Meta-Analysis of Dietary Interventions for Enteric Methane Mitigation in Ruminants Through Methodological Advancements and Implementation Pathways
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Quality Assessment Framework
2.4. Data Extraction
2.5. Standardization of Methane Measurement Methods
2.6. Statistical Analysis
2.6.1. Effect Size Calculation
2.6.2. Handling Dependencies in Meta-Analysis Models
2.6.3. Heterogeneity Assessment
2.6.4. Expanded Meta-Regression Analysis
2.6.5. Network Meta-Analysis
2.6.6. Publication Bias Assessment
2.6.7. Mechanistic Framework for Combination Analysis
2.6.8. Temporal Trend Analysis
2.6.9. Implementation Factor Analysis with Uncertainty Quantification
2.6.10. Sensitivity Analyses
3. Results
3.1. Characteristics of Included Studies
3.2. Comparative Efficacy for Dietary Interventions
3.3. Dose–Response Relationships and Animal-Specific Effects
3.4. Network Meta-Analysis
3.5. Publication Bias and Temporal Trends
3.6. Combination Analysis for Synergy Assessment
3.7. Implementation Factor Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMI | Dry matter intake |
| NDF | Neutral detergent fiber |
| ADF | Acid detergent fiber |
| LD | Linear dichroism |
| 3-NOP | 3-Nitrooxypropanol |
| RVE | Robust variance estimation |
| SF6 | Sulfur hexafluoride |
| DM | Dry matter |
| CP | Crude protein |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| VFA | Volatile fatty acids |
References
- IPCC. Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Allen, M.R.; Peters, G.P.; Shine, K.P.; Azar, C.; Balcombe, P.; Boucher, O.; Cain, M.; Ciais, P.; Collins, W.; Forster, P.M.; et al. Indicate separate contributions of long-lived and short-lived greenhouse gases in emission targets. NPJ Clim. Atmos. Sci. 2022, 5, 5. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Suong, N.T.M.; Paengkoum, S.; Schonewille, J.T.; Paengkoum, P. Dietary inclusion of anthocyanin-rich black cane silage treated with ferrous sulfate heptahydrate reduces oxidative stress and promotes tender meat production in goats. Front. Vet. Sci. 2022, 9, 969321. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Metabolic Hydrogen Flows in Rumen Fermentation: Principles and Possibilities of Interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Meo-Filho, P.; Ramirez-Agudelo, J.F.; Kebreab, E. Mitigating methane emissions in grazing beef cattle with a seaweed-based feed additive: Implications for climate-smart agriculture. Proc. Natl. Acad. Sci. USA 2024, 121, e2410863121. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.F.; Cueva, S.F.; Wasson, D.E.; Almeida, C.V.; Eifert, C.; de Ondarza, M.B.; Tricarico, J.M.; Hristov, A.N. Effects of dose, dietary nutrient composition, and supplementation period on the efficacy of methane mitigation strategies in dairy cows: A meta-analysis. J. Dairy Sci. 2024, 107, 9289–9308. [Google Scholar] [CrossRef]
- Almeida, A.K.; Hegarty, R.S.; Cowie, A. Meta-analysis quantifying the potential of dietary additives and rumen modifiers for methane mitigation in ruminant production systems. Anim. Nutr. 2021, 7, 1219–1230. [Google Scholar] [CrossRef]
- Purba, R.A.P.; Paengkoum, P.; Paengkoum, S. The links between supplementary tannin levels and conjugated linoleic acid (CLA) formation in ruminants: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0216187. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Benchaar, C. Diet supplementation with cinnamon oil, cinnamaldehyde, or monensin does not reduce enteric methane production of dairy cows. Animal 2016, 10, 418–425. [Google Scholar] [CrossRef]
- Angellotti, M.; Lindberg, M.; Ramin, M.; Krizsan, S.J.; Danielsson, R. Asparagopsis taxiformis supplementation to mitigate enteric methane emissions in dairy cows—Effects on performance and metabolism. J. Dairy Sci. 2024, 108, 2503–2516. [Google Scholar] [CrossRef]
- Muizelaar, W.; van Duinkerken, G.; Khan, Z.; Dijkstra, J. Evaluation of 3 northwest European seaweed species on enteric methane production and lactational performance of Holstein-Friesian dairy cows. J. Dairy Sci. 2023, 106, 4622–4633. [Google Scholar] [CrossRef]
- Eikanger, K.S.; Kjær, S.T.; Dörsch, P.; Iwaasa, A.D.; Alemu, A.W.; Schei, I.; Pope, P.B.; Hagen, L.H.; Kidane, A. Asparagopsis taxiformis inclusion in grass silage-based diets fed to Norwegian red dairy cows: Effects on ruminal fermentation, milk yield, and enteric methane emission. Livest. Sci. 2024, 285, 105495. [Google Scholar] [CrossRef]
- Roque, B.M.; Venegas, M.; Kinley, R.D.; de Nys, R.; Duarte, T.L.; Yang, X.; Kebreab, E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS ONE 2021, 16, e0247820. [Google Scholar] [CrossRef]
- Hristov, A.N.; Lee, C.; Cassidy, T.; Heyler, K.; Tekippe, J.A.; Varga, G.A.; Corl, B.; Brandt, R.C. Effect of Origanum vulgare L. leaves on rumen fermentation, production, and milk fatty acid composition in lactating dairy cows. J. Dairy Sci. 2013, 96, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Magnani, E.; Silva, T.H.; Sakamoto, L.; Manella, M.Q.; Dias, F.M.G.N.; Mercadante, M.E.; Henry, D.; Marcatto, J.O.S.; Paula, E.M.; Branco, R.H. Tannin-based product in feedlot diet as a strategy to reduce enteric methane emissions of Nellore cattle finished under tropical conditions. Transl. Anim. Sci. 2023, 7, txad048. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Magnani, E.; de Andrade, J.G.P.; Amâncio, B.R.; Meurer, G.W.; Reolon, H.G.; Benedeti, P.D.B.; Paula, E.M.; Branco, R.H. Evaluation of increasing levels of condensed tannin extracted from Acacia mearnsii on performance, carcass traits, meat quality, methane emission, and health of finishing Nellore bulls. Anim. Feed Sci. Technol. 2024, 315, 116046. [Google Scholar] [CrossRef]
- Teobaldo, R.W.; Cardoso, A.D.; Brito, T.R.; Leite, R.G.; Romanzini, E.P.; Granja-Salcedo, Y.T.; Reis, R.A. Response of phytogenic additives on enteric methane emissions and animal performance of nellore bulls raised in grassland. Sustainability 2022, 14, 9395. [Google Scholar] [CrossRef]
- Stefenoni, H.A.; Räisänen, S.E.; Cueva, S.F.; Wasson, D.E.; Lage, C.F.A.; Melgar, A.; Fetter, M.E.; Smith, P.; Hennessy, M.; Vecchiarelli, B.; et al. Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J. Dairy Sci. 2021, 104, 4157–4173. [Google Scholar] [CrossRef]
- Kirwan, S.F.; Tamassia, L.F.M.; Walker, N.D.; Karagiannis, A.; Kindermann, M.; Waters, S.M. Effects of dietary supplementation with 3-nitrooxypropanol on enteric methane production, rumen fermentation, and performance in young growing beef cattle offered a 50:50 forage:concentrate diet. J. Anim. Sci. 2024, 102, skad399. [Google Scholar] [CrossRef]
- Souza, W.L.; Niehues, M.B.; Cardoso, A.D.; Carvalho, V.V.; Perdigão, A.; Acedo, T.S.; Costa, D.F.; Tamassia, L.F.; Kindermann, M.; Reis, R.A. Effect of 3-nitrooxypropanol combined with different feed additives on growth performance, carcass traits, enteric methane emissions, and physiological responses in feedlot beef cattle fed a high-concentrate finishing diet. Animals 2024, 14, 3488. [Google Scholar] [CrossRef]
- Machmüller, A.; Ossowski, D.A.; Kreuzer, M. Comparative evaluation of the effects of coconut oil, oilseeds and crystalline fat on methane release, digestion and energy balance in lambs. Anim. Feed Sci. Technol. 2000, 85, 41–60. [Google Scholar] [CrossRef]
- Machmüller, A.; Dohme, F.; Soliva, C.R.; Wanner, M.; Kreuzer, M. Diet composition affects the level of ruminal methane suppression by medium-chain fatty acids. Aust. J. Agric. Res. 2001, 52, 713–722. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M. Methane emissions from beef cattle: Effects of fumaric acid, essential oil, and canola oil. J. Anim. Sci. 2006, 84, 1489–1496. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M.; Petit, H.V. Methane abatement strategies for cattle: Lipid supplementation of diets. Can. J. Anim. Sci. 2007, 87, 431–440. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M.; Benchaar, C.; Holtshausen, L. Crushed sunflower, flax, or canola seeds in lactating dairy cow diets: Effects on methane production, rumen fermentation, and milk production. J. Dairy Sci. 2009, 92, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Hess, H.D.; Beuret, R.A.; Lötscher, M.; Hindrichsen, I.K.; Machmüller, A.; Carulla, J.E.; Lascano, C.E.; Kreuzer, M. Ruminal fermentation, methanogenesis and nitrogen utilization of sheep receiving tropical grass hay-concentrate diets offered with Sapindus saponaria fruits and Cratylia argentea foliage. Anim. Sci. 2016, 79, 177–189. [Google Scholar] [CrossRef]
- Benchaar, C.; Hassanat, F.; Martineau, R.; Gervais, R. Linseed oil supplementation to dairy cows fed diets based on red clover silage or corn silage: Effects on methane production, rumen fermentation, nutrient digestibility, N balance, and milk production. J. Dairy Sci. 2015, 98, 7993–8008. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.H.; Hegarty, R.S.; Woodgate, R. Persistence of defaunation effects on digestion and methane production in ewes. Aust. J. Agric. Res. 2008, 48, 152–155. [Google Scholar] [CrossRef]
- Caetano, M.; Wilkes, M.J.; Pitchford, W.S.; Lee, S.J.; Hynd, P.I. Effect of ensiled crimped grape marc on energy intake, performance and gas emissions of beef cattle. Anim. Feed Sci. Technol. 2019, 247, 166–172. [Google Scholar] [CrossRef]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Aust. J. Exp. Agric. 2005, 56, 961–970. [Google Scholar] [CrossRef]
- Carvalho, I.P.C.D.; Fiorentini, G.; Berndt, A.; Castagnino, P.D.S.; Messana, J.D.; Frighetto, R.T.S.; Reis, R.A.; Berchielli, T.T. Performance and methane emissions of Nellore steers grazing tropical pasture supplemented with lipid sources. Rev. Bras. Zootec. 2016, 45, 760–767. [Google Scholar] [CrossRef]
- Chung, Y.H.; Walker, N.D.; McGinn, S.M.; Beauchemin, K.A. Differing effects of 2 active dried yeast (Saccharomyces cerevisiae) strains on ruminal acidosis and methane production in nonlactating dairy cows. J. Dairy Sci. 2011, 94, 2431–2439. [Google Scholar] [CrossRef]
- Cooprider, K.L.; Mitloehner, F.M.; Famula, T.R.; Kebreab, E.; Zhao, Y.; Van Eenennaam, A.L. Feedlot efficiency implications on greenhouse gas emissions and sustainability. J. Anim. Sci. 2011, 89, 2643–2656. [Google Scholar] [CrossRef]
- Cosgrove, G.P.; Waghorn, G.C.; Anderson, C.B.; Peters, J.S.; Smith, A.; Molano, G.; Deighton, M. The effect of oils fed to sheep on methane production and digestion of ryegrass pasture. Aust. J. Agric. Res. 2008, 48, 189–192. [Google Scholar] [CrossRef]
- Ding, X.; Long, R.; Zhang, Q.; Huang, X.; Guo, X.; Mi, J. Reducing methane emissions and the methanogen population in the rumen of Tibetan sheep by dietary supplementation with coconut oil. Trop. Anim. Health Prod. 2012, 44, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Duthie, C.A.; Troy, S.M.; Hyslop, J.J.; Ross, D.W.; Roehe, R.; Rooke, J.A. The effect of dietary addition of nitrate or increase in lipid concentrations, alone or in combination, on performance and methane emissions of beef cattle. Animal 2018, 12, 280–287. [Google Scholar] [CrossRef] [PubMed]
- El-Zaiat, H.M.; Araujo, R.C.; Soltan, Y.A.; Morsy, A.S.; Louvandini, H.; Pires, A.V.; Patino, H.O.; Correa, P.S.; Abdalla, A.L. Encapsulated nitrate and cashew nut shell liquid on blood and rumen constituents, methane emission, and growth performance of lambs. J. Anim. Sci. 2014, 92, 2214–2224. [Google Scholar] [CrossRef]
- Fiorentini, G.; Carvalho, I.P.C.; Messana, J.D.; Castagnino, P.S.; Berndt, A.; Canesin, R.C.; Frighetto, R.T.S.; Berchielli, T.T. Effect of lipid sources with different fatty acid profiles on the intake, performance, and methane emissions of feedlot Nellore steers. J. Anim. Sci. 2014, 92, 1613–1620. [Google Scholar] [CrossRef]
- Hristov, A.N.; Kebreab, E.; Niu, M.; Oh, J.; Bannink, A.; Bayat, A.R.; Boland, T.M.; Brito, A.F.; Casper, D.P.; Crompton, L.A.; et al. Symposium review: Uncertainties in enteric methane inventories, measurement techniques, and prediction models. J. Dairy Sci. 2018, 101, 6655–6674. [Google Scholar] [CrossRef]
- Suong, N.T.M.; Paengkoum, S.; Purba, R.A.P.; Paengkoum, P. Optimizing anthocyanin-rich black cane (Saccharum sinensis robx.) silage for ruminants using molasses and iron sulphate: A sustainable alternative. Fermentation 2022, 8, 248. [Google Scholar] [CrossRef]
- Hedges, L.V.; Tipton, E.; Johnson, M.C. Robust variance estimation in meta-regression with dependent effect size estimates. Res. Synth. Methods 2010, 1, 39–65. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, S. Fixed effects and variance components estimation in three-level meta-analysis. Res. Synth. Methods 2011, 2, 61–76. [Google Scholar] [CrossRef]
- Chen, Z.; Berger, J.S.; Castellucci, L.A.; Farkouh, M.; Goligher, E.C.; Hade, E.M.; Hunt, B.J.; Kornblith, L.Z.; Lawler, P.R.; Leifer, E.S.; et al. A comparison of computational algorithms for the Bayesian analysis of clinical trials. Clin. Trials 2024, 21, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. Recent advances in measurement and dietary mitigation of enteric methane emissions in ruminants. Front. Vet. Sci. 2016, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Magnusson, M.; Paul, N.A.; de Nys, R.; Tomkins, N. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS ONE 2014, 9, e85289. [Google Scholar] [CrossRef]
- Duin, E.C.; Wagner, T.; Shima, S.; Prakash, D.; Cronin, B.; Yanez-Ruiz, D.R.; Duval, S.; Rumbeli, R.; Stemmler, R.T.; Thauer, R.K.; et al. Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc. Natl. Acad. Sci. USA 2016, 113, 6172–6177. [Google Scholar] [CrossRef]
- Roque, B.M.; Brooke, C.G.; Ladau, J.; Polley, T.; Marsh, L.J.; Najafi, N.; Pandey, P.; Singh, L.; Kinley, R.; Salwen, J.K.; et al. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Anim. Microbiome 2019, 1, 3. [Google Scholar] [CrossRef]
- Dijkstra, J.; Bannink, A.; France, J.; Kebreab, E.; van Gastelen, S. Short communication: Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J. Dairy Sci. 2018, 101, 9041–9047. [Google Scholar] [CrossRef]
- Veneman, J.B.; Saetnan, E.R.; Clare, A.J.; Newbold, C.J. MitiGate; an online meta-analysis database for quantification of mitigation strategies for enteric methane emissions. Sci. Total Environ. 2016, 572, 1166–1174. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef]
- Lean, I.J.; Rabiee, A.R.; Duffield, T.F.; Dohoo, I.R. Invited review: Use of meta-analysis in animal health and reproduction: Methods and applications. J. Dairy Sci. 2009, 92, 3545–3565. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.A.; Haberl, H.; Harper, R.; House, J.; Jafari, M. Agriculture, forestry and other land use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 811–922. [Google Scholar]
- van Gastelen, S.; Dijkstra, J.; Bannink, A. Are dietary strategies to mitigate enteric methane emission equally effective across dairy cattle, beef cattle, and sheep? J. Dairy Sci. 2019, 102, 6109–6130. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.J.; Uwizeye, A.; Schulte, R.P.O.; Opio, C.I.; de Boer, I.J.M. Nutrient use efficiency: A valuable approach to benchmark the sustainability of nutrient use in global livestock production? Curr. Opin. Environ. Sustain. 2014, 9–10, 122–130. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, s2–s16. [Google Scholar] [CrossRef] [PubMed]

| Study | Animal Type | Dietary Intervention | Measurement Method | Sample Size | Methane Reduction % | Quality Score |
|---|---|---|---|---|---|---|
| [10] | Dairy | Phytochemicals, ionophores | SF6 | 8 | 21.4 | 9 |
| [11] | Dairy | Seaweed | GreenFeed | 20 | 18.8 | 13 |
| [12] | Dairy | Seaweed | GreenFeed | 48 | 20.8 | 18 |
| [13] | Dairy | Seaweed | SF6 | 10 | 18 | 16 |
| [14] | Beef | Seaweed | GreenFeed | 14 | 74.9 | 17 |
| [15] | Dairy | Phytochemicals | SF6 | 8 | 26.1 | 12 |
| [16] | Beef | Phytochemicals | SF6 | 6 | 13.3 | 19 |
| [17] | Beef | Phytochemicals | SF6 | 60 | 21.1 | 21 |
| [18] | Beef | Phytochemicals | SF6 | 24 | 17.9 | 22 |
| [19] | Dairy | Phytochemicals | GreenFeed | 8 | 60 | 23 |
| [20] | Beef | 3-NOP | GreenFeed | 34 | 30.6 | 24 |
| [21] | Beef | 3-NOP, ionophores | GreenFeed | 22 | 38 | 25 |
| [22] | Sheep | Oil, defanuation | Chamber | 3 | 21.2 | 9 |
| [23] | Sheep | Oil, defanuation | Chamber | 3 | 22.8 | 10 |
| [24] | Beef | Phytochemicals, oil, organic acid | Chamber | 8 | 16.6 | 5 |
| [25] | Beef | Oil | Chamber | 4 | 18.2 | 7 |
| [26] | Dairy | Oil, defanuation | Chamber | 4 | 19.8 | 8 |
| [27] | Sheep | Phytochemicals, defanuation | Chamber | 6 | 19.7 | 8 |
| [28] | Dairy | Oil, defanuation | Chamber | 6 | 23 | 10 |
| [29] | Sheep | Defanuation | Chamber | 7 | 24.6 | 11 |
| [30] | Beef | Phytochemicals | GreenFeed | 10 | 26.2 | 13 |
| [31] | Sheep | Phytochemicals, defanuation | Chamber | 6 | 27.8 | 14 |
| [32] | Beef | Oil | Chamber | 9 | 29.4 | 15 |
| [33] | Beef | Phytochemicals, defanuation | Chamber | 8 | 31 | 16 |
| [34] | Beef | Ionophores | Chamber | 4 | 32.6 | 17 |
| [35] | Sheep | Oil | SF6 | 2 | 34.2 | 19 |
| [36] | Sheep | Oil | other | 3 | 35.8 | 20 |
| [37] | Beef | NO3− | Chamber | 18 | 30.4 | 21 |
| [38] | Sheep | Phytochemicals, NO3− | Chamber | 6 | 31.2 | 22 |
| [39] | Beef | Oil, defanuation | SF6 | 9 | 40.6 | 23 |
| Intervention | Studies | Effect Ratio [95% CI] | Reduction (%) | Z | p | I2 (%) | Τ2 |
|---|---|---|---|---|---|---|---|
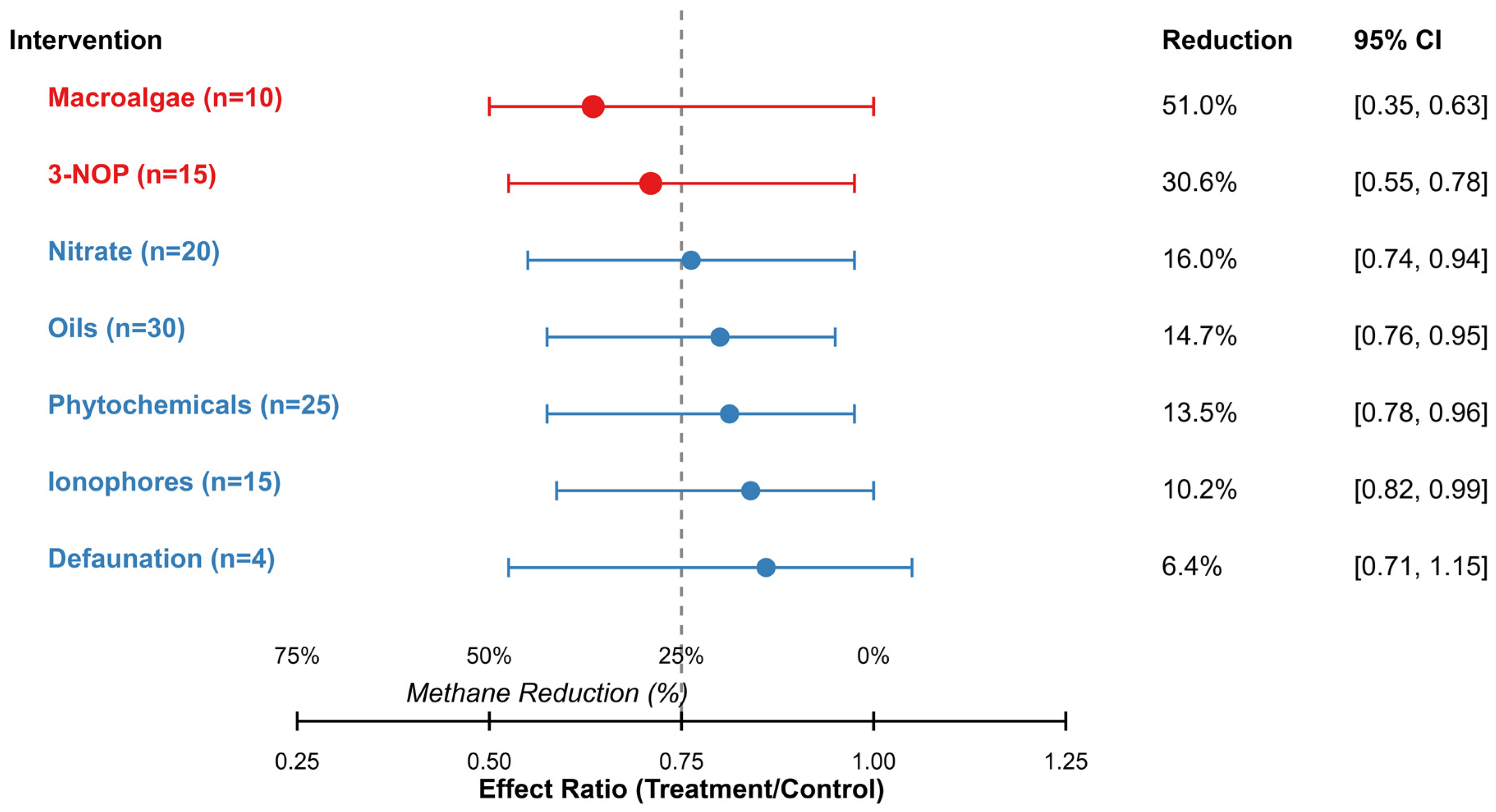
| Macroalgae | 10 | 0.49 [0.37, 0.63] | 51.0 | −5.92 | <0.001 | 86.3 | 0.125 |
| 3-NOP | 15 | 0.69 [0.55, 0.78] | 30.6 | −5.14 | <0.001 | 74.5 | 0.064 |
| Nitrate | 20 | 0.84 [0.74, 0.94] | 16.0 | −3.12 | 0.002 | 68.6 | 0.042 |
| Oils | 30 | 0.85 [0.76, 0.95] | 14.7 | −2.89 | 0.004 | 72.4 | 0.053 |
| Phytochemicals | 25 | 0.87 [0.78, 0.96] | 13.5 | −2.68 | 0.007 | 80.2 | 0.061 |
| Ionophores | 15 | 0.90 [0.82, 0.99] | 10.2 | −2.15 | 0.031 | 62.5 | 0.033 |
| Defaunation | 4 | 0.94 [0.71, 1.15] | 6.4 | −0.75 | 0.451 | 71.2 | 0.058 |
| Intervention | Studies | Moderator | Coefficient | SE | 95% CI | p-Value | R2 (%) |
|---|---|---|---|---|---|---|---|
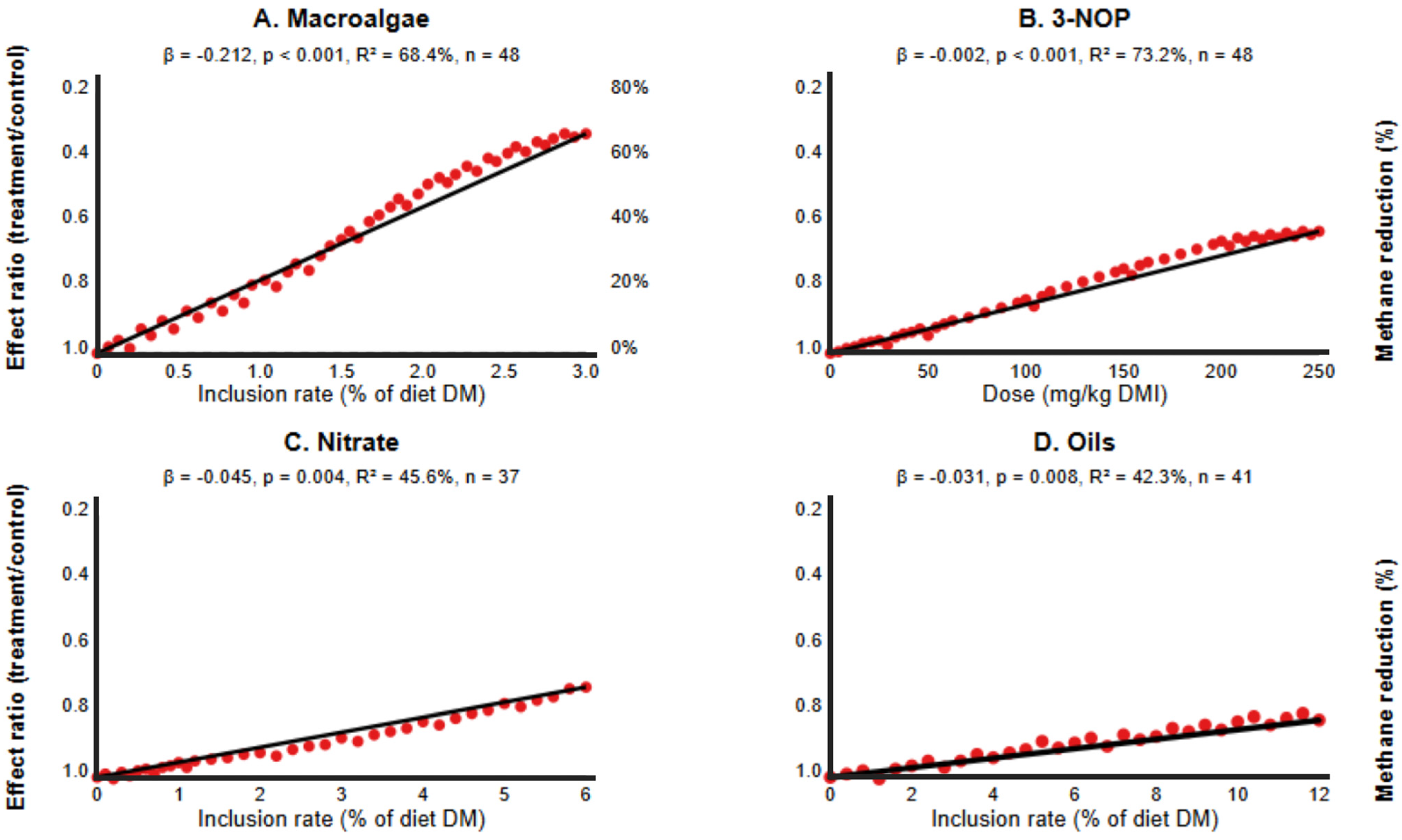
| Macroalgae | 10 | Dose | −0.212 | 0.043 | [−0.296, −0.128] | <0.001 | 68.4 |
| 3-NOP | 15 | Dose | −0.002 | 0.0004 | [−0.003, −0.001] | <0.001 | 73.2 |
| Nitrate | 20 | Dose | −0.045 | 0.015 | [−0.075, −0.015] | 0.004 | 45.6 |
| Oils | 30 | Dose | −0.031 | 0.011 | [−0.053, −0.009] | 0.008 | 38.5 |
| Phytochemicals | 22 | Dose | −0.034 | 0.019 | [−0.071, 0.003] | 0.075 | 18.2 |
| Macroalgae | 8 | Forage Proportion | 0.004 | 0.002 | [0.000, 0.008] | 0.048 | 32.3 |
| 3-NOP | 12 | Forage Proportion | 0.001 | 0.001 | [−0.001, 0.003] | 0.322 | 9.7 |
| Nitrate | 18 | Forage Proportion | −0.002 | 0.001 | [−0.004, 0.000] | 0.088 | 16.8 |
| Oils | 28 | Forage Proportion | 0.003 | 0.001 | [0.001, 0.005] | 0.012 | 28.4 |
| Phytochemicals | 22 | Forage Proportion | 0.001 | 0.001 | [−0.001, 0.003] | 0.284 | 6.3 |
| Macroalgae | 10 | Baseline CH4 | −0.005 | 0.003 | [−0.011, 0.001] | 0.105 | 19.2 |
| 3-NOP | 15 | Baseline CH4 | −0.012 | 0.004 | [−0.020, −0.004] | 0.003 | 47.6 |
| Nitrate | 20 | Baseline CH4 | −0.008 | 0.003 | [−0.014, −0.002] | 0.014 | 34.8 |
| Oils | 30 | Baseline CH4 | −0.006 | 0.002 | [−0.010, −0.002] | 0.006 | 36.7 |
| Phytochemicals | 25 | Baseline CH4 | −0.007 | 0.003 | [−0.013, −0.001] | 0.022 | 28.9 |
| Intervention | Animal Type | Studies | Effect Ratio [95% CI] | Reduction (%) |
|---|---|---|---|---|
| Macroalgae | Dairy Cattle | 6 | 0.58 [0.42, 0.74] | 42.0 |
| Macroalgae | Beef Cattle | 4 | 0.38 [0.29, 0.47] | 62.0 |
| 3-NOP | Dairy Cattle | 9 | 0.72 [0.56, 0.89] | 28.0 |
| 3-NOP | Beef Cattle | 6 | 0.65 [0.46, 0.84] | 35.0 |
| Nitrate | Dairy Cattle | 10 | 0.87 [0.76, 0.98] | 13.0 |
| Nitrate | Beef Cattle | 8 | 0.80 [0.68, 0.92] | 20.0 |
| Nitrate | Small Ruminants | 2 | 0.85 [0.66, 1.04] | 15.0 |
| Oils | Dairy Cattle | 14 | 0.89 [0.79, 0.99] | 11.0 |
| Oils | Beef Cattle | 10 | 0.83 [0.73, 0.93] | 17.0 |
| Oils | Small Ruminants | 6 | 0.79 [0.69, 0.89] | 21.0 |
| Phytochemicals | Dairy Cattle | 11 | 0.89 [0.79, 0.99] | 11.0 |
| Phytochemicals | Beef Cattle | 7 | 0.85 [0.74, 0.96] | 15.0 |
| Phytochemicals | Small Ruminants | 7 | 0.82 [0.71, 0.93] | 18.0 |
| Ionophores | Dairy Cattle | 6 | 0.93 [0.82, 1.04] | 7.0 |
| Ionophores | Beef Cattle | 9 | 0.87 [0.76, 0.98] | 13.0 |
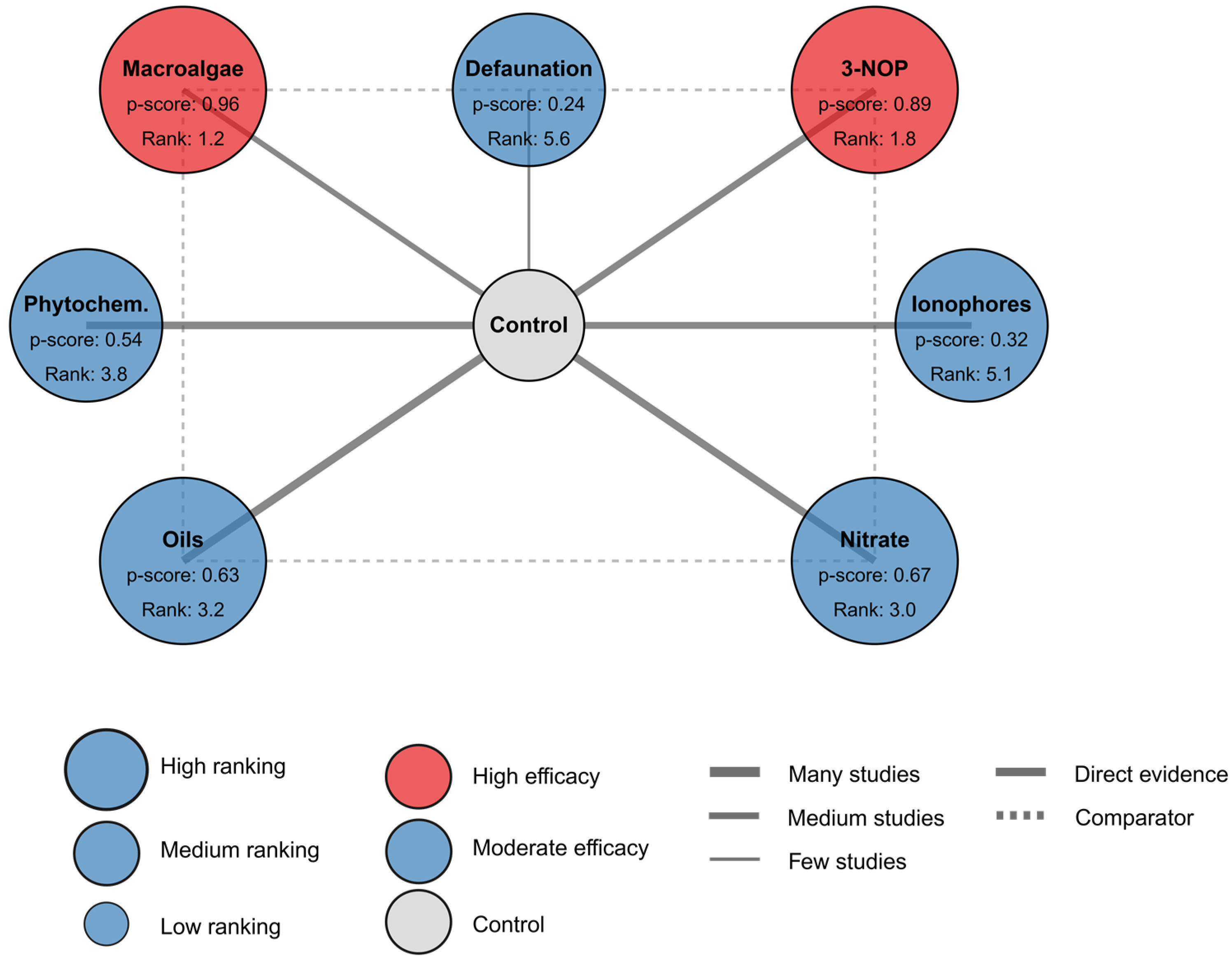
| Intervention | Direct Effect Ratio [95% CI] | Network Effect Ratio [95% CI] | P-Score | Mean Rank | Best Rank Probability |
|---|---|---|---|---|---|
| Macroalgae | 0.49 [0.37, 0.63] | 0.46 [0.35, 0.61] | 0.96 | 1.2 | 0.63 |
| 3-NOP | 0.69 [0.55, 0.78] | 0.65 [0.52, 0.81] | 0.89 | 1.8 | 0.32 |
| Nitrate | 0.84 [0.74, 0.94] | 0.83 [0.74, 0.93] | 0.67 | 3.0 | 0.03 |
| Oils | 0.85 [0.76, 0.95] | 0.84 [0.76, 0.93] | 0.63 | 3.2 | 0.01 |
| Phytochemicals | 0.87 [0.78, 0.96] | 0.86 [0.77, 0.96] | 0.54 | 3.8 | 0.01 |
| Ionophores | 0.90 [0.82, 0.99] | 0.91 [0.83, 0.99] | 0.32 | 5.1 | 0.00 |
| Defaunation | 0.94 [0.71, 1.15] | 0.93 [0.74, 1.15] | 0.24 | 5.6 | 0.00 |
| Control | 1.00 [1.00, 1.00] | 1.00 [1.00, 1.00] | 0.00 | 8.0 | 0.00 |
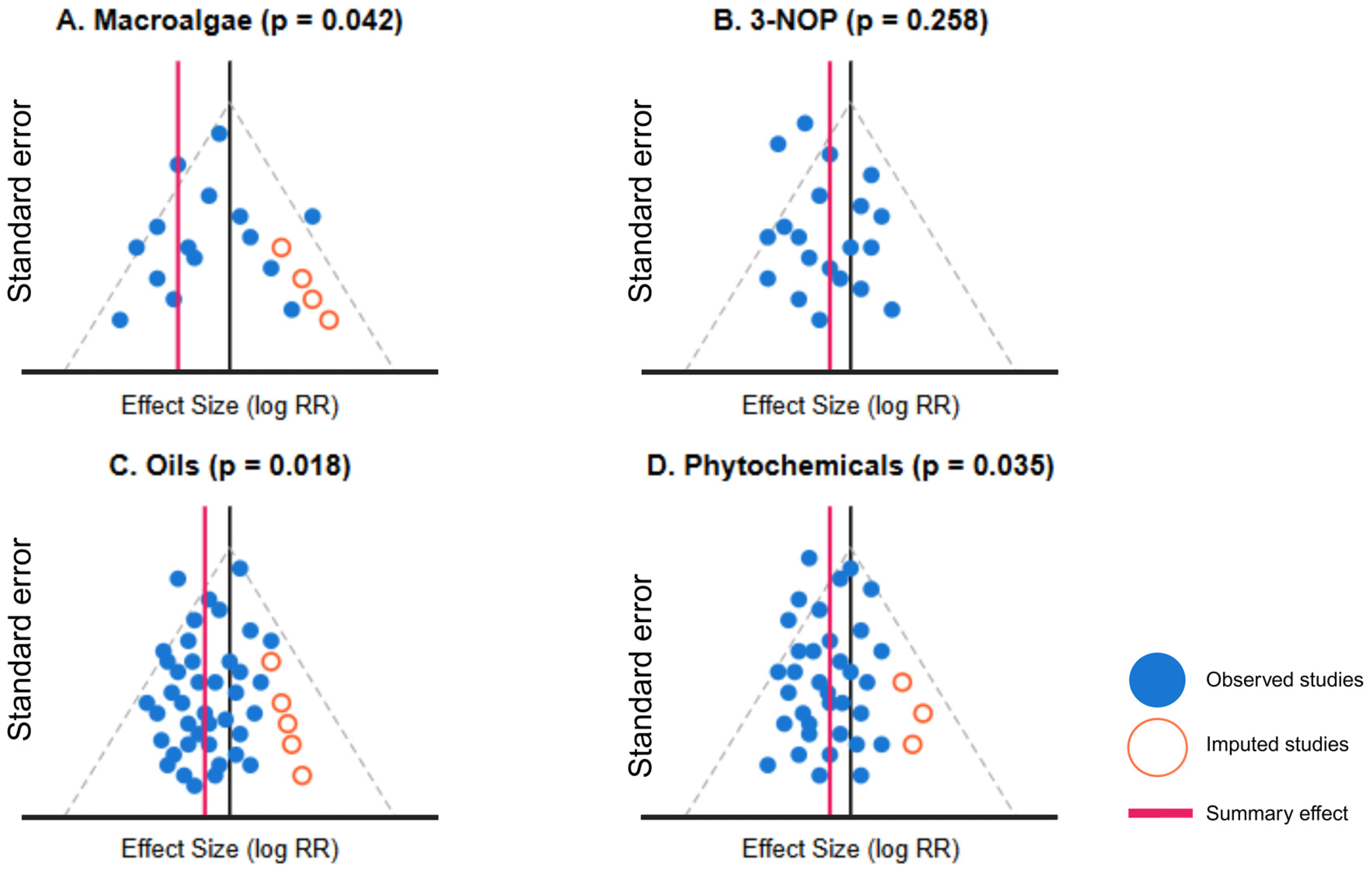
| Intervention | Studies | Egger’s Test (p) | Imputed Studies | Original Effect [95% CI] | Original Reduction (%) | Adjusted Effect [95% CI] | Adjusted Reduction (%) | Percent Change |
|---|---|---|---|---|---|---|---|---|
| Macroalgae | 10 | 0.036 | 3 | 0.49 [0.37, 0.63] | 51.0 | 0.56 [0.42, 0.74] | 44.0 | +14.3% |
| 3-NOP | 15 | 0.092 | 2 | 0.69 [0.55, 0.78] | 30.6 | 0.72 [0.58, 0.91] | 28.0 | +4.3% |
| Nitrate | 20 | 0.246 | 0 | 0.84 [0.74, 0.94] | 16.0 | 0.84 [0.74, 0.94] | 16.0 | 0.0% |
| Oils | 30 | 0.328 | 0 | 0.85 [0.76, 0.95] | 14.7 | 0.85 [0.76, 0.95] | 14.7 | 0.0% |
| Phytochemicals | 25 | 0.042 | 4 | 0.87 [0.78, 0.96] | 13.5 | 0.91 [0.81, 1.01] | 9.0 | +4.6% |
| Ionophores | 15 | 0.189 | 1 | 0.90 [0.82, 0.99] | 10.2 | 0.91 [0.83, 1.00] | 9.0 | +1.1% |
| Defaunation | 4 | 0.625 | 0 | 0.94 [0.71, 1.15] | 6.4 | 0.94 [0.71, 1.15] | 6.4 | 0.0% |
| Intervention | 2000–2009 Effect [95% CI] | 2010–2019 Effect [95% CI] | 2020–2024 Effect [95% CI] | Trend Coefficient | p-Value |
|---|---|---|---|---|---|
| Macroalgae | Not available | 0.58 [0.44, 0.76] | 0.41 [0.31, 0.54] | −0.082 | 0.012 |
| 3-NOP | Not available | 0.73 [0.58, 0.92] | 0.65 [0.52, 0.81] | −0.045 | 0.084 |
| Nitrate | 0.92 [0.83, 1.02] | 0.82 [0.72, 0.93] | 0.80 [0.70, 0.92] | −0.036 | 0.048 |
| Oils | 0.88 [0.79, 0.98] | 0.85 [0.76, 0.95] | 0.83 [0.74, 0.93] | −0.022 | 0.105 |
| Phytochemicals | 0.93 [0.84, 1.03] | 0.87 [0.78, 0.97] | 0.82 [0.73, 0.92] | −0.038 | 0.037 |
| Ionophores | 0.95 [0.86, 1.05] | 0.89 [0.80, 0.99] | 0.87 [0.78, 0.97] | −0.025 | 0.079 |
| Defaunation | 0.98 [0.88, 1.09] | 0.90 [0.81, 1.00] | Not available | −0.031 | 0.212 |
| Combination | Expected Reduction (%) | Observed Reduction (%) | Ratio | Interaction | Mechanism A | Mechanism B | Compatible |
|---|---|---|---|---|---|---|---|
| Tannin + Nitrate | 25.2 | 31.5 | 1.25 | Synergistic | Protein binding | Alternative H-sink | Yes |
| 3-NOP + Macroalgae | 64.9 | 72.7 | 1.12 | Synergistic | Direct enzyme inhibit | Biohydrogenation/Toxicity | Yes |
| Nitrate + 3-NOP | 40.4 | 44.4 | 1.10 | Synergistic | Alternative H-sink | Direct enzyme inhibition | Yes |
| Essential Oil + Ionophore | 9.8 | 11.3 | 1.15 | Synergistic | Membrane disruption | Propionate enhancement | Yes |
| Essential Oil + 3-NOP | 33.3 | 34.3 | 1.03 | Additive | Membrane disruption | Direct enzyme inhibition | Yes |
| Oil + 3-NOP | 39.3 | 41.3 | 1.05 | Additive | Biohydrogenation | Direct enzyme inhibition | Yes |
| Saponin + Nitrate | 23.6 | 23.1 | 0.98 | Additive | Defaunation | Alternative H-sink | No |
| Tannin + Saponin | 19.0 | 18.1 | 0.95 | Additive | Protein binding | Defaunation | No |
| Saponin + Oil | 22.7 | 21.6 | 0.95 | Additive | Defaunation | Biohydrogenation | No |
| Nitrate + Oil | 28.6 | 26.6 | 0.93 | Additive | Alternative H-sink | Biohydrogenation | No |
| Tannin + Oil | 24.4 | 20.7 | 0.85 | Antagonistic | Protein binding | Biohydrogenation | No |
| Essential Oil + Oil | 20.1 | 16.1 | 0.80 | Antagonistic | Membrane disruption | Biohydrogenation | No |
| Combination | Primary Mechanism A | Primary Mechanism B | Secondary Mechanisms | Biochemical Interaction | Ecological Interaction |
|---|---|---|---|---|---|
| Tannin + Nitrate | Protein binding | Alternative H-sink | Reduced H availability, altered fermentation | Non-overlapping targets (methanogenesis vs. H production) | Complementary microbial targeting (methanogens vs. H producers) |
| 3-NOP + Macroalgae | Direct enzyme inhibition | Biohydrogenation/Toxicity | Multiple methanogen inhibition | Multiple points of methanogenesis pathway inhibition | Different methanogen species targeted |
| Nitrate + 3-NOP | Alternative H-sink | Direct enzyme inhibition | Reduced methanogenesis + H diversion | Simultaneous substrate reduction and enzyme inhibition | Complementary ecological niches |
| Essential Oil + Ionophore | Membrane disruption | Propionate enhancement | Altered fermentation, multiple antimicrobial | Different membrane targeting mechanisms | Different microbial targeted (gram-positive vs. diverse) |
| Essential Oil + 3-NOP | Membrane disruption | Direct enzyme inhibition | Multiple methanogen inhibition | Membrane permeability may enhance 3-NOP access | Complementary targeting (community structure vs. specific enzyme) |
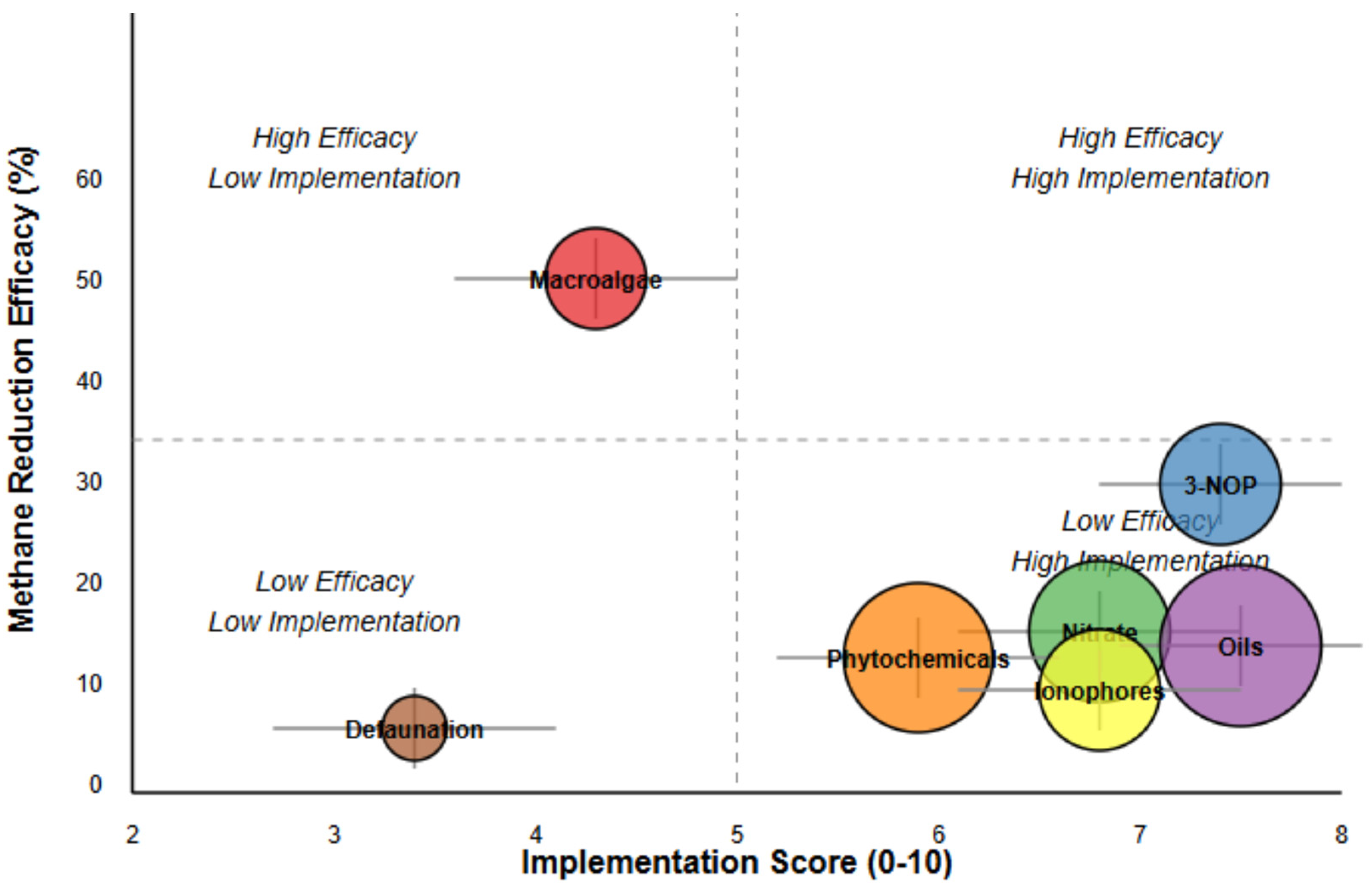
| Intervention | Cost Score | Regulatory Score | Production Impact | Intensive Systems | Grazing Systems | Overall Score | CI |
|---|---|---|---|---|---|---|---|
| Macroalgae | 3.2 | 4.5 | 5.2 | 6.2 | 2.5 | 4.3 | [3.6, 5.1] |
| 3-NOP | 6.5 | 7.8 | 8.5 | 8.2 | 6.0 | 7.4 | [6.8, 8.0] |
| Nitrate | 7.8 | 8.5 | 4.5 | 7.5 | 5.0 | 6.8 | [6.1, 7.5] |
| Oils | 8.2 | 9.2 | 6.8 | 7.2 | 5.8 | 7.5 | [6.9, 8.1] |
| Phytochemicals | 6.5 | 7.2 | 5.5 | 5.5 | 4.5 | 5.9 | [5.2, 6.5] |
| Ionophores | 8.5 | 8.8 | 6.2 | 6.0 | 3.5 | 6.8 | [6.1, 7.4] |
| Defaunation | 2.5 | 3.5 | 2.8 | 5.5 | 2.8 | 3.4 | [2.7, 4.2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purba, R.A.P.; Sangsawad, P. Meta-Analysis of Dietary Interventions for Enteric Methane Mitigation in Ruminants Through Methodological Advancements and Implementation Pathways. Vet. Sci. 2025, 12, 372. https://doi.org/10.3390/vetsci12040372
Purba RAP, Sangsawad P. Meta-Analysis of Dietary Interventions for Enteric Methane Mitigation in Ruminants Through Methodological Advancements and Implementation Pathways. Veterinary Sciences. 2025; 12(4):372. https://doi.org/10.3390/vetsci12040372
Chicago/Turabian StylePurba, Rayudika Aprilia Patindra, and Papungkorn Sangsawad. 2025. "Meta-Analysis of Dietary Interventions for Enteric Methane Mitigation in Ruminants Through Methodological Advancements and Implementation Pathways" Veterinary Sciences 12, no. 4: 372. https://doi.org/10.3390/vetsci12040372
APA StylePurba, R. A. P., & Sangsawad, P. (2025). Meta-Analysis of Dietary Interventions for Enteric Methane Mitigation in Ruminants Through Methodological Advancements and Implementation Pathways. Veterinary Sciences, 12(4), 372. https://doi.org/10.3390/vetsci12040372







