First Serologic Evidence of West Nile Virus and Usutu Virus Circulation Among Dogs in the Bulgarian Danube Region and Analysis of Some Risk Factors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Collection of Samples
2.2. Serological Assays
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Postler, T.S.; Beer, M.; Blitvich, B.J.; Bukh, J.; de Lamballerie, X.; Drexler, J.F.; Imrie, A.; Kapoor, A.; Karganova, G.G.; Lemey, P.; et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol. 2023, 168, 224. [Google Scholar] [CrossRef] [PubMed]
- Huston, N.C.; Tsao, L.H.; Brackney, D.E.; Pyle, A.M. The West Nile virus genome harbors essential riboregulatory elements with conserved and host-specific functional roles. Proc. Natl. Acad. Sci. USA 2024, 121, e2312080121. [Google Scholar] [CrossRef]
- Fagre, A.C.; Lyons, S.; Staples, J.E.; Lindsey, N. West Nile Virus and Other Nationally Notifiable Arboviral Diseases—United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 901–906. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; LaDeau, S.L.; Marra, P.P. Ecology of West Nile virus transmission and its impact on birds in the Western hemisphere. Auk 2007, 124, 1121–1136. [Google Scholar] [CrossRef]
- Jourdain, E.; Toussaint, Y.; Leblond, A.; Bicout, D.J.; Sabatier, P.; Gauthier-Clerc, M. Bird species potentially involved in introduction, amplification, and spread of West Nile virus in a Mediterranean wetland, the Camargue (Southern France). Vector-Borne Zoonotic Dis. 2007, 7, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, M.; Heesterbeek, H.; Martínez-de la Puente, J.; Jiménez-Clavero, M.Á.; Vázquez, A.; Ruiz, S.; Llorente, F.; Roiz, D.; Vernooij, H.; Soriguer, R.; et al. The role of different Culex mosquito species in the transmission of West Nile virus and avian malaria parasites in Mediterranean areas. Transbound. Emerg. Dis. 2021, 68, 920–930. [Google Scholar] [CrossRef]
- Bowen, R.A.; Nemeth, N.M. Experimental infections with West Nile virus. Curr. Opin. Infect. Dis. 2007, 20, 293–297. [Google Scholar] [CrossRef]
- García-Carrasco, J.M.; Muñoz, A.R.; Olivero, J.; Segura, M.; Real, R. Mapping the risk for West Nile virus transmission, Africa. Emerg. Infect. Dis. 2022, 28, 777–785. [Google Scholar] [CrossRef]
- Hayes, E.B.; Sejvar, J.J.; Zaki, S.R.; Lanciotti, R.S.; Bode, A.V.; Campbell, G.L. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg. Infect. Dis. 2005, 119, 1174–1179. [Google Scholar] [CrossRef]
- Sejvar, J.J. Clinical manifestations and outcomes of West Nile virus infection. Viruses 2014, 6, 606–623. [Google Scholar] [CrossRef]
- Cantile, C.; Del Piero, F.; Di Guardo, G.; Arispici, M. Pathologic and immunohistochemical findings in naturally occuring West Nile virus infection in horses. Vet. Pathol. 2001, 38, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Olivares, J.; Wood, J. West Nile virus infection of horses. Vet. Res. 2004, 35, 467–483. [Google Scholar] [CrossRef]
- Austgen, L.E.; Bowen, R.A.; Bunning, M.L.; Davis, B.S.; Mitchell, C.J.; Chang, G.J. Experimental infection of cats and dogs with West Nile virus. Emerg. Infect. Dis. 2004, 10, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Lichtensteiger, C.A.; Heinz-Taheny, K.; Osborne, T.S.; Novak, R.J.; Lewis, B.A.; Firth, M.L. West Nile virus encephalitis and myocarditis in wolf and dog. Emerg. Infect. Dis. 2003, 9, 1303–1306. [Google Scholar] [CrossRef]
- Read, R.W.; Rodriguez, D.B.; Summers, B.A. West Nile virus encephalitis in a dog. Vet. Pathol. 2005, 42, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Abbo, S.R.; Visser, T.M.; Westenberg, M.; Geertsema, C.; Fros, J.J.; Koenraadt, C.J.M.; Pijlman, G.P. Competition between Usutu virus and West Nile virus during simultaneous and sequential infection of Culex pipiens mosquitoes. Emerg. Microbes Infect. 2020, 9, 2642–2652. [Google Scholar] [CrossRef]
- Gaibani, P.; Rossini, G. An overview of Usutu virus. Microbes Infect. 2017, 19, 382–387. [Google Scholar] [CrossRef]
- Nelson, A.N.; Ploss, A. Emerging mosquito-borne flaviviruses. mBio 2024, 15, e0294624. [Google Scholar] [CrossRef]
- Lan, D.; Ji, W.; Yu, D.; Chu, J.; Wang, C.; Yang, Z.; Hua, X. Serological evidence of West Nile virus in dogs and cats in China. Arch. Virol. 2011, 156, 893–895. [Google Scholar] [CrossRef]
- Durand, B.; Haskouri, H.; Lowenski, S.; Vachiery, N.; Beck, C.; Lecollinet, S. Seroprevalence of West Nile and Usutu viruses in military working horses and dogs, Morocco, 2012: Dog as an alternative WNV sentinel species? Epidemiol. Infect. 2016, 144, 1857–1864. [Google Scholar] [CrossRef]
- Lan, D.L.; Wang, C.S.; Deng, B.; Zhou, J.P.; Cui, L.; Tang, C.; Yue, H.; Hua, X.G. Serological investigations on West Nile virus in birds and horses in Shanghai, China. Epidemiol. Infect. 2013, 141, 596–600. [Google Scholar] [CrossRef]
- Rocheleau, J.P.; Michel, P.; Lindsay, L.R.; Drebot, M.; Dibernardo, A.; Ogden, N.H.; Fortin, A.; Arsenault, J. Emerging arboviruses in Quebec, Canada: Assessing public health risk by serology in humans, horses and pet dogs. Epidemiol. Infect. 2017, 145, 2940–2948. [Google Scholar] [CrossRef] [PubMed]
- Pham-Thanh, L.; Nguyen-Tien, T.; Magnusson, U.; Bui-Nghia, V.; Bui-Ngoc, A.; Le-Thanh, D.; Lundkvist, Å.; Can-Xuan, M.; Nguyen-Thi, T.T.; Vu-Thi Bich, H.; et al. Dogs as sentinels for flavivirus exposure in Urban, Peri-Urban and Rural Hanoi, Vietnam. Viruses 2021, 13, 507. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.N.; Mosena, A.C.S.; Baumbach, L.F.; da Silva, M.S.; Canova, R.; Dos Santos, D.R.L.; Budaszewski, R.D.F.; de Oliveira, L.V.; Soane, M.M.; Saraiva, N.B.; et al. Serologic evidence of West Nile virus and Saint Louis encephalitis virus in horses from Southern Brazil. Braz. J. Microbiol. 2021, 52, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Constant, O.; Gil, P.; Barthelemy, J.; Bolloré, K.; Foulongne, V.; Desmetz, C.; Leblond, A.; Desjardins, I.; Pradier, S.; Joulié, A.; et al. One Health surveillance of West Nile and Usutu viruses: A repeated cross-sectional study exploring seroprevalence and endemicity in Southern France, 2016 to 2020. Euro Surveill. 2022, 27, 2200068. [Google Scholar] [CrossRef]
- Gothe, L.M.R.; Ganzenberg, S.; Ziegler, U.; Obiegala, A.; Lohmann, K.L.; Sieg, M.; Vahlenkamp, T.W.; Groschup, M.H.; Hörügel, U.; Pfeffer, M. Horses as sentinels for the circulation of flaviviruses in Eastern-Central Germany. Viruses 2023, 15, 1108. [Google Scholar] [CrossRef]
- Ben-Mostafa, K.K.; Savini, G.; Di Gennaro, A.; Teodori, L.; Leone, A.; Monaco, F.; Alaoqib, M.M.A.; Rayes, A.A.; Dayhum, A.; Eldaghayes, I. Evidence of West Nile virus circulation in horses and dogs in Libya. Pathogens 2023, 13, 41. [Google Scholar] [CrossRef]
- Mohammed, M.N.; Yasmin, A.R.; Ramanoon, S.Z.; Noraniza, M.A.; Ooi, P.T.; Ain-Najwa, M.Y.; Natasha, J.A.; Nur-Fazila, S.H.; Arshad, S.S.; Mohammed, H.O. Serological and molecular surveillance of West Nile virus in domesticated mammals of peninsular Malaysia. Front. Vet. Sci. 2023, 10, 1126199. [Google Scholar] [CrossRef]
- Yuen, N.K.Y.; Harrison, J.J.; Wang, A.S.W.; McMahon, I.E.; Habarugira, G.; Coyle, M.P.; Bielefeldt-Ohmann, H. Orthoflavivirus circulation in South-East Queensland, Australia, before and during the 2021–2022 incursion of Japanese encephalitis virus assessed through sero-epidemiological survey of a sentinel equine population. One Health 2024, 19, 100930. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 13th ed.; World Organisation for Animal Health: Paris, France, 2024; Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ (accessed on 7 March 2025).
- Prince, H.E.; Hogrefe, W.R. Assays for detecting West Nile Virus antibodies in human serum, plasma, and cerebrospinal fluid. Clin. Appl. Immunol. Rev. 2005, 5, 45–63. [Google Scholar] [CrossRef]
- Kuno, G. Serodiagnosis of flaviviral infections and vaccinations in humans. Adv. Virus Res. 2003, 61, 3–65. [Google Scholar] [PubMed]
- Maezono, K.; Kobayashi, S.; Tabata, K.; Yoshii, K.; Kariwa, H. Development of a highly specific serodiagnostic ELISA for West Nile virus infection using subviral particles. Sci. Rep. 2021, 11, 9213. [Google Scholar] [CrossRef] [PubMed]
- Khare, B.; Kuhn, R.J. The Japanese Encephalitis Antigenic Complex Viruses: From Structure to Immunity. Viruses 2022, 14, 2213. [Google Scholar] [CrossRef]
- Klaus, C.; Ziegle, U.; Kalthoff, D.; Hoffmann, B.; Beer, M. Tick-borne encephalitis virus (TBEV)—Findings on cross reactivity and longevity of TBEV antibodies in animal sera. BMC Vet. Res. 2014, 10, 78. [Google Scholar] [CrossRef]
- Maeki, T.; Tajima, S.; Ando, N.; Wakimoto, Y.; Hayakawa, K.; Kutsuna, S.; Kato, F.; Taniguchi, S.; Nakayama, E.; Lim, C.K.; et al. Analysis of cross-reactivity among flaviviruses using sera of patients with dengue showed the importance of neutralization tests with paired serum samples for the correct interpretations of serological test results for dengue. J. Infect. Chemother. 2023, 29, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z. Mosquito-borne viruses in Europe. Parasitol. Res. 2008, 103 (Suppl. S1), 29–43. [Google Scholar] [CrossRef]
- Tamba, M.; Caminiti, A.; Prosperi, A.; Desprès, P.; Lelli, D.; Galletti, G.; Moreno, A.; Paternoster, G.; Santi, A.; Licata, E.; et al. Accuracy estimation of an indirect ELISA for the detection of West Nile Virus antibodies in wild birds using a latent class model. J. Virol. Methods 2017, 248, 202–206. [Google Scholar] [CrossRef]
- Baymakova, M.; Trifonova, I.; Panayotova, E.; Dakova, S.; Pacenti, M.; Barzon, L.; Lavezzo, E.; Hristov, Y.; Ramshev, K.; Plochev, K.; et al. Fatal case of West Nile neuroinvasive disease in Bulgaria. Emerg. Infect. Dis. 2016, 22, 2203–2204. [Google Scholar] [CrossRef]
- Christova, I.; Panayotova, E.; Tchakarova, S.; Taseva, E.; Trifonova, I.; Gladnishka, T.A. Nationwide seroprevalence screening for West Nile virus and Tick-borne encephalitis virus in the population of Bulgaria. J. Med. Virol. 2017, 89, 1875–1878. [Google Scholar] [CrossRef]
- Panayotova, E.; Christova, I.; Trifonova, I.; Taseva, E.; Gladnishka, T.; Ivanova, V. Seroprevalence of West Nile virus in Bulgaria, 2018. Probl. Infect. Parasit. Dis. 2019, 47, 15–17. [Google Scholar] [CrossRef]
- Christova, I.; Papa, A.; Trifonova, I.; Panayotova, E.; Pappa, S.; Mikov, O. West Nile virus lineage 2 in humans and mosquitoes in Bulgaria, 2018-2019. J. Clin. Virol. 2020, 127, 104365. [Google Scholar] [CrossRef]
- Trifonova, I.; Christova, I.; Ivanova-Aleksandrova, N.; Gladnishka, T.; Ivanova, V.; Panayotova, E.; Taseva, E.; Dimitrov, D.; Marinov, M.; Kamenov, G.; et al. Survey of Borrelia Burgdorferi Sensu Lato and West Nile Fever virus in wild birds in Bulgaria. Biologia 2022, 77, 3519–3524. [Google Scholar] [CrossRef]
- Rusenova, N.; Rusenov, A.; Monaco, F.A. Retrospective Study on the Seroprevalence of West Nile Virus Among Donkeys and Mules in Bulgaria. Vector-Borne Zoonotic Dis. 2024, 24, 274–277. [Google Scholar] [CrossRef]
- Rusenova, N.; Rusenov, A.; Chervenkov, M.; Sirakov, I. Seroprevalence of West Nile Virus among Equids in Bulgaria in 2022 and Assessment of Some Risk Factors. Vet. Sci. 2024, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- DiGennaro, A.; Lorusso, A.; Casaccia, C.; Conte, A.; Monaco, F.; Savini, G. Serum neutralization assay can efficiently replace plaque reduction neutralization test for detection and quantitation of West Nile virus antibodies in human and animal serum samples. Clin. Vaccine Immunol. 2014, 21, 1460–1462. [Google Scholar] [CrossRef]
- Laidoudi, Y.; Durand, G.; Watier-Grillot, S.; Dessimoulie, A.S.; Labarde, C.; Normand, T.; Andréo, V.; Guérin, P.; Grard, G.; Davoust, B. Evidence of Antibodies against the West Nile Virus and the Usutu Virus in Dogs and Horses from the Southeast of France. Transbound. Emerg. Dis. 2023, 2023, 779723. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Popovic, N.; Milošević, B.; Urošević, A.; Poluga, J.; Lavadinović, L.; Nedelijković, J.; Jevtović, D.; Dulović, O. Outbreak of West Nile virus infection among humans in Serbia, August to October 2012. Euro Surveill. 2013, 18, 30613. [Google Scholar] [CrossRef]
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile virus in Europe: Emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 2013, 19, 699–704. [Google Scholar] [CrossRef]
- ECDC. Epidemiological Update: West Nile Virus Transmission Season in Europe, 2023. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2023-0 (accessed on 21 February 2025).
- Matsuda, M.; Yamanaka, A.; Yato, K.; Yoshii, K.; Watashi, K.; Aizaki, H.; Konishi, E.; Takasaki, T.; Kato, T.; Muramatsu, M. High-throughput neutralization assay for multiple flaviviruses based on single-round infectious particles using dengue virus type 1 reporter replicon. Sci. Rep. 2018, 8, 16624. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.R.; Ismail, A.A.; Thergarajan, G.; Raju, C.S.; Yam, H.C.; Rishya, M.; Sekaran, S.D. Serological cross-reactivity among common flaviviruses. Front. Cell. Infect. Microbiol. 2022, 12, 975398. [Google Scholar] [CrossRef] [PubMed]
- Vasic, A.; Răileanu, C.; Körsten, C.; Vojinovi′c, D.; Mani′c, M.; Uroševi′c, A.; Nikoli′c, N.; Dulovi′c, O.; Tews, B.A.; Petrovi′c, T.; et al. West Nile Virus in the Republic of Serbia-Diagnostic Performance of Five Serological Tests in Dog and Horse Sera. Transbound. Emerg. Dis. 2022, 69, e2506–e2515. [Google Scholar] [CrossRef]
- Trozzi, G.; Adjadj, N.R.; Vervaeke, M.; Matthijs, S.; Sohier, C.; De Regge, N. Comparison of Serological Methods for Tick-Borne Encephalitis Virus-Specific Antibody Detection in Wild Boar and Sheep: Impact of the Screening Approach on the Estimated Seroprevalence. Viruses 2023, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Lowenski, S.; Durand, B.; Bahuon, C.; Zientara, S.; Lecollinet, S. Improved Reliability of Serological Tools for the Diagnosis of West Nile Fever in Horses within Europe. PLoS Negl. Trop. Dis. 2017, 11, e0005936. [Google Scholar] [CrossRef]
- Vista, F.E.S.; Tantengco, O.A.G.; Dispo, M.D.; Opiso, D.M.S.; Badua, C.L.D.C.; Gerardo, J.P.Z.; Perez, J.R.M.; Baldo, K.A.T.; Chao, D.Y.; Dalmacio, L.M.M. Trends in ELISA-based flavivirus IgG serosurveys: A systematic review. Trop. Med. Infect. Dis. 2023, 8, 224. [Google Scholar] [CrossRef]
- Tsankov, K. On the names of the islands in the Danube. State Probl. Bulg. Onomast. 2018, 15, 65–77. [Google Scholar]
- Papa, A.; Anastasiadou, A.; Delianidou, M. West Nile virus IgM and IgG antibodies three years post-infection. Hippokratia 2015, 9, 34–36. [Google Scholar]
- Buckweitz, S.; Kleiboeker, S.; Marioni, K.; Ramos-Vara, J.; Rottinghaus, A.; Schwabenton, B.; Johnson, G. Serological, reverse transcriptase-polymerase chain reaction, andimmunohistochemical detection ofWest Nile virus in a clinically affected dog. J. Vet. Diagn. Investig. 2003, 15, 324–329. [Google Scholar] [CrossRef]
- Suka, M.; Cirkovic, V.; Siljic, M.; Jankovic, M.; Loncar, A.; Rajkovic, M.; Stamenkovic, G.; Vukicevic-Radic, O.; Stanojevic, M. Dynamics of West Nile Virus Lineage 2 Spread in the Balkans in the Context of Global Spatio-Temporal Dispersal. J. Med. Virol. 2024, 96, e70092. [Google Scholar] [CrossRef]
- Knap, N.; Korva, M.; Ivović, V.; Kalan, K.; Jelovšek, M.; Sagadin, M.; Zakotnik, S.; Strašek Smrdel, K.; Slunečko, J.; Avšič-Županc, T. West Nile Virus in Slovenia. Viruses 2020, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Montagnaro, S.; Piantedosi, D.; Ciarcia, R.; Loponte, R.; Veneziano, V.; Fusco, G.; Amoroso, M.G.; Ferrara, G.; Damiano, S.; Iovane, G.; et al. Serological Evidence of Mosquito-Borne Flaviviruses Circulation in Hunting Dogs in Campania Region, Italy. Vector-Borne Zoonotic Dis. 2019, 19, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.P.; Grunenwald, P.; Blackmar, D.; Hailey, C.; Bueno, R.; Murray, K.O. Juvenile dogs as potential sentinels for West Nile virus surveillance. Zoonoses Public Health 2008, 55, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Oslobanu, E.L.; Crivei, L.A.; Ratoi, I.A.; Crivei, I.; Savuţa, G. Prevalence of West Nile virus antibodies in indoor dogs from an urban area in Iași, Romania: Indicators of viral presence and urban transmission potential. J. Appl. Life Sci. Environ. 2023, 56, 221–230. [Google Scholar] [CrossRef]

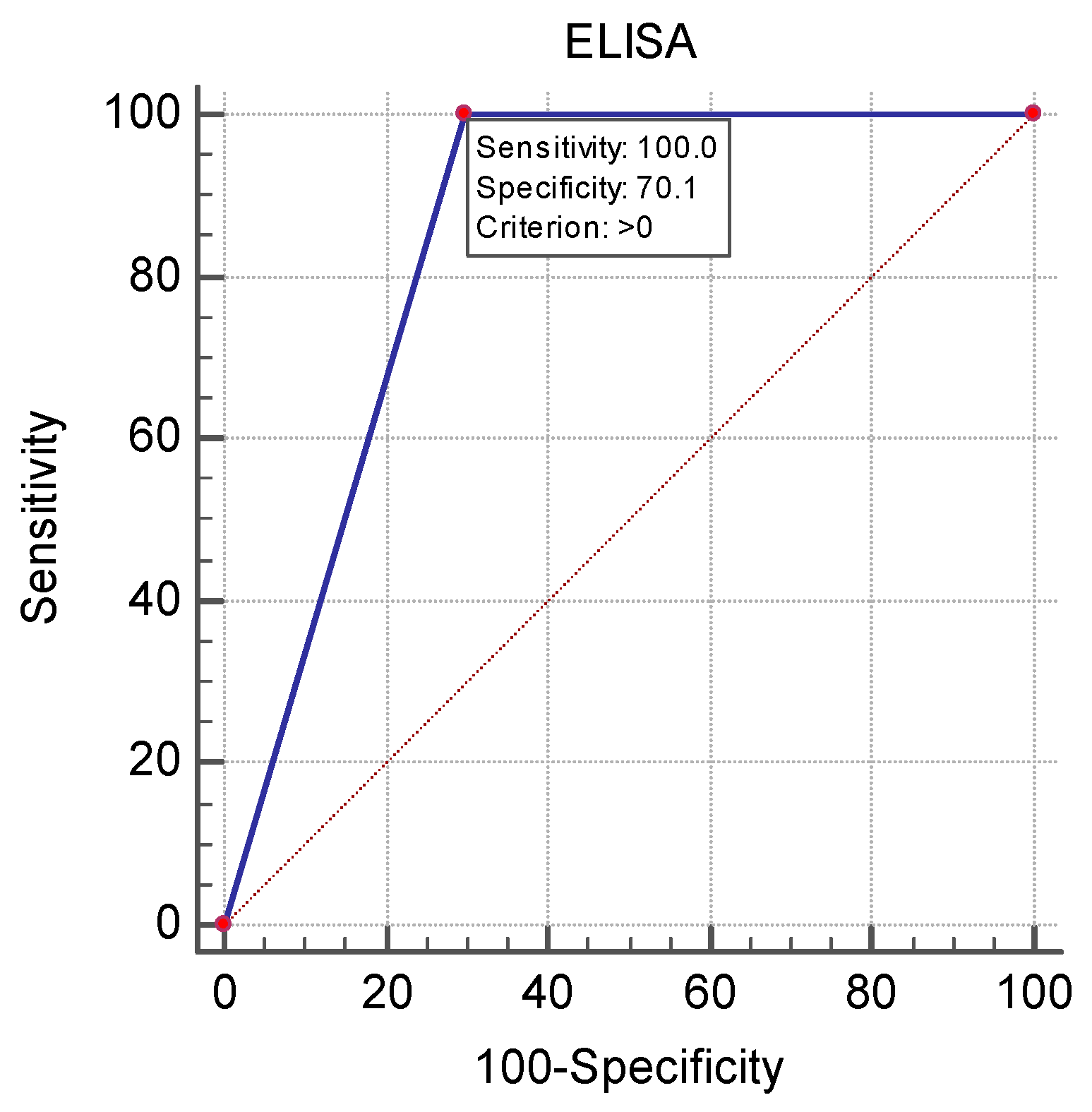
| Parameter | Negative, n = 110 Median (IQR) or Number (%) | Positive, n = 91 Median (IQR) or Number (%) | p-Value | |
|---|---|---|---|---|
| Region | Northwestern | 50 (47.2) | 56 (52.8) | 0.0635 |
| North-central | 33 (66.0) | 17 (34.0) | ||
| Northeastern | 27 (60.0) | 18 (40.0) | ||
| District | Vratsa | 20 (55.6) | 16 (44.4) | 0.0700 |
| Pleven | 30 (42.9) | 40 (57.1) | ||
| Ruse | 33 (66.0) | 17 (34.0) | ||
| Silistra | 27 (60.0) | 18 (40.0) | ||
| Age, years | 4 (1–5) | 5 (3–6) | 0.0073 | |
| Sex | Female | 63 (52.1) | 58 (47.9) | 0.4312 |
| Male | 47 (58.7) | 33 (41.2) | ||
| Parameter | Negative, n = 157 Median (IQR) or Number (%) | Positive, n = 44 Median (IQR) or Number (%) | p-Value | |
|---|---|---|---|---|
| Region | Northwestern | 77 (72.6) | 29 (27.4) | 0.0500 |
| North-central | 45 (90.0) | 5 (10.0) | ||
| Northeastern | 35 (78.8) | 10 (22.2) | ||
| District | Vratsa | 26 (72.2) | 10 (27.8) | 0.1118 |
| Pleven | 51 (72.9) | 19 (27.1) | ||
| Ruse | 45 (90.0) | 5 (10.0) | ||
| Silistra | 35 (78.8) | 10 (22.2) | ||
| Age, years | 4 (2–6) | 4.5 (3.5–6) | 0.1129 | |
| Sex | Female | 93 (76.9) | 28 (23.1) | 0.7242 |
| Male | 64 (80.0) | 16 (20.0) | ||
| N Samples | VNT-WNV Titre | VNT-USUV Titre | Interpretation |
|---|---|---|---|
| 13 | 1:10 | ND | WNV |
| 16 | 1:20 | ND | WNV |
| 1 | 1:20 | 1:5 | WNV |
| 7 | 1:40 | ND | WNV |
| 1 | 1:40 | 1:10 | WNV |
| 3 | 1:80 | ND | WNV |
| 1 | 1:80 | 1:10 | WNV |
| 1 | 1:80 | 1:20 | WNV |
| 1 | 1:160 | 1:10 | WNV |
| 6 | ND | 1:10 | USUV |
| 2 | ND | 1:20 | USUV |
| 2 | ND | 1:40 | USUV |
| 1 | 1:10 | 1:40 | USUV |
| 1 | 1:40 | 1:160 | USUV |
| 2 | 1:10 | 1:10 | WNV + USUV |
| 1 | 1:10 | 1:20 | WNV + USUV |
| 1 | 1:20 | 1:10 | WNV + USUV |
| 3 | 1:20 | 1:20 | WNV + USUV |
| 1 | 1:40 | 1:20 | WNV + USUV |
| 1 | 1:40 | 1:40 | WNV + USUV |
| 26 | ND | ND | UD * |
| Total 91 | 44 WNV, 12 USUV, 9 WNV/USUV, 26 UD |
| Parameter | Negative, n = 189 Median (IQR) or Number (%) | Positive, n = 12 Median (IQR) or Number (%) | p-Value | |
|---|---|---|---|---|
| Region | Northwestern | 98 (92.5) | 8 (7.5) | 0.6060 |
| North-central | 48 (96.0) | 2 (4.0) | ||
| Northeastern | 43 (95.6) | 2 (4.4) | ||
| District | Vratsa | 36 (100.0) | - | 0.0884 |
| Pleven | 62 (88.6) | 8 (11.4) | ||
| Ruse | 48 (96.0) | 2 (4.0) | ||
| Silistra | 43 (95.6) | 2 (4.4) | ||
| Age, years | 4 (2–6) | 4.5 (3–5.5) | 0.6025 | |
| Sex | Female | 114 (94.2) | 7 (5.8) | 0.8666 |
| Male | 75 (93.7) | 5 (6.2) | ||
| Variable | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Region | Northwestern | 3.3896 | 1.2249 to 9.3800 | 0.0187 |
| North-central | - | |||
| Northeastern | 2.5714 | 0.8054 to 8.2098 | 0.1108 | |
| District | Vratsa | 1.0324 | 0.4199 to 2.5385 | 0.9446 |
| Pleven | - | |||
| Ruse | 0.2982 | 0.1030 to 0.8639 | 0.0258 | |
| Silistra | 0.7669 | 0.3186 to 1.8458 | 0.5537 | |
| Age * | <2 y | |||
| ≥2 y | 3.0472 | 1.2101 to 7.6734 | 0.0180 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusenova, N.; Rusenov, A. First Serologic Evidence of West Nile Virus and Usutu Virus Circulation Among Dogs in the Bulgarian Danube Region and Analysis of Some Risk Factors. Vet. Sci. 2025, 12, 373. https://doi.org/10.3390/vetsci12040373
Rusenova N, Rusenov A. First Serologic Evidence of West Nile Virus and Usutu Virus Circulation Among Dogs in the Bulgarian Danube Region and Analysis of Some Risk Factors. Veterinary Sciences. 2025; 12(4):373. https://doi.org/10.3390/vetsci12040373
Chicago/Turabian StyleRusenova, Nikolina, and Anton Rusenov. 2025. "First Serologic Evidence of West Nile Virus and Usutu Virus Circulation Among Dogs in the Bulgarian Danube Region and Analysis of Some Risk Factors" Veterinary Sciences 12, no. 4: 373. https://doi.org/10.3390/vetsci12040373
APA StyleRusenova, N., & Rusenov, A. (2025). First Serologic Evidence of West Nile Virus and Usutu Virus Circulation Among Dogs in the Bulgarian Danube Region and Analysis of Some Risk Factors. Veterinary Sciences, 12(4), 373. https://doi.org/10.3390/vetsci12040373






