Development of Dog Immune System: From in Uterus to Elderly
Abstract
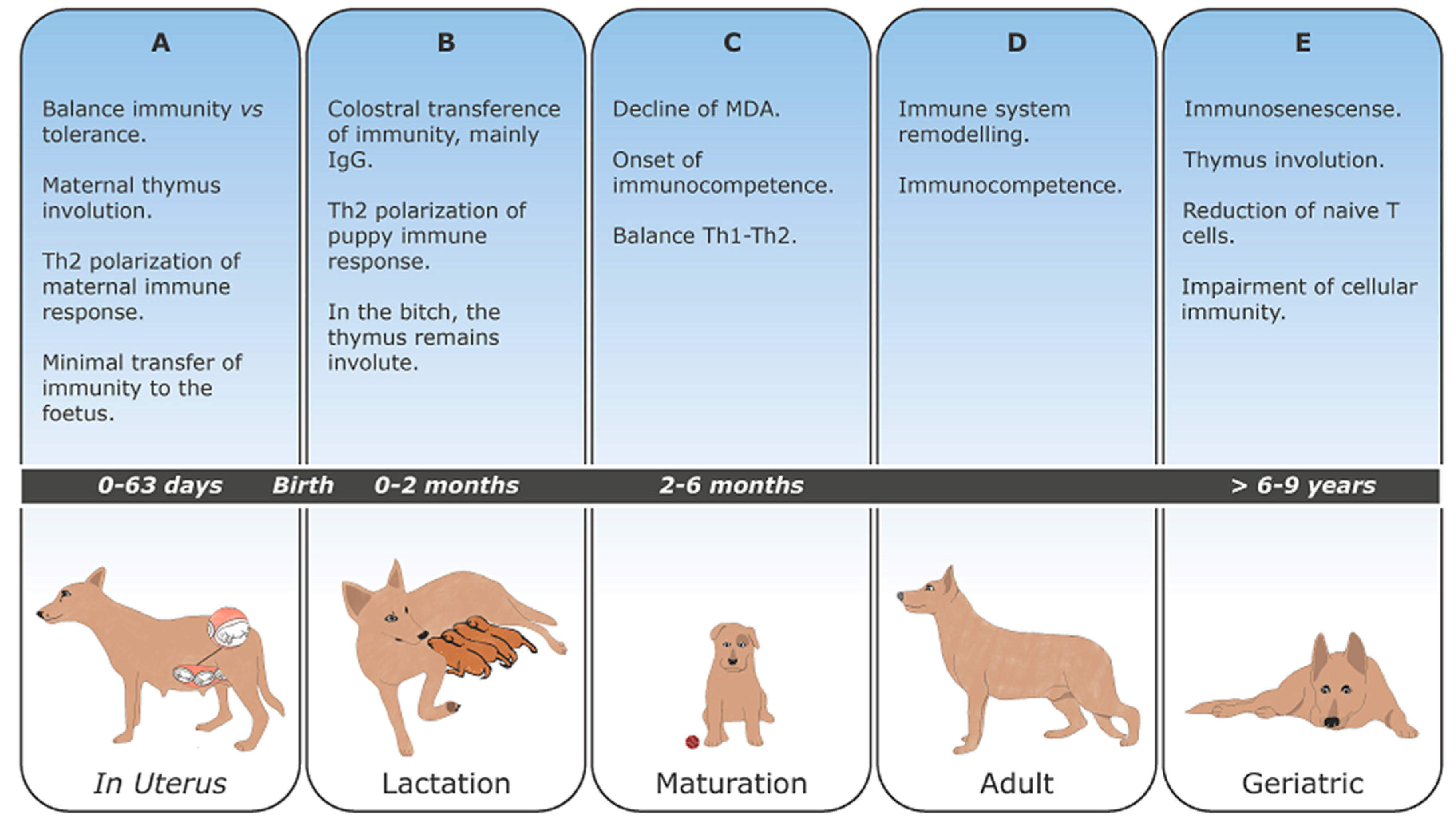
1. Introduction
2. Immune System Overview
3. Canine Pregnancy
4. Maternally Derived Antibodies (MDA)
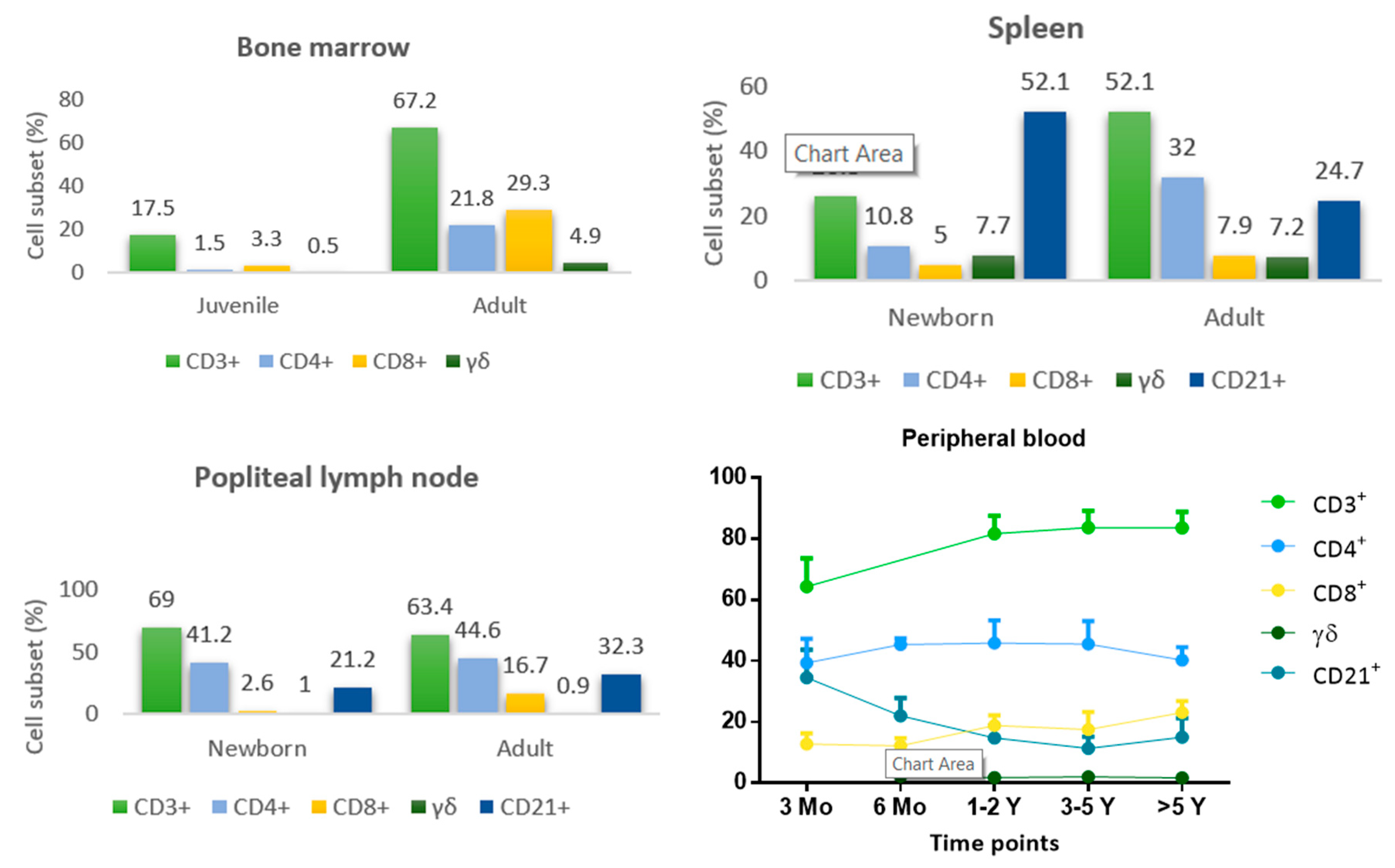
5. Development and Maturation of Lymphoid Organs
6. Development and Maturation of the Immune Response
7. Immunosenescense
8. Concluding Remarks and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Tizard, I.R. Veterinary Immunology, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Borregaard, N. Neutrophils, from Marrow to Microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Braber, I.D.; Vrisekoop, N.; Kwast, L.M.; De Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S. Another look at the life of a neutrophil. World J. Hematol. 2013, 2, 44–58. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez-Pomares, L. Physiological roles of macrophages. Pflug. Arch. Eur. J. Physiol. 2017, 469, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, F.; Lehmbecker, A.; Raddatz, B.B.; Kegler, K.; Tipold, A.; Stein, V.M.; Kalkuhl, A.; Deschl, U.; Baumgärtner, W.; Ulrich, R.; et al. Morphologic, phenotypic, and transcriptomic characterization of classically and alternatively activated canine blood-derived macrophages in vitro. PLoS ONE 2017, 12, e0183572. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Alexandre-Pires, G.; Câmara, M.; Santos, M.; Martins, C.; Rodrigues, A.; Adriana, J.; Passero, L.F.D.; Da Fonseca, I.P.; Santos-Gomes, G. Canine neutrophils cooperate with macrophages in the early stages of Leishmania infantum in vitro infection. Parasite Immunol. 2019, 41, e12617. [Google Scholar] [CrossRef]
- Freitas, A.A.; Rocha, B. Population Biology of Lymphocytes: The Flight for Survival. Annu. Rev. Immunol. 2000, 18, 83–111. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M. Survival and maturation of lymphocytes in peripheral lymphoid tissues. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Júnior, D.M.; Araújo, J.A.P.; Catelan, T.T.T.; De Souza, A.W.S.; Cruvinel, W.D.M.; Andrade, L.E.C.; Da Silva, N.P. Sistema imunitário—Parte II: Fundamentos da resposta imunológica mediada por linfócitos T e B. Rev. Bras. Reumatol. 2010, 50, 552–580. [Google Scholar] [CrossRef]
- Olivry, T.; Naydan, D.K.; Moore, P.F. Characterization of the Cutaneous Inflammatory Infiltrate in Canine Atopic Dermatitis. Am. J. Dermatopathol. 1997, 19, 477–486. [Google Scholar] [CrossRef]
- German, A.J.; Hall, E.J. The distribution of lymphocytes expressing alphabeta and gammadelta T-cell receptors, and the expression of mucosal addressin cell adhesion molecule-1 in the canine intestine. J. Comp. Pathol. 1999, 121, 249–263. [Google Scholar] [CrossRef]
- Galler, A.; Rütgen, B.; Haas, E.; Saalmüller, A.; Hirt, R.; Gerner, W.; Schwendenwein, I.; Richter, B.; Thalhammer, J.; Luckschander-Zeller, N. Immunophenotype of Peripheral Blood Lymphocytes in Dogs with Inflammatory Bowel Disease. J. Vet. Intern. Med. 2017, 31, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.; Medeiros, L.J. Hepatosplenic and other gammadelta T-cell lymphomas. Am. J. Clin. Pathol. 2007, 127, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Takeishi, M.; Takagi, K.; Kuyama, T.; Yukawa, M.; Ishida, M.; Kuramochi, T. Cross-reactivity between human and canine helper and suppressor T cell antigens using monoclonal antibodies RPA-T4 and HuLy-m8. Cell. Mol. Boil. 1989, 35, 271–278. [Google Scholar]
- Deeg, H.J.; Raff, R.F.; Severns, E.; Szer, J.; Storb, R. Two monoclonal antibodies recognizing subpopulations of canine T lymphocytes with or without suppressor/cytotoxic functions. Methods Find. Exp. Clin. Pharmacol. 1987, 9, 755–759. [Google Scholar] [PubMed]
- Szer, J.; Deeg, H.J.; Rieber, P.; Storb, R. Monoclonal antibody to human cytotoxic-suppressor T-lymphocytes cross-reacts with canine lymphocytes and inhibits cell-mediated lympholysis of canine cells. Exp. Hematol. 1985, 13, 641–646. [Google Scholar] [PubMed]
- Concannon, P.W. Canine Pregnancy: Predicting Parturition and Timing Events of Gestation. In Recent Advances in Small Animal Reproduction; Concannon, P.W., England, E., Verstegen, J., Eds.; International Veterinary Information Service: Ithaca, NY, USA, 2001. [Google Scholar]
- Beceriklisoy, H.; Budik, S.; Kanca, H.; Aksoy, O.; Polat, B.; Cetin, Y.; Ay, S.; Aslan, S.; Schäfer-Somi, S. Expression of Genes in the Canine Pre-implantation Uterus and Embryo: Implications for an Active Role of the Embryo Before and During Invasion. Reprod. Domest. Anim. 2008, 43, 656–663. [Google Scholar]
- Schäfer-Somi, S.; Sabitzer, S. Is Apoptosis a Regulatory Mechanism During Early Canine Pregnancy? Reprod. Domest. Anim. 2012, 47, 169–172. [Google Scholar] [CrossRef]
- Clarke, A.G.; Kendall, M.D. The thymus in pregnancy: The interplay of neural, endocrine and immune influences. Immunol. Today 1994, 15, 545–552. [Google Scholar] [CrossRef]
- Tibbetts, T.A.; DeMayo, F.; Rich, S.; Conneely, O.M.; O’Malley, B.W. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc. Natl. Acad. Sci. USA 1999, 96, 12021–12026. [Google Scholar] [CrossRef]
- Laan, M.; Haljasorg, U.; Kisand, K.; Salumets, A.; Peterson, P. Pregnancy-induced thymic involution is associated with suppression of chemokines essential for T-lymphoid progenitor homing. Eur. J. Immunol. 2016, 46, 2008–2017. [Google Scholar] [CrossRef]
- Bodey, B.; Bodey, B., Jr. Involution of the mammalian thymus, one of the leading regulators of aging. In Vivo 1997, 11, 421–440. [Google Scholar] [PubMed]
- Schumacher, A.; Costa, S.D.; Zenclussen, A.C. Endocrine Factors Modulating Immune Responses in Pregnancy. Front. Immunol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, M.; Piccinno, M. Evaluation of serum concentrations of interleukin (IL)-4, IL-10, and IL-12 during pregnancy in bitches. Theriogenology 2012, 79, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Horzinek, M.C.; Schultz, R.D. Vaccination Guidelines Group (VGG) of the World Small Anima Veterinary Association (WSAVA). WSAVA Guidelines for the vaccination of dogs and cats. J. Small Anim. Pract. 2016, 57, E1–E45. [Google Scholar] [CrossRef]
- Wilbur, L.A.; Evermann, J.F.; Levings, R.L.; Stoll, I.R.; Starling, D.E.; Spillers, C.A.; Gustafson, G.A.; McKeirnan, A.J. Abortion and death in pregnant bitches associated with a canine vaccine contaminated with bluetongue virus. J. Am. Vet. Med. Assoc. 1994, 204, 1762–1765. [Google Scholar]
- Schreiber, P.; Sanquer, A. Safety of Canigen® DHPPi/L(R) Vaccines for Pregnant Bitches and their Offspring. J. Vet. Sci. Med. 2015, 3, 6. [Google Scholar]
- Mila, H.; Feugier, A. Variability of mortality risk factors with age in puppies. In Proceedings of the Annual Meeting of the Society for Veterinary Epidemiology and Preventive Medicine, Gand, Belgium, 25–27 March 2005. [Google Scholar]
- Nelson, R.W.; Couto, C.G. Small Animal Internal Medicine, 2nd ed.; Mosby: St. Louis, MO, USA, 2009; p. 892. [Google Scholar]
- Bouchard, G.; Plata-Madrid, H.; Youngquist, R.S.; Buening, G.M.; Ganjam, V.K.; Krause, G.F.; Allen, G.K.; Paine, A.L. Absorption of an alternate source of immunoglobulin in pups. Am. J. Vet. Res. 1992, 53, 230–233. [Google Scholar]
- Poffenbarger, E.M.; Olson, P.N.; Chandler, M.L.; Seim, H.B.; Varman, M. Use of adult dog serum as a substitute for colostrum in the neonatal dog. Am. J. Vet. Res. 1991, 52, 1221–1224. [Google Scholar]
- Stoffel, M.H.; Friess, A.E.; Hartmann, S.H. Ultrastructural evidence of transplacental transport of immunoglobulin G in bitches. Reproduction 2000, 118, 315–326. [Google Scholar] [CrossRef][Green Version]
- Chappuis, G. Neonatal immunity and immunisation in early age: Lessons from veterinary medicine. Vaccine 1998, 16, 1468–1472. [Google Scholar] [CrossRef]
- Krakowka, S. Handbook of Veterinary Vertebrate Immunology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 261–272. [Google Scholar]
- Felsburg, P.J. Overview of immune system development in the dog: Comparison with humans. Hum. Exp. Toxicol. 2002, 21, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Day, M. Immune System Development in the Dog and Cat. J. Comp. Pathol. 2007, 137, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Freyburger, L.; Marcheteau, E.; Thoumire, S.; Ravier, J.; Reynaud, K.; Chastant-Maillard, S. Timing of the Intestinal Barrier Closure in Puppies. Reprod. Domest. Anim. 2012, 47, 190–193. [Google Scholar]
- Vaerman, J. The immunoglobulins of the dog—II. The immunoglobulins of canine secretions. Immunochemistry 1969, 6, 779–780. [Google Scholar] [CrossRef]
- Reynolds, H.Y.; Johnson, J.S. Quantitation of canine immunoglobulins. J. Immunol. 1970, 105, 698–703. [Google Scholar]
- Ricks, J.; Roberts, M.; Patterson, R. Canine secretory immunoglobulins: Identification of secretory component. J. Immunol. 1970, 105, 1327–1333. [Google Scholar]
- Heddle, R.J.; Rowley, D. Dog Immunoglobulins. Immunology 1975, 29, 185–195. [Google Scholar]
- Schäfer-Somi, S.; Bär-Schadler, S.; Aurich, J.E. Immunoglobulins in nasal secretions of dog puppies from birth to six weeks of age. Res. Vet. Sci. 2005, 78, 143–150. [Google Scholar] [CrossRef]
- Chastant-Maillard, S.; Aggouni, C. Canine and feline colostrum. Reprod. Domest. Anim. 2017, 52, 148–152. [Google Scholar] [CrossRef]
- Mila, H.; Feugier, A.; Grellet, A.; Anné, J.; Gonnier, M.; Martin, M.; Rossig, L.; Chastant-Maillard, S. Immunoglobulin G concentration in canine colostrum: Evaluation and variability. J. Reprod. Immunol. 2015, 112, 24–28. [Google Scholar] [CrossRef]
- Handl, S.; Wehr, U.; Zentek, J.; Krammer-Lukas, S. Histological and immunohistochemical evaluation of duodenal and colonic biopsies after oral bovine lactoferrin supplementation in beagle puppies. J. Anim. Physiol. Anim. Nutr. 2009, 93, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Treneva, M.; Peroni, D.G.; Colicino, S.; Chow, L.; Dissanayeke, S.; Abrol, P.; Sheth, S.; Pampura, A.; Boner, A.L.; et al. Colostrum and Mature Human Milk of Women from London, Moscow, and Verona: Determinants of Immune Composition. Nutrients 2016, 8, 695. [Google Scholar] [CrossRef] [PubMed]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune components of bovine colostrum and milk. J. Anim. Sci. 2009, 87, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, D.B.; Buddington, K.K.; Buddington, R.K. Dimensions and histologic characteristics of the small intestine of dogs during postnatal development. Am. J. Vet. Res. 2003, 64, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, L.E.; Robson, D.S.; Barnes, F.D. Transfer and Decline of Maternal Infectious Canine Hepatitis Antibody in Puppies. Exp. Biol. Med. 1962, 109, 677–681. [Google Scholar] [CrossRef]
- Chappuis, G. Control of canine distemper. Vet. Microbiol. 1995, 44, 351–358. [Google Scholar] [CrossRef]
- Morein, B.; Abusugra, I. Immunity in neonates. Vet. Immunol. Immunopathol. 2002, 87, 207–213. [Google Scholar] [CrossRef]
- De Cramer, K.; Stylianides, E.; Van Vuuren, M. Efficacy of vaccination at 4 and 6 weeks in the control of canine parvovirus. Vet. Microbiol. 2011, 149, 126–132. [Google Scholar] [CrossRef]
- Nandi, S.; Kumar, M. Canine Parvovirus: Current Perspective. Indian J. Virol. 2010, 21, 31–44. [Google Scholar] [CrossRef]
- DeCaro, N.; Campolo, M.; Desario, C.; Elia, G.; Martella, V.; Lorusso, E.; Buonavoglia, C. Maternally-derived antibodies in pups and protection from canine parvovirus infection. Biologicals 2005, 33, 261–267. [Google Scholar] [CrossRef]
- Pardo, M.; Tanner, P.; Bauman, J.; Silver, K.; Fischer, L. Immunization of Puppies in the Presence of Maternally Derived Antibodies Against Canine Distemper Virus. J. Comp. Pathol. 2007, 137, S72–S75. [Google Scholar] [CrossRef] [PubMed]
- Chastant, S.; Mila, H. Passive immune transfer in puppies. Anim. Reprod. Sci. 2019, 207, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Bodey, B.; Calvo, W.; Prümmer, O.; Fliedner, T.; Borysenko, M. Development and histogenesis of the thymus in dog. A light and electron microscopical study. Dev. Comp. Immunol. 1987, 11, 227–238. [Google Scholar] [CrossRef]
- Klein, A.K.; Dyck, J.A. Characterization of canine fetal lymphohematopoiesis. Exp. Hematol. 1983, 11, 263–274. [Google Scholar]
- Day, M.J. Clinical Immunology of the Dog and Cat, 2nd ed.; Manson Publishing: London, UK, 2011; pp. 21–24. [Google Scholar]
- Boyd, R.L.; Tucek, C.L. The thymic microenvironment. Immunol. Today 1993, 14, 445–459. [Google Scholar] [CrossRef]
- Samuelson Don, A. Tratado de Histologia Veterinária, 1st ed.; Elsevier: Rio de Jneiro, Brasil, 2007; pp. 145–150. [Google Scholar]
- Faldyna, M.; Sinkora, J.; Knotigova, P.; Rehakova, Z.; Moravkova, A.; Toman, M. Flow cytometric analysis of bone marrow leukocytes in neonatal dogs. Vet. Immunol. Immunopathol. 2003, 95, 165–176. [Google Scholar] [CrossRef]
- Yang, T.J.; Gawlak, S.L. Lymphoid organ weights and organ: Body weight ratios of growing beagles. Lab. Anim. 1989, 23, 143–146. [Google Scholar] [CrossRef]
- HogenEsch, H.; Hahn, F.F. Pathobiology of the Aging Dog, 1st ed.; Mohr, U., Carlton, W.W., Dungworth, D.L., Eds.; Iowa State University Press: Ames, IA, USA, 2001; Volume 2, pp. 127–135. [Google Scholar]
- Haley, P.J. Species differences in the structure and function of the immune system. Toxicology 2003, 188, 49–71. [Google Scholar] [CrossRef]
- Somberg, R.L.; Robinson, J.P.; Felsburg, P.J. T lymphocyte development and function in dogs with X-linked severe combined immunodeficiency. J. Immunol. 1994, 153, 4006–4015. [Google Scholar]
- Bismarck, D.; Schütze, N. Canine CD4+ CD8+ double positive T cell in peripheral blood have features of activated T cells. Vet. Immunol. Immunopathol. 2012, 149, 157–166. [Google Scholar] [CrossRef]
- Faldyna, M.; Sinkora, J. Characterization of CD34⁺ thymocytes in newborn dogs. Vet. Immunol. Immunopathol. 2012, 147, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.H.; Chalasani, G. Role of secondary lymphoid tissues in primary and memory T-cell responses to a transplanted organ. Transplant. Rev. 2009, 24, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Faldyna, M.; Sinkora, J.; Knotigova, P.; Leva, L.; Toman, M. Lymphatic organ development in dogs: Major lymphocyte subsets and activity. Vet. Immunol. Immunopathol. 2005, 104, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Delves, P.J.; Roitt, I.M. The immune system. First of two parts. N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [CrossRef]
- Toman, M.; Faldyna, M.; Knotigova, P.; Pokorova, D.; Sinkora, J. Postnatal development of leukocyte subset composition and activity in dogs. Vet. Immunol. Immunopathol. 2002, 87, 321–326. [Google Scholar] [CrossRef]
- Faldyna, M.; Leva, L.; Knötigová, P.; Toman, M. Lymphocyte subsets in peripheral blood of dogs—A flow cytometric study. Vet. Immunol. Immunopathol. 2001, 82, 23–37. [Google Scholar] [CrossRef]
- Holt, P.G. Regulation of antigen-presenting cell function(s) in lung and airway tissues. Eur. Respir. J. 1993, 6, 120–129. [Google Scholar]
- Hiller, A.S.; Tschernig, T.; Kleemann, W.J.; Pabst, R. Bronchus-Associated Lymphoid Tissue (BALT) and Larynx-Associated Lymphoid Tissue (LALT) are Found at Different Frequencies in Children, Adolescents and Adults. Scand. J. Immunol. 1998, 47, 159–162. [Google Scholar] [CrossRef]
- Debertin, A.S.; Tschernig, T.; Tönjes, H.; Kleemann, W.J.; Tröger, H.D.; Pabst, R. Nasal-associated lymphoid tissue (NALT): Frequency and localization in young children. Clin. Exp. Immunol. 2003, 134, 503–507. [Google Scholar] [CrossRef]
- Hall, E.J. Mucosal Immunology—Why it’s important. In Proceedings of the World Small Animal Veterinary Association, Sydney, Australia, 19–23 August 2007. [Google Scholar]
- HogenEsch, H.; Felsburg, P.J. Immunohistology of Peyer’s patches in the dog. Vet. Immunol. Immunopathol. 1992, 30, 147–160. [Google Scholar] [CrossRef]
- HogenEsch, H.; Felsburg, P.J. Isolation and phenotypic and functional characterization of cells from Peyer’s patches in the dog. Vet. Immunol. Immunopathol. 1992, 31, 1–10. [Google Scholar] [CrossRef]
- Rørtveit, R.; Saevik, B.K.; Eggertsdóttir, A.V.; Skancke, E.; Lingaas, F.; Thoresen, S.I.; Jansen, J.H.; Sævik, B.K. Age-related changes in hematologic and serum biochemical variables in dogs aged 16–60 days. Vet. Clin. Pathol. 2015, 44, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Surendran, N. Immune responses in neonates. Expert Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.O.; Dennis, R.A.; Griesemer, R.A. Development of immunity in fetal dogs: Humoral responses. Am. J. Vet. Res. 1969, 30, 1503–1510. [Google Scholar] [PubMed]
- Gerber, J.D.; Brown, A.L. Effect of Development and Aging on the Response of Canine Lymphocytes to Phytohemagglutinin. Infect. Immun. 1974, 10, 695–699. [Google Scholar]
- Nova, B.V.; Cunha, E.; Sepúlveda, N.; Oliveira, M.; Braz, B.S.; Tavares, L.; Almeida, V.; Gil, S. Evaluation of the humoral immune response induced by vaccination for canine distemper and parvovirus: A pilot study. BMC Vet. Res. 2018, 14, 348. [Google Scholar]
- Holmes, M.A.; Lunn, D.P. Variation of MHC II expression on canine lymphocytes with age. Tissue Antigens 1994, 43, 179–183. [Google Scholar] [CrossRef]
- Marshall-Clarke, S.; Tasker, L.; Parkhouse, R.M.E. Immature B lymphocytes from adult bone marrow exhibit a selective defect in induced hyperexpression of major histocompatibility complex class II and fail to show B7.2 induction. Immunology 2000, 100, 141–151. [Google Scholar] [CrossRef]
- Marshall-Clarke, S.; Reen, D.; Tasker, L.; Hassan, J. Neonatal immunity: How well has it grown up? Immunol. Today 2000, 21, 35–41. [Google Scholar] [CrossRef]
- Rook, G.A.W.; Stanford, J.L. Give us this day our daily germs. Immunol. Today 1998, 19, 113–116. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Nakajima, Y.; Nariai, Y.; Asanuma, H.; Kuwabara, M.; Yukawa, M. Th1/Th2 balance in canine peripheral blood lymphocytes—A flow cytometric study. Vet. Immunol. Immunopathol. 2007, 118, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kraft, W. Geriatrics in canine and feline internal medicine. Eur. J. Med. Res. 1998, 3, 31–41. [Google Scholar] [PubMed]
- Rosato, E.; Salsano, F. Immunity, autoimmunity and autoimmune diseases in older people. J. Biol. Regul. Homeost. Agents 2008, 22, 217–224. [Google Scholar] [PubMed]
- Day, M.J. Ageing, Immunosenescense and Inflammageing in the Dog and Cat. J. Comp. Pathol. 2010, 142, 60–69. [Google Scholar] [CrossRef]
- Holder, A.; Mirczuk, S.M. Perturbation of the T cell receptor repertoire occurs with increasing age in dogs. Dev. Comp. Immunol. 2018, 79, 150–157. [Google Scholar] [CrossRef]
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing 2012, 9, 15. [Google Scholar] [CrossRef]
- Greeley, E.; Kealy, R.; Ballam, J.; Lawler, D.; Segre, M. The influence of age on the canine immune system. Vet. Immunol. Immunopathol. 1996, 55, 1–10. [Google Scholar] [CrossRef]
- Greeley, E.H.; Ballam, J.M.; Harrison, J.M.; Kealy, R.D.; Lawler, D.F.; Segre, M. The influence of age and gender on the immune system: A longitudinal study in Labrador Retriever dogs. Vet. Immunol. Immunopathol. 2001, 82, 57–71. [Google Scholar] [CrossRef]
- Strasser, A.; Teltscher, A.; Sanders, C.; Niedermüller, H.; May, B. Age-associated Changes in the Immune System of German Shepherd Dogs. J. Vet. Med. Ser. A 2000, 47, 181–192. [Google Scholar] [CrossRef]
- Heaton, P.R.; Blount, D.G.; Devlin, P.; Koelsch, S.; Mann, S.J.; Smith, B.H.E.; Stevenson, J.; Harper, E.J. Assessing age-related changes in peripheral blood leukocyte phenotypes in Labrador retriever dogs using flow cytometry. J. Nutr. 2002, 132, 1655–1657. [Google Scholar] [CrossRef]
- HogenEsch, H.; Thompson, S.; Dunham, A.; Ceddia, M.; Hayek, M. Effect of age on immune parameters and the immune response of dogs to vaccines: A cross-sectional study. Vet. Immunol. Immunopathol. 2004, 97, 77–85. [Google Scholar] [CrossRef]
- Blount, D.G.; Pritchard, D.I.; Heaton, P.R. Age-related alterations to immune parameters in Labrador retriever dogs. Vet. Immunol. Immunopathol. 2005, 108, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Withers, S.S.; Moore, P.F.; Chang, H.; Choi, J.W.; McSorley, S.J.; Kent, M.S.; Monjazeb, A.M.; Canter, R.J.; Murphy, W.J.; Sparger, E.E.; et al. Multi-color flow cytometry for evaluating age-related changes in memory lymphocyte subsets in dogs. Dev. Comp. Immunol. 2018, 87, 64–74. [Google Scholar] [CrossRef]
- Mouzin, D.E.; Lorenzen, M.J.; Haworth, J.D.; King, V.L. Duration of serologic response to five viral antigens in dogs. J. Am. Vet. Med. Assoc. 2004, 224, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Gow, S.; Rhodes, C.; Lacoste, S.; Kong, L.; Musil, K.; Snead, E. Serum antibody responses to vaccinal antigens in lean and obese geriatric dogs. Can. Vet. J. 2016, 57, 531–534. [Google Scholar] [PubMed]
- Kennedy, L.J.; Lunt, M.; Barnes, A.; McElhinney, L.; Fooks, A.R.; Baxter, D.N.; Ollier, W.E. Factors influencing the antibody response of dogs vaccinated against rabies. Vaccine 2007, 25, 8500–8507. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.E.; Colyer, A. Understanding How Dogs Age: Longitudinal Analysis of Markers of Inflammation, Immune Function, and Oxidative Stress. J. Gerontol. Ser. A 2018, 73, 720–728. [Google Scholar] [CrossRef] [PubMed]
| 16–24 Days | 28–46 Days | 46–60 Days | Adult (Reference Range) | |
|---|---|---|---|---|
| WBC (×109/L) | 11.0 (2.9) | 13.1 (2.7) | 13.7 (3.8) | 6–18 |
| Neutrophils (×109/L) | 5.5 (2.4) | 7.0 (1.8) | 7.1 (2.1) | 3.6–13.0 |
| Lymphocytes (×109/L) | 4.2 (1.4) | 4.6 (1.1) | 5.0 (2.1) | 0.8–5.8 |
| Monocytes (×109/L) | 0.6 (0.18) | 0.9 (0.2) | 0.9 (0.3) | 0–1.6 |
| Eosinophils (×109/L) | 0.5 (0.26) | 0.4 (0.25) | 0.5 (0.39) | 0–1.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.; Valério-Bolas, A.; Saraiva-Marques, C.; Alexandre-Pires, G.; Pereira da Fonseca, I.; Santos-Gomes, G. Development of Dog Immune System: From in Uterus to Elderly. Vet. Sci. 2019, 6, 83. https://doi.org/10.3390/vetsci6040083
Pereira M, Valério-Bolas A, Saraiva-Marques C, Alexandre-Pires G, Pereira da Fonseca I, Santos-Gomes G. Development of Dog Immune System: From in Uterus to Elderly. Veterinary Sciences. 2019; 6(4):83. https://doi.org/10.3390/vetsci6040083
Chicago/Turabian StylePereira, Maria, Ana Valério-Bolas, Cátia Saraiva-Marques, Graça Alexandre-Pires, Isabel Pereira da Fonseca, and Gabriela Santos-Gomes. 2019. "Development of Dog Immune System: From in Uterus to Elderly" Veterinary Sciences 6, no. 4: 83. https://doi.org/10.3390/vetsci6040083
APA StylePereira, M., Valério-Bolas, A., Saraiva-Marques, C., Alexandre-Pires, G., Pereira da Fonseca, I., & Santos-Gomes, G. (2019). Development of Dog Immune System: From in Uterus to Elderly. Veterinary Sciences, 6(4), 83. https://doi.org/10.3390/vetsci6040083







