease diagnosis focused on diagnostic confirmation, early detection, and prediction of unfavorable outcomes in feedlot cattle. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Amrine, D.E.; Larson, R.L.; Theurer, M.E.; White, B.J. Determining relevant risk factors associated with mid- and late-feeding-stage bovine respiratory disease morbidity in cohorts of beef feedlot cattle. Appl. Anim. Sci. 2022, 38, 373–379. [Google Scholar] [CrossRef]
- Bøysen, S.; Gommeren, K.; Chalhoub, S. The essentials of veterinary point-of-care ultrasound. In Pleural Space and Lung, 1st ed.; Edra: Palm Beach Gardens, FL, USA, 2022. [Google Scholar]
- Johnston, R.; Jones, K.; Manley, D. Confounding and collinearity in regression analysis: A cautionary tale and an alternative procedure, illustrated by studies of British voting behaviour. Qual. Quant. 2018, 52, 1957–1976. [Google Scholar] [CrossRef]
- Babcock, A.H.; Renter, D.G.; White, B.J.; Dubnicka, S.R.; Scott, H.M. Temporal distributions of respiratory disease events within cohorts of feedlot cattle and associations with cattle health and performance indices. Prev. Vet. Med. 2010, 97, 198–219. [Google Scholar] [CrossRef]
- Vogel, G.J.; Bokenkroger, C.D.; Rutten-Ramos, S.C.; Bargen, J.L. Retrospective evaluation of animal mortality in US feedlots. Bov. Pract. 2015, 49, 113–123. [Google Scholar] [CrossRef]
- Babcock, A.H.; Cernicchiaro, N.; White, B.J.; Dubnicka, S.R.; Thomson, D.U.; Ives, S.E.; Scott, H.M.; Milliken, G.A.; Renter, D.G. A multivariable assessment quantifying effects of cohort-level factors associated with combined mortality and culling risk in cohorts of U.S. commercial feedlot cattle. Prev. Vet. Med. 2013, 108, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.R.; Ollivett, T.L.; Renaud, D.L.; Leslie, K.E.; LeBlanc, S.J.; Duffield, T.F.; Kelton, D.F. The effect of lung consolidation, as determined by ultrasonography, on first-lactation milk production in Holstein dairy calves. J. Dairy. Sci. 2018, 101, 5404–5410. [Google Scholar] [CrossRef]
- Ollivett, T.L.; Hewson, J.; Shubotz, R.; Caswell, J. Ultrasonographic progression of lung consolidation after experimental infection with Mannheimia haemolytica in Holstein bull calves. In Proceedings of the American Association of Bovine Practitioners Conference Proceedings, Ashland, OH, USA, 19 September 2013; p. 147. [Google Scholar] [CrossRef]
- Teixeira, A.G.V.; McArt, J.A.A.; Bicalho, R.C. Thoracic ultrasound assessment of lung consolidation at weaning in Holstein dairy heifers: Reproductive performance and survival. J. Dairy. Sci. 2017, 100, 2985–2991. [Google Scholar] [CrossRef]
- Adams, E.A.; Buczinski, S. Short communication: Ultrasonographic assessment of lung consolidation postweaning and survival to the first lactation in dairy heifers. J. Dairy. Sci. 2016, 99, 1465–1470. [Google Scholar] [CrossRef]
- Parri, N.; Allinovi, M.; Giacalone, M.; Corsini, I. To B or not to B. The rationale for quantifying B-lines in pediatric lung diseases. Pediatr. Pulmonol. 2023, 58, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.A.; Minami, T.; Ma, I.W.Y.; Yasukawa, K. Lung ultrasound for pleural line abnormalities, confluent B-Lines, and consolidation: Expert Reproducibility and a Method of Standardization. J. Ultrasound Med. 2022, 41, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, B.; Hunskaar, S. Point-of-care ultrasound in primary care: A systematic review of generalist performed point-of-care ultrasound in unselected populations. Ultrasound J. 2019, 11, 31. [Google Scholar] [CrossRef]
- Mojoli, F.; Bouhemad, B.; Mongodi, S.; Lichtenstein, D. Lung ultrasound for critically ill patients. Am. J. Respir. Crit. Care Med. 2019, 199, 701–714. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. Ultrasound in the management of thoracic disease. Crit. Care Med. 2007, 35, S250–S261. [Google Scholar] [CrossRef]
- Allinovi, M.; Parise, A.; Giacalone, M.; Amerio, A.; Delsante, M.; Odone, A.; Franci, A.; Gigliotti, F.; Amadasi, S.; Delmonte, D.; et al. Lung Ultrasound may support diagnosis and monitoring of COVID-19 pneumonia. Ultrasound Med. Biol. 2020, 46, 2908–2917. [Google Scholar] [CrossRef]
- Omer, T.; Cousins, C.; Lynch, T.; Le, N.N.; Sajed, D.; Mailhot, T. Lung Ultrasound findings in COVID-19: A descriptive retrospective study. Cureus 2022, 14, e23375. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Mussa, A.; Garofalo, G.; Cardinale, L.; Casoli, G.; Perotto, F.; Fava, C.; Frascisco, M. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am. J. Emerg. Med. 2006, 24, 689–696. [Google Scholar] [CrossRef]
- Gargani, L. Ultrasound of the Lungs: More than a room with a view. Heart Fail. Clin. 2019, 15, 297–303. [Google Scholar] [CrossRef]
| 60-Day Outcome | ||
|---|---|---|
| Recovered 1 | DNF 2 | |
| Sex | ||
| Heifer | 348 | 126 |
| Steer | 280 | 65 |
| Days on feed | ||
| 0 to 42 | 481 | 92 |
| 43 to 71 | 77 | 27 |
| >71 | 70 | 72 |
| Bodyweight, average (SD), kg | 353.1 (±73.9) | 389.9 (±115.2) |
| Bodyweight, kg | ||
| <272 | 63 | 27 |
| 272 to 361 | 324 | 68 |
| 362 to 453 | 188 | 36 |
| >453 | 53 | 60 |
| Real-time measurements | ||
| Ultrasound lung score, head * | ||
| 1 | 78 | 15 |
| 2 | 245 | 52 |
| 3 | 224 | 61 |
| 4 | 67 | 39 |
| 5 | 14 | 24 |
| Blood oxygen saturation (SD), % *,§ | 85.7 (±8.5) | 83.1 (±9.4) |
| Pulse, average (SD) *,§ | 74.5 (±22.2) | 75.9 (±25.1) |
| Auscultation score (cranioventral), head *,§ | ||
| 1 | 12 | 1 |
| 2 | 103 | 9 |
| 3 | 137 | 12 |
| 4 | 89 | 20 |
| 5 | 39 | 21 |
| Auscultation score (caudo-dorsal), head *,§ | ||
| 1 | 103 | 6 |
| 2 | 129 | 13 |
| 3 | 83 | 14 |
| 4 | 54 | 19 |
| 5 | 11 | 11 |
| A-line count, head * | ||
| 0 to 2 | 243 | 117 |
| ≥3 | 389 | 70 |
| B-line count, head * | ||
| 0 to 2 | 482 | 67 |
| ≥3 | 150 | 120 |
| Pleural effusion, head * | ||
| Absent | 530 | 134 |
| Present | 102 | 53 |
| Moth sign †, head * | ||
| Absent | 491 | 77 |
| Present | 141 | 110 |
| Post hoc measurements ‡ | ||
| B-line area, cm2, average (SEM) | 16.11 (±13.1) | 19.7 (±18.2) |
| First Treatment 60-Day Outcome | ||
|---|---|---|
| Failure 1 | Success 2 | |
| Sex | ||
| Heifer | 205 | 269 |
| Steer | 122 | 223 |
| Days on feed | ||
| 0 to 42 | 210 | 363 |
| 43 to 71 | 36 | 68 |
| >71 | 81 | 61 |
| Bodyweight, average (SD), kg | 363.9 (±100.4) | 360.1 (±75.8) |
| Bodyweight, kg | ||
| <272 | 43 | 47 |
| 272 to 361 | 156 | 236 |
| 362 to 453 | 65 | 159 |
| >453 | 63 | 50 |
| Real-time measurements | ||
| Ultrasound lung score, head * | ||
| 1 | 30 | 62 |
| 2 | 101 | 197 |
| 3 | 108 | 177 |
| 4 | 61 | 45 |
| 5 | 27 | 11 |
| Blood oxygen saturation (SD), % *,§ | 84.1 (±9.2) | 85.9 (±8.3) |
| Pulse, average (SD) *,§ | 75.1 (±23.2) | 74.6 (±22.3) |
| Auscultation score (cranioventral), head *,§ | ||
| 1 | 4 | 9 |
| 2 | 34 | 78 |
| 3 | 39 | 110 |
| 4 | 42 | 67 |
| 5 | 31 | 29 |
| Auscultation score (caudo-dorsal), head *,§ | ||
| 1 | 27 | 82 |
| 2 | 39 | 103 |
| 3 | 35 | 62 |
| 4 | 34 | 39 |
| 5 | 15 | 7 |
| A-line count, head * | ||
| 0 to 2 | 181 | 179 |
| ≥3 | 137 | 322 |
| B-line count, head * | ||
| 0 to 2 | 163 | 386 |
| ≥3 | 156 | 114 |
| Pleural effusion, head * | ||
| Absent | 245 | 419 |
| Present | 74 | 81 |
| Moth sign †, head * | ||
| Absent | 166 | 403 |
| Present | 152 | 98 |
| Post hoc measurements ‡ | ||
| B-line area, cm2, average (SEM) | 18.9 (±17.3) | 15.4 ± 12.2 |
| 60-day Outcome Models * | ||||||
|---|---|---|---|---|---|---|
| Did Not Finish 1 | First Treatment Failure 2 | |||||
| Items | Probability | SE | p-Value | Probability | SE | p-Value |
| Sex | ||||||
| Heifer | 0.402 | 0.047 | 0.542 | 0.041 | ||
| Steer | 0.322 | 0.052 | 0.223 | 0.483 | 0.051 | 0.235 |
| Days on feed | ||||||
| 0 to 42 | 0.178 | 0.030 | 0.433 | 0.042 | ||
| 43 to 71 | 0.319 | 0.069 | 0.018 | 0.437 | 0.068 | 0.983 |
| >71 | 0.639 | 0.057 | 0.001 | 0.662 | 0.058 | 0.004 |
| Bodyweight, kg | ||||||
| <272 | 0.612 | 0.102 | 0.603 | 0.079 | ||
| 272 to 361 | 0.337 | 0.062 | 0.005 | 0.535 | 0.053 | 0.397 |
| 362 to 453 | 0.247 | 0.048 | 0.001 | 0.385 | 0.047 | 0.008 |
| >453 | 0.477 | 0.076 | 0.324 | 0.527 | 0.065 | 0.444 |
| Ultrasound Lung Score | ||||||
| 1 | 0.185 | 0.053 | 0.355 | 0.060 | ||
| 2 | 0.237 | 0.040 | 0.402 | 0.371 | 0.040 | 0.798 |
| 3 | 0.283 | 0.043 | 0.146 | 0.408 | 0.040 | 0.416 |
| 4 | 0.382 | 0.065 | 0.017 | 0.574 | 0.056 | 0.006 |
| 5 | 0.740 | 0.081 | 0.001 | 0.745 | 0.074 | 0.001 |
| B-line | ||||||
| 0 to 2 | 0.242 | 0.040 | 0.430 | 0.042 | ||
| ≥3 | 0.605 | 0.058 | 0.001 | 0.638 | 0.046 | 0.001 |
| Moth sign | ||||||
| Absent | 0.2375 | 0.037 | 0.371 | 0.040 | ||
| Present | 0.506 | 0.053 | 0.001 | 0.652 | 0.043 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feitoza, L.F.B.B.; White, B.J.; Larson, R.L.; Spore, T.J. Associations Between Thoracic Ultrasound Chute-Side Evaluations and 60-Day Outcomes in Feedyard Cattle at Time of First Treatment for Respiratory Disease. Vet. Sci. 2025, 12, 369. https://doi.org/10.3390/vetsci12040369
Feitoza LFBB, White BJ, Larson RL, Spore TJ. Associations Between Thoracic Ultrasound Chute-Side Evaluations and 60-Day Outcomes in Feedyard Cattle at Time of First Treatment for Respiratory Disease. Veterinary Sciences. 2025; 12(4):369. https://doi.org/10.3390/vetsci12040369
Chicago/Turabian StyleFeitoza, Luis F. B. B., Brad J. White, Robert L. Larson, and Tyler J. Spore. 2025. "Associations Between Thoracic Ultrasound Chute-Side Evaluations and 60-Day Outcomes in Feedyard Cattle at Time of First Treatment for Respiratory Disease" Veterinary Sciences 12, no. 4: 369. https://doi.org/10.3390/vetsci12040369
APA StyleFeitoza, L. F. B. B., White, B. J., Larson, R. L., & Spore, T. J. (2025). Associations Between Thoracic Ultrasound Chute-Side Evaluations and 60-Day Outcomes in Feedyard Cattle at Time of First Treatment for Respiratory Disease. Veterinary Sciences, 12(4), 369. https://doi.org/10.3390/vetsci12040369







