Gastric Infusion of Short-Chain Fatty Acids Improves Health via Enhance Liver and Intestinal Immune Response and Antioxidant Capacity in Goats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval Statement
2.2. Goat Management and Experimental Design
2.3. Sampling Collections
2.4. Antioxidant Capacity Analysis
2.5. ELISA Analysis of Cytokines and Tight-Junction Proteins
2.6. Statistical Analysis
3. Results
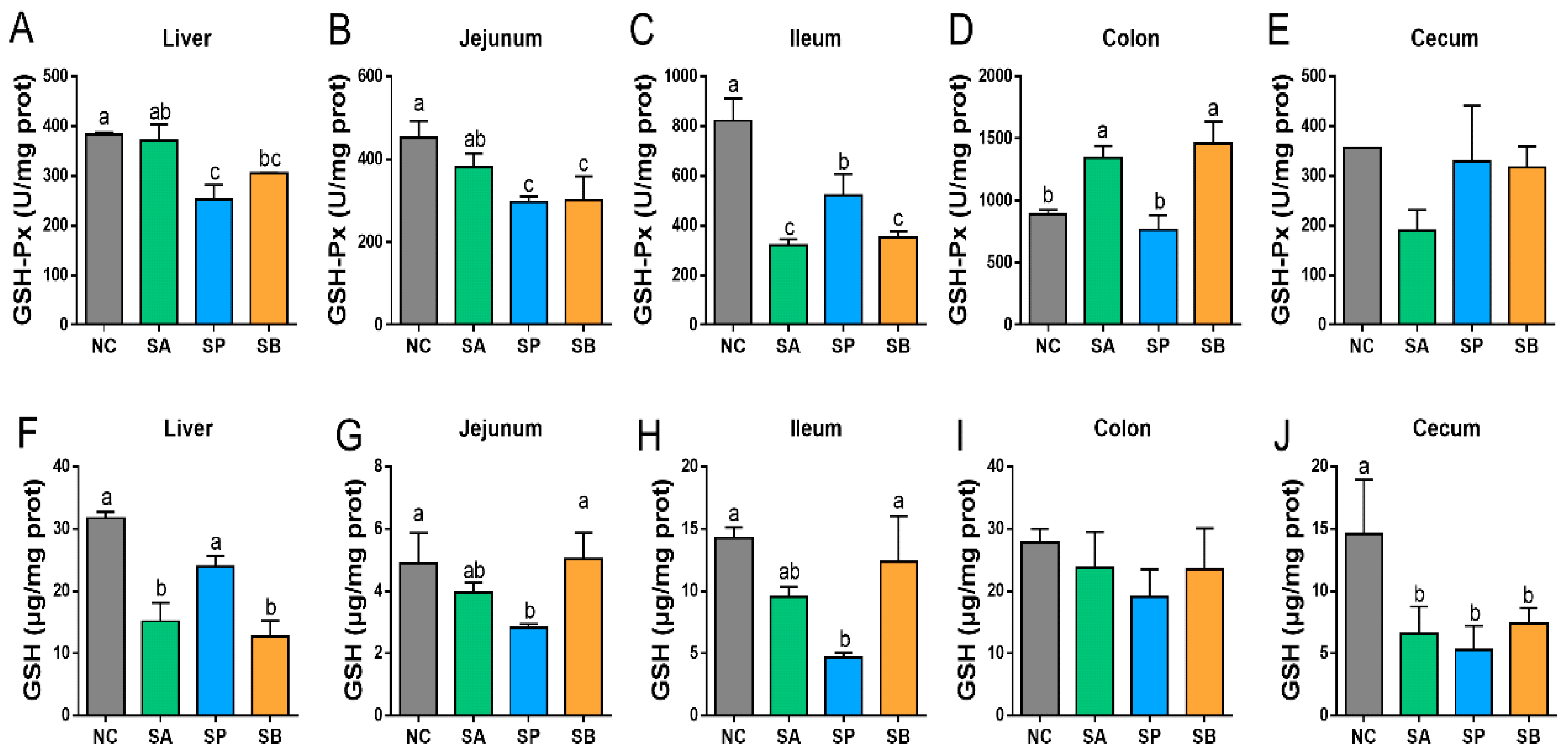
3.1. Effects of SCFAs on Antioxidant Activity in the Liver and Intestine
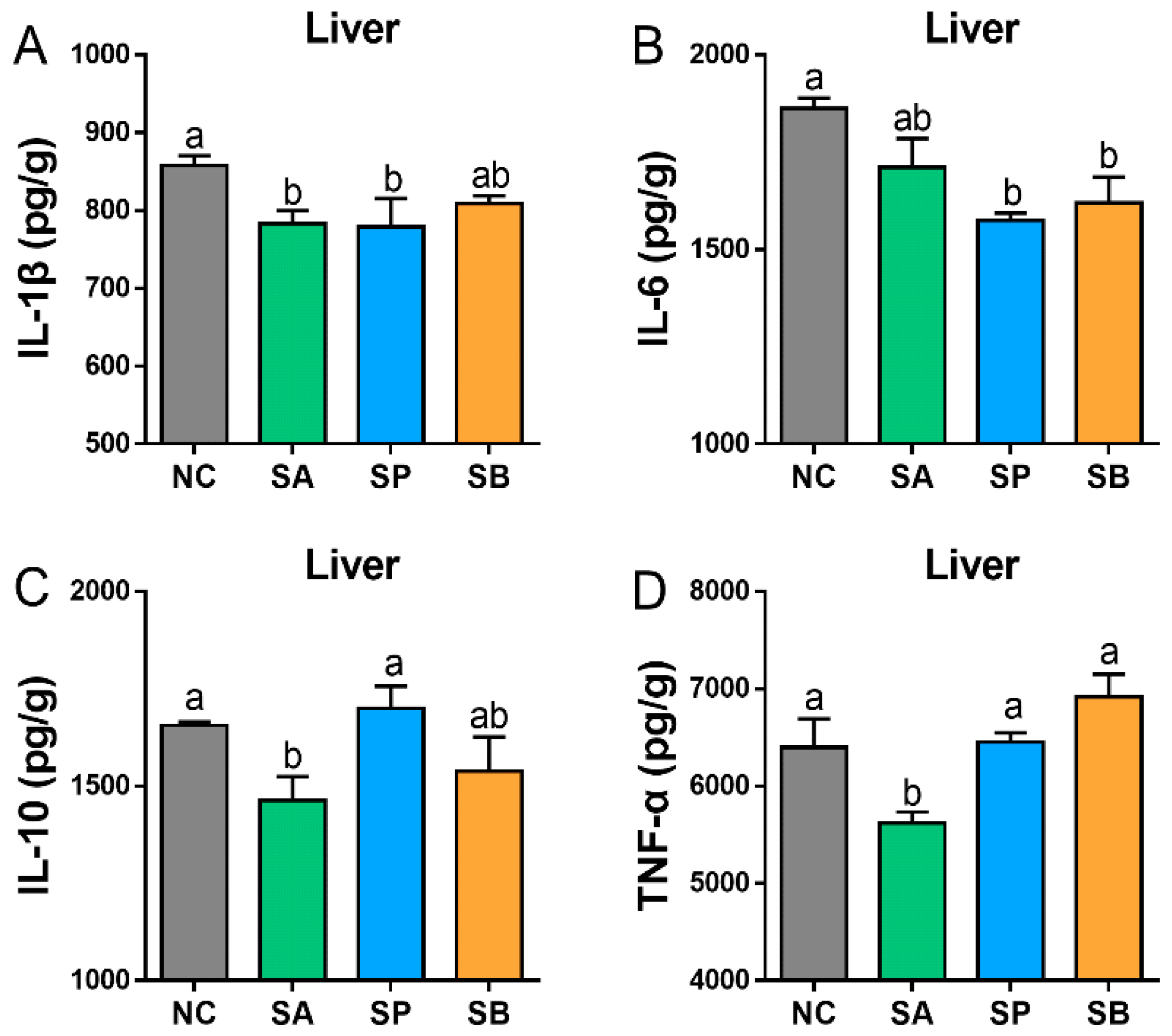
3.2. Effects of SCFAs on Cytokines Concentration in the Liver and Intestine
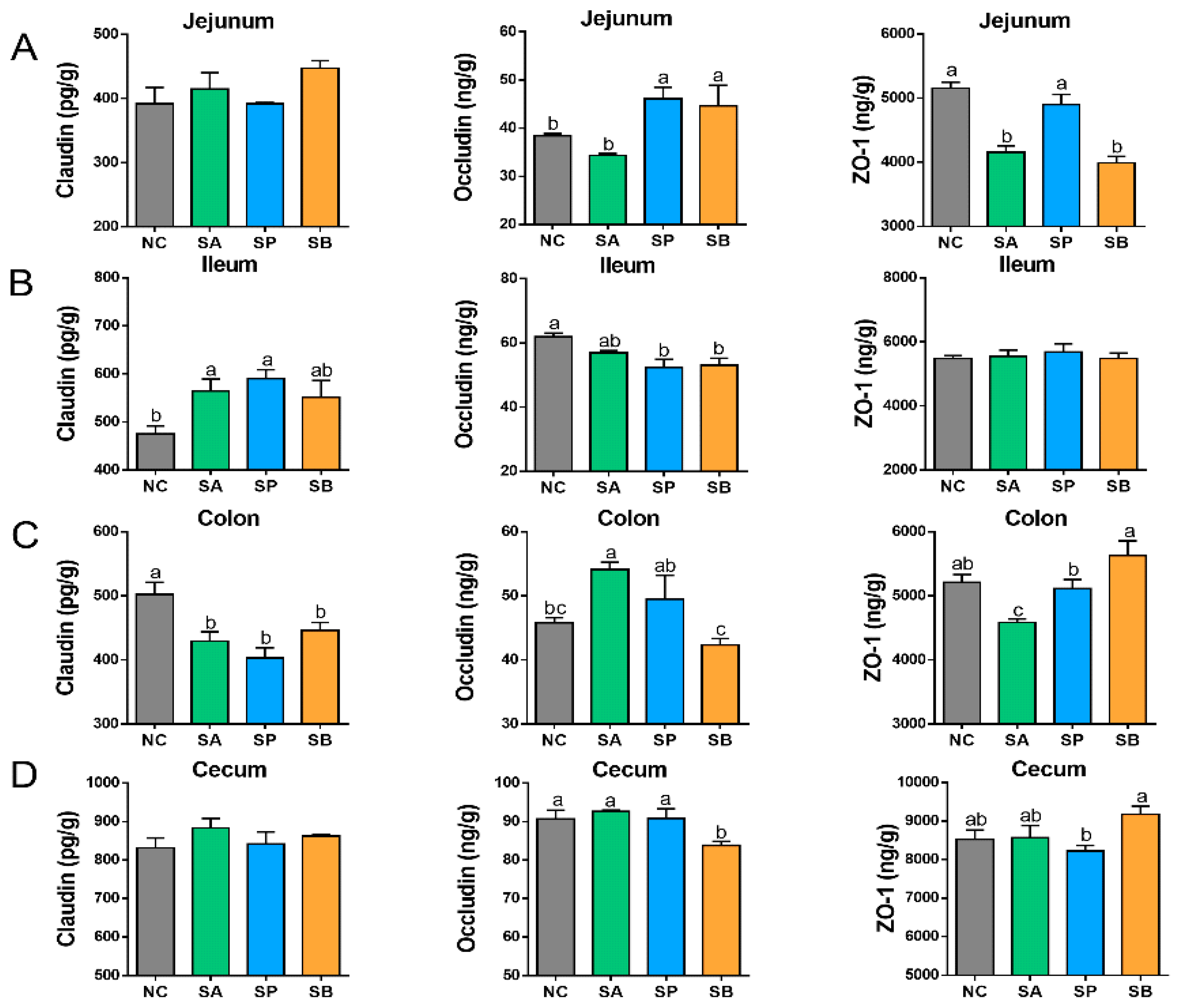
3.3. Effects of SCFAs on Intestinal Tight Junction Proteins
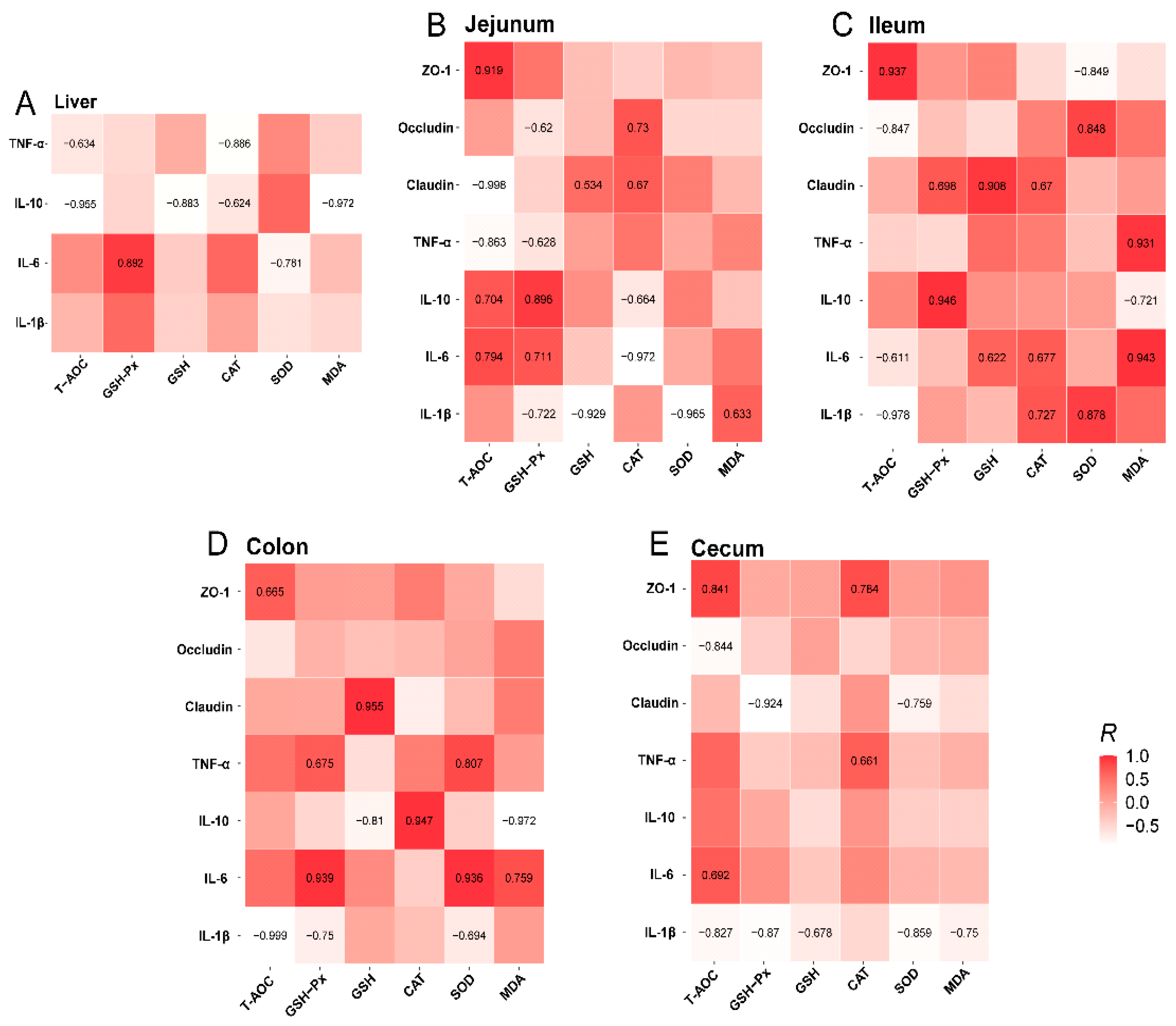
3.4. Relationship Among Intestinal Antioxidant, Inflammatory Cytokine, and Tight-Junction Protein Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.L.; Yap, Y.A.; McLeod, K.H.; Mackay, C.R.; Mariño, E. Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clin. Transl. Immunol. 2016, 5, e82. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; De Vos, P.; Hermoso, M.A. Impact of bacterial metabolites on gut barrier function and host immunity: A focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khalil, A.A.; Rahman, U.-U.; Khalid, A.; Naz, S.; Shariati, M.A.; Rebezov, M.; Urtecho, E.Z.; de Albuquerque, R.D.D.G.; Anwar, S. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6034–6054. [Google Scholar] [CrossRef]
- Pant, K.; Venugopal, S.K.; Pisarello, M.J.L.; Gradilone, S.A. The role of gut microbiome-derived short chain fatty acid butyrate in hepatobiliary diseases. Am. J. Pathol. 2023, 193, 1455–1467. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of intestinal barrier function by microbial metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the intestinal barrier: The involvement of epithelial cells and microbiota—A mutual relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef]
- Khoshbin, K.; Camilleri, M. Effects of dietary components on intestinal permeability in health and disease. Am. J. Physiol. Liver Physiol. 2020, 319, G589–G608. [Google Scholar] [CrossRef]
- Panwar, S.; Sharma, S.; Tripathi, P. Role of barrier integrity and dysfunctions in maintaining the healthy gut and their health outcomes. Front. Physiol. 2021, 12, 715611. [Google Scholar] [CrossRef]
- Vachharajani, V.T.; DeJong, M.P.; Dunn, A.R. PDZ Domains from the Junctional Proteins Afadin and ZO-1 Act as Mechanosensors. bioRxiv 2023. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Liu, J.; Zhong, F.; Zhang, C.; Chen, Y.; Sun, T.; Ji, C.; Ma, D. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat. Commun. 2022, 13, 2522. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Naqvi, S.; Liu, Q.; Pan, H.; Ma, Z.; Kong, N.; Chen, Y.; Shi, D.; Kulyar, M.-A.; Khan, J.A. Short Chain Fatty Acids (SCFAs) Are the Potential Immunomodulatory Metabolites in Controlling Staphylococcus aureus-Mediated Mastitis. Nutrients 2022, 14, 3687. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Erickson, A.; Moreau, R. Divergent regulation of inflammatory cytokines by mTORC1 in THP-1–derived macrophages and intestinal epithelial Caco-2 cells. Life Sci. 2021, 284, 119920. [Google Scholar] [CrossRef] [PubMed]
- Lalles, J.-P.; Montoya, C.A. Dietary alternatives to in-feed antibiotics, gut barrier function and inflammation in piglets post-weaning: Where are we now? Anim. Feed Sci. Technol. 2021, 274, 114836. [Google Scholar] [CrossRef]
- Li, S.; Heng, X.; Guo, L.; Lessing, D.J.; Chu, W. SCFAs improve disease resistance via modulate gut microbiota, enhance immune response and increase antioxidative capacity in the host. Fish Shellfish Immunol. 2022, 120, 560–568. [Google Scholar] [CrossRef]
- Toschi, A.; Piva, A.; Grilli, E. Phenol-rich botanicals modulate oxidative stress and epithelial integrity in intestinal epithelial cells. Animals 2022, 12, 2188. [Google Scholar] [CrossRef]
- Shastak, Y.; Gordillo, A.; Pelletier, W. The relationship between vitamin A status and oxidative stress in animal production. J. Appl. Anim. Res. 2023, 51, 546–553. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Hui, X.; Ma, Y.; Yin, Z.; Chen, Q.; Chen, C.; Wu, H.; Wu, X. Protective Effects of Mulberry (Morus atropurpurea Roxb.) Leaf Protein Hydrolysates and Their In Vitro Gastrointestinal Digests on AAPH-Induced Oxidative Stress in Human Erythrocytes. Foods 2023, 12, 3468. [Google Scholar] [CrossRef]
- Ma, N.; Abaker, J.A.; Bilal, M.S.; Dai, H.; Shen, X. Sodium butyrate improves antioxidant stability in sub-acute ruminal acidosis in dairy goats. BMC Vet. Res. 2018, 14, 275. [Google Scholar] [CrossRef]
- Zhen, Y.; Xi, Z.; Hu, L.; Chen, Y.; Ge, L.; Wei, W.; Loor, J.J.; Yang, Q.; Wang, M. Impacts of circadian gene Period2 knockout on intestinal metabolism and hepatic antioxidant and inflammation state in mice. Oxid. Med. Cell. Longev. 2022, 2022, 7896371. [Google Scholar] [CrossRef]
- Patlevič, P.; Vašková, J.; Švorc, P., Jr.; Vaško, L.; Švorc, P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr. Med. Res. 2016, 5, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals: Antioxidant action, animal health, and product quality—Invited review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, J.; Kong, Y.; Zhao, C.; Liu, S.; Liu, Y.; Li, L.; Yang, J.; Zhu, X.; Zhao, B. Oxidative status in dairy goats: Periparturient variation and changes in subclinical hyperketonemia and hypocalcemia. BMC Vet. Res. 2021, 17, 238. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef]
- He, P.; Lei, Y.; Zhang, K.; Zhang, R.; Bai, Y.; Li, Z.; Jia, L.; Shi, J.; Cheng, Q.; Ma, Y. Dietary oregano essential oil supplementation alters meat quality, oxidative stability, and fatty acid profiles of beef cattle. Meat Sci. 2023, 205, 109317. [Google Scholar] [CrossRef]
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519. [Google Scholar] [CrossRef]
- Diao, H.; Jiao, A.R.; Yu, B.; Mao, X.B.; Chen, D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr. 2019, 14, 4. [Google Scholar] [CrossRef]
- Liu, W.; La, A.L.T.Z.; Evans, A.; Gao, S.; Yu, Z.; Bu, D.; Ma, L. Supplementation with sodium butyrate improves growth and antioxidant function in dairy calves before weaning. J. Anim. Sci. Biotechnol. 2021, 12, 2. [Google Scholar] [CrossRef]
- Georgieva, N.V.; Gabrashanska, M.; Koinarski, V.; Yaneva, Z. Zinc Supplementation against Eimeria acervulina-Induced Oxidative Damage in Broiler Chickens. Vet. Med. Int. 2011, 2011, 647124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Jiang, Y.; Zhu, Q.F.; Gao, F.; Dai, S.F.; Chen, J.; Zhou, G.H. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 2011, 52, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Hong, Z.; Jian, H.; Xu, Q.; Liu, Y.; Wang, X.; Li, Y.; Dong, X.; Zou, X. Alterations in intestinal antioxidant and immune function and cecal microbiota of laying hens fed on coated sodium butyrate supplemented diets. Animals 2022, 12, 545. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Hoseinifar, S.H.; Nejadmoghadam, S.; Khalili, M. Non-specific immune parameters, immune, antioxidant and growth-related genes expression of common carp (Cyprinus carpio L.) fed sodium propionate. Aquac. Res. 2017, 48, 4470–4478. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, X.; Ding, Q.; Duan, M.; Wang, C. Combined effects of dietary phytase and organic acid on growth and phosphorus utilization of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 2014, 430, 1–8. [Google Scholar] [CrossRef]
- Safari, R.; Hoseinifar, S.H.; Kavandi, M. Modulation of antioxidant defense and immune response in zebra fish (Danio rerio) using dietary sodium propionate. Fish Physiol. Biochem. 2016, 42, 1733–1739. [Google Scholar] [CrossRef]
- Chase, C.; Kaushik, R.S. Mucosal immune system of cattle: All immune responses begin here. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 431–451. [Google Scholar] [CrossRef]
- de Oliveira Santos, J.G.; Migueis, D.P.; do Amaral, J.B.; Bachi, A.L.L.; Boggi, A.C.; Thamboo, A.; Voegels, R.L.; Pezato, R. Impact of SARS-CoV-2 on saliva: TNF-⍺, IL-6, IL-10, lactoferrin, lysozyme, IgG, IgA, and IgM. J. Oral Biosci. 2022, 64, 108–113. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Dawood, M.A.O.; Moghadam, M.S.; Sheikhzadeh, N.; Hoseinifar, S.H.; Musthafa, M.S. Modulation of immune parameters and antioxidant defense in zebrafish (Danio rerio) using dietary apple cider vinegar. Aquaculture 2019, 513, 734412. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; Khattaby, A.E.-R.A.; Samir, F.; Awad, S.M.M.; Abdel-Tawwab, M. Stimulatory effect of dietary butyrate on growth, immune response, and resistance of Nile tilapia, Oreochromis niloticus against Aeromonas hydrophila infection. Anim. Feed Sci. Technol. 2019, 254, 114212. [Google Scholar] [CrossRef]
- Du, Y.; Cheng, L.; Zhao, J.; de Cruz, C.R.; Xu, H.; Wang, L.; Xu, Q. Effects of clostridium butyricum and sodium butyrate on growth performance, immunity, and gut microbiota of mirror carp cyprinus carpio fed with soybean meal based diet. Aquac. Rep. 2023, 29, 101501. [Google Scholar] [CrossRef]
- Tan, H.Y.; Chen, S.-W.; Hu, S.-Y. Improvements in the growth performance, immunity, disease resistance, and gut microbiota by the probiotic Rummeliibacillus stabekisii in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 92, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Busti, S.; Rossi, B.; Volpe, E.; Ciulli, S.; Piva, A.; D’Amico, F.; Soverini, M.; Candela, M.; Gatta, P.P.; Bonaldo, A. Effects of dietary organic acids and nature identical compounds on growth, immune parameters and gut microbiota of European sea bass. Sci. Rep. 2020, 10, 21321. [Google Scholar] [CrossRef] [PubMed]
- Zabana, Y.; Lorén, V.; Domènech, E.; Aterido, A.; Garcia-Jaraquemada, A.; Julià, A.; Vicario, M.; Pedrosa, E.; Ferreiro, M.; Troya, J. Transcriptomic identification of TMIGD1 and its relationship with the ileal epithelial cell differentiation in Crohn’s disease. Am. J. Physiol. Liver Physiol. 2020, 319, G109–G120. [Google Scholar] [CrossRef]
- Andrade, M.E.R.; Araújo, R.S.; de Barros, P.A.V.; Soares, A.D.N.; Abrantes, F.A.; de Vasconcelos Generoso, S.; Fernandes, S.O.A.; Cardoso, V.N. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin. Nutr. 2015, 34, 1080–1087. [Google Scholar] [CrossRef]
- Morhardt, T.L.; Hayashi, A.; Ochi, T.; Quirós, M.; Kitamoto, S.; Nagao-Kitamoto, H.; Kuffa, P.; Atarashi, K.; Honda, K.; Kao, J.Y. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci. Rep. 2019, 9, 1223. [Google Scholar] [CrossRef]
- Wang, C.C.; Wu, H.; Lin, F.H.; Gong, R.; Xie, F.; Peng, Y.; Feng, J.; Hu, C.H. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 2018, 24, 40–46. [Google Scholar] [CrossRef]
- Plöger, S.; Stumpff, F.; Penner, G.B.; Schulzke, J.; Gäbel, G.; Martens, H.; Shen, Z.; Günzel, D.; Aschenbach, J.R. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann. N. Y. Acad. Sci. 2012, 1258, 52–59. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Dai, J.; Yang, P.; Xu, W.; Ai, Q.; Zhang, W.; Zhang, Y.; Zhang, Y.; Mai, K. Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): Effects on inflammatory status, mucosal barriers and microbiota in the intestine. Fish Shellfish Immunol. 2019, 88, 65–75. [Google Scholar] [CrossRef]
- Yu, L.; Yu, Y.; Yin, R.; Duan, H.; Qu, D.; Tian, F.; Narbad, A.; Chen, W.; Zhai, Q. Dose-dependent effects of lead induced gut injuries: An in vitro and in vivo study. Chemosphere 2021, 266, 129130. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Xu, Q.; Chen, J.; Xu, Y.; Pang, J.; Peng, X.; Tang, Z.; Sun, W.; Sun, Z. Effects of Different Combinations of Sodium Butyrate, Medium-Chain Fatty Acids and Omega-3 Polyunsaturated Fatty Acids on the Reproductive Performance of Sows and Biochemical Parameters, Oxidative Status and Intestinal Health of Their Offspring. Animals 2023, 13, 1093. [Google Scholar] [CrossRef] [PubMed]




| Ingredients | % of DM | Nutrients | g/kg of DM |
|---|---|---|---|
| Oat hay | 41.00 | DE (MJ/kg) | 10.25 |
| Corn | 29.50 | CP | 155.60 |
| Soybean meal | 14.50 | NDF | 352.60 |
| Wheat bran | 10.00 | ADF | 196.25 |
| Stone dust | 0.35 | EE | 28.94 |
| CaH2PO4 | 0.15 | Ca | 4.08 |
| NaCl | 0.50 | P | 4.65 |
| Premix | 4.00 | ||
| Total | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdu, S.M.N.; Abdalla, I.M.; Zhen, Y.; Zhang, C.; Xi, Z.; Ma, J.; Zhong, Y.; Lin, J.; Ali, R.; Wang, M. Gastric Infusion of Short-Chain Fatty Acids Improves Health via Enhance Liver and Intestinal Immune Response and Antioxidant Capacity in Goats. Vet. Sci. 2025, 12, 395. https://doi.org/10.3390/vetsci12050395
Abdu SMN, Abdalla IM, Zhen Y, Zhang C, Xi Z, Ma J, Zhong Y, Lin J, Ali R, Wang M. Gastric Infusion of Short-Chain Fatty Acids Improves Health via Enhance Liver and Intestinal Immune Response and Antioxidant Capacity in Goats. Veterinary Sciences. 2025; 12(5):395. https://doi.org/10.3390/vetsci12050395
Chicago/Turabian StyleAbdu, Shaima Mohmed Nasr, Ismail Mohamed Abdalla, Yongkang Zhen, Chong Zhang, Zanna Xi, Jianjun Ma, Yuhong Zhong, Jiaqi Lin, Rahmat Ali, and Mengzhi Wang. 2025. "Gastric Infusion of Short-Chain Fatty Acids Improves Health via Enhance Liver and Intestinal Immune Response and Antioxidant Capacity in Goats" Veterinary Sciences 12, no. 5: 395. https://doi.org/10.3390/vetsci12050395
APA StyleAbdu, S. M. N., Abdalla, I. M., Zhen, Y., Zhang, C., Xi, Z., Ma, J., Zhong, Y., Lin, J., Ali, R., & Wang, M. (2025). Gastric Infusion of Short-Chain Fatty Acids Improves Health via Enhance Liver and Intestinal Immune Response and Antioxidant Capacity in Goats. Veterinary Sciences, 12(5), 395. https://doi.org/10.3390/vetsci12050395







