Mongoose (Herpestes auropunctatus) May Not Be Reservoir Hosts for Mycobacterium bovis in Fiji Despite High Population Density and Direct Contact with Cattle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Design
2.2. Animal Ethics Approval
2.3. Trapping and Euthanasia
2.4. Post-Mortem Examination
3. Results
3.1. Trapping Results and Population Data
3.2. Post-Mortem Findings
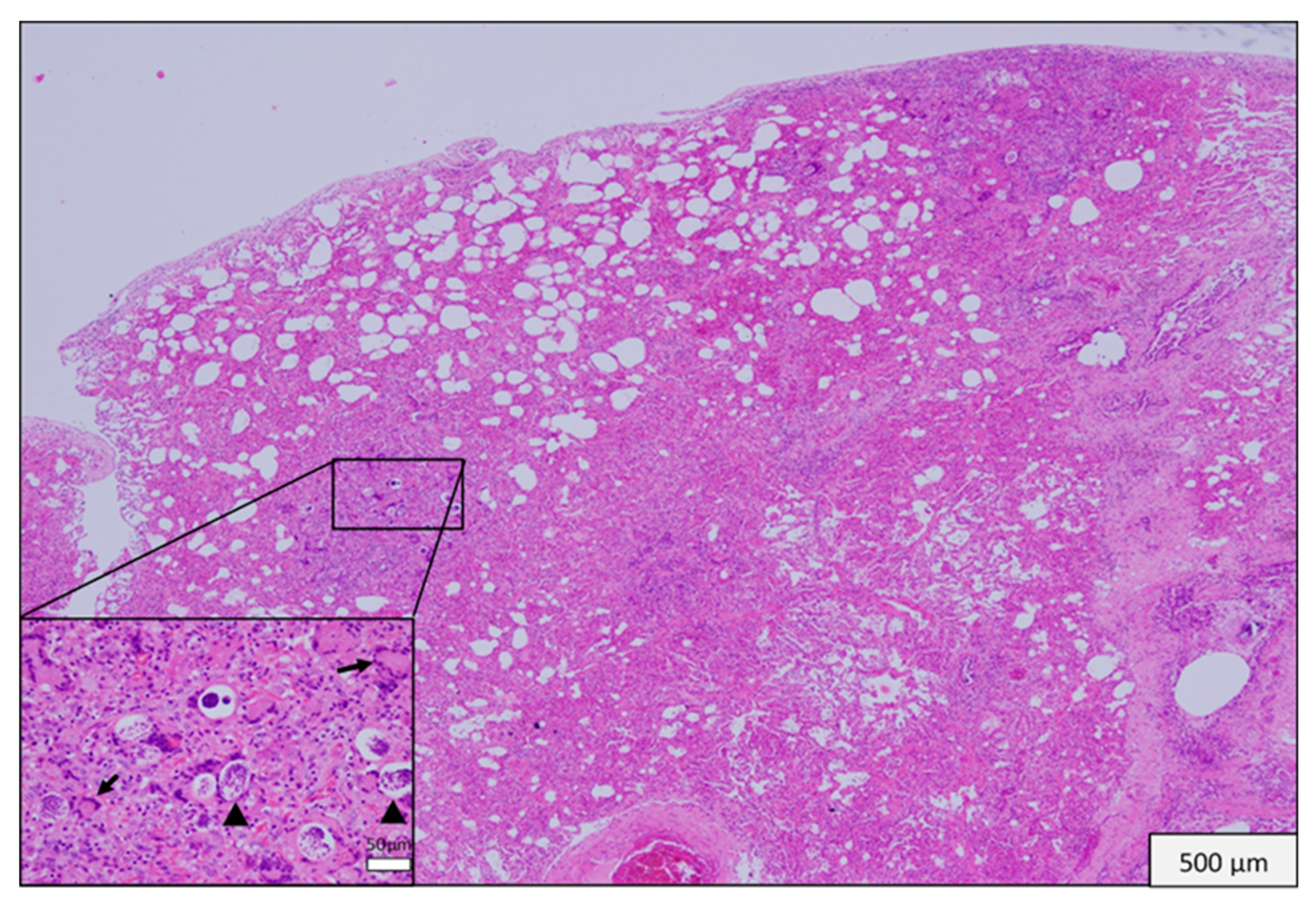
3.3. Histopathological Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zachary, J.F. Pathologic Basis of Veterinary Disease, 6th ed.; Elsevier: St Louis, MO, USA, 2017. [Google Scholar]
- Borja, E.; Borja, L.F.; Prasad, R.; Tunabuna, T.; Toribio, J.-A.L.M.L. A retrospective study on bovine tuberculosis in cattle on Fiji: Study findings and stakeholder responses. Front. Vet. Sci. 2018, 5, 270. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Targets Elimination of TB in Over 30 Countries—WHO/ERS Joint News Release; World Health Organisation/European Respiratory Society (ERS): Rome, Italy, 2014; Available online: http://www.who.int/mediacentre/news/releases/2014/tb-elimination/en/ (accessed on 1 October 2019).
- WHO. Global Tuberculosis Report, 20th ed.; WHO: Geneva, Switzerland, 2015; 192p, Available online: http://www.who.int/iris/handle/10665/191102 (accessed on 1 October 2019).
- Pessoli, L.; Gounder, S.; Tamani, T.; Daulako, M.R.; Underwood, F.; Mainaalala, S.; Nawadra-Taylor, V.; Rafai, E.; Gillini, L. Tuberculosis, Fiji, 2002–2013. Emerg. Infect. Dis. 2016, 22, 547–549. [Google Scholar] [CrossRef] [PubMed]
- WHO; FAO; OIE. Roadmap for Zoonotic Tuberculosis; WHO: Geneva, Switzerland, 2017; 20p. [Google Scholar]
- Sauvakacolo, S. $2m for Animal TB Eradication. The Fiji Times. 25 October 2016. Available online: http://www.fijitimes.com/story.aspx?id=321929 (accessed on 1 October 2019).
- Krebs, J.; Anderson, R.; Clutton-Brock, T.; Morrison, I.; Young, D.; Donnelley, C. Bovine Tuberculosis in Cattle and Badgers; MAFF: Tokyo, Japan, 1997.
- Cheeseman, C.L.; Jones, G.W.; Gallagher, J.; Mallinson, P.J. The population structure, density and prevalence of tuberculosis (Mycobacterium bovis) in badgers (Meles meles) from four areas in South-West England. J. Appl Ecol. 1981, 18, 795–804. [Google Scholar] [CrossRef]
- Livingstone, P.G.; Hancox, N.; Nugent, G.; De Lisle, G.W. Toward eradication: The effect of Mycobacterium bovis infection in wildlife on the evolution and future direction of bovine tuberculosis management in New Zealand. N. Z. Vet. J. 2015, 63, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.S.; Sweeney, S.J.; Stärk, K.D.C. Mycobacterium bovis (bovine tuberculosis) infection in North American wildlife: Current status and opportunities for mitigation of risks of further infection in wildlife populations. Epidemiol. Infect. 2013, 141, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Higa, H.H.; Fujinaka, I.T. Prevalence of rodent and mongoose leptospirosis on the Island of Oahu. Public Health Rep. 1976, 91, 171–177. [Google Scholar] [PubMed]
- Sato, S.; Kabeya, H.; Shigematsu, Y.; Sentsui, H.; Une, Y.; Minami, M.; Murata, K.; Ogura, G.; Maruyama, S. Small Indian mongooses and masked palm civets serve as new reservoirs of Bartonella henselae and potential sources of infection for humans. Clin. Microbiol. Infect. 2013, 19, 1181–1187. [Google Scholar] [CrossRef]
- Berentsen, A.R.; Johnson, S.R.; Gilbert, A.T.; Vercauteren, K.C. Exposure to rabies in small Indian mongooses (Herpestes auropunctatus) from two regions in Puerto Rico. J. Wildl. Dis. 2015, 51, 896–900. [Google Scholar] [CrossRef]
- Brown, P.; Daigneault, A. Managing the invasive small Indian mongoose in Fiji. Agric. Resour. Econ. Rev. 2015, 44, 275–290. [Google Scholar] [CrossRef]
- Bruns, A.C.; Tanner, M.; Williams, M.C.; Botha, L.; O’Brien, A.; Fosgate, G.T.; Van Helden, P.D.; Clarke, J.; Michel, A.L. Diagnosis and implications of Mycobacterium bovis infection in banded mongooses (Mungos mungo) in the Kruger national park, South Africa. J. Wildl. Dis. 2017, 53, 19–29. [Google Scholar] [CrossRef]
- Matos, A.C. Mycobacterium bovis in an Egyptian mongoose. Vet. Rec. 2013, 173, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Sanderson, C.E.; Larsen, M.H.; Robbe-Austerman, S.; Williams, M.C.; Palmer, M.V. Emerging tuberculosis pathogen hijacks social communication behavior in the group-living banded mongoose (Mungos mungo). Mbio 2016, 7, e00281-16. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.D.C.; Drewe, J.A.; Gey Van Pittius, N.C.; Warren, R.M.; Van Helden, P.D. Novel cause of tuberculosis in meerkats, South Africa. Emerg. Infect. Dis. 2013, 19, 2004–2007. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.D.; Widmer, K.W.; Ivey, L.J.; Coker, K.C.; Newman, E.; Lingsweiler, S.; Baca, D.; Kelley, M.; Davis, D.S.; Silvy, N.J.; et al. Failure to identify non-bovine reservoirs of Mycobacterium bovis in a region with a history of infected dairy-cattle herds. Prev. Vet. Med. 2000, 43, 53–62. [Google Scholar] [CrossRef]
- Matos, A.C.; Figueira, L.; Martins, M.H.; Matos, M.; Álvares, S.; Mendes, A.; Pinto, M.L.; Coelho, A.C. Detection of Mycobacterium avium subsp. paratuberculosis in kidney samples of red deer (Cervus elaphus) in Portugal: Evaluation of different methods. J. Vet. Med. Sci. 2017, 79, 692–698. [Google Scholar]
- Liang-Sheng, Y. A redescription of Pulmostrongylus herpestis (S. Khera, 1956) n. comb, from the lung of a mongoose, Herpestes sp., from Suva, Fiji. J. Helminthol. 1958, 32, 93–98. [Google Scholar] [CrossRef]
- Huizinga, H.W.; Cosgrove, G.E.; Sturrock, R.F. Renal capillariasis in the small Indian mongoose, Herpestes auropunctatus. J. Wildl. Dis. 1976, 12, 93–96. [Google Scholar] [CrossRef]
- Wedlock, D.N.; Skinner, M.A.; De Lisle, G.W.; Buddle, B.M. Control of Mycobacterium bovis infections and the risk to human populations. Microbes Infect. 2002, 4, 471–480. [Google Scholar] [CrossRef]
- O’Reilly, L.M.; Daborn, C.J. The epidemiology of Mycobacterium bovis infections in animals and man: A review. Tuber. Lung Dis. 1995, 76, 1–46. [Google Scholar] [CrossRef]
- Dankner, W.M.; Davis, C.E. Mycobacterium bovis as a significant cause of tuberculosis in children residing along the United States–Mexico border in the Baja California region. Pediatrics 2000, 105, e79. [Google Scholar] [CrossRef]
- VerCauteren, K.C.; Lavelle, M.J.; Campa, H. Persistent spillback of bovine tuberculosis from white-tailed deer to cattle in Michigan, USA: Status, strategies, and needs. Front. Vet. Sci. 2018, 5, 301. [Google Scholar] [CrossRef] [PubMed]
- Anon. Department of Agriculture Annual Report; Appendix to Journals of House of Representatives; Department of Agriculture New Zealand: Wellington, New Zealand, 1955.
- Nugent, G. The Role of Wild Deer in the Epidemiology and Management of Bovine Tuberculosis in New Zealand. Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2005. [Google Scholar]
- Julian, A. Tuberculosis in the possum Trichosurus vulpecula. In Proceedings of the First Symposium on Marsupials in New Zealand; Victoria University: Wellington, New Zealand, 1981; pp. 163–174. [Google Scholar]
- Garcia-Saenz, A.; Napp, S.; Lopez, S.; Casal, J.; Allepuz, A. Estimation of the individual slaughterhouse surveillance sensitivity for bovine tuberculosis in Catalonia (North-Eastern Spain). Prev. Vet. Med. 2015, 121, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Biffa, D.; Bogale, A.; Skjerve, E. Diagnostic efficiency of abattoir meat inspection service in Ethiopia to detect carcasses infected with Mycobacterium bovis: Implications for public health. BMC Public Health 2010, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Corner, L.A. Post mortem diagnosis of Mycobacterium bovis infection in cattle. Vet. Microbiol. 1994, 40, 53–63. [Google Scholar] [CrossRef]
- Alexander, K.A.; Laver, P.N.; Williams, M.C.; Sanderson, C.E.; Kanipe, C.; Palmer, M.V. Pathology of the emerging Mycobacterium tuberculosis complex pathogen, Mycobacterium mungi, in the banded mongoose (Mungos mungo). Vet. Pathol. 2018, 55, 303–309. [Google Scholar] [CrossRef]
- Drewe, J.A.; Foote, A.K.; Sutcliffe, R.L.; Pearce, G.P. Pathology of Mycobacterium bovis infection in wild meerkats (Suricata suricatta). J. Comp. Pathol. 2009, 140, 12–24. [Google Scholar] [CrossRef]
- Cross, M.L.; Labes, R.E.; Mackintosh, C.G. Oral infection of ferrets with virulent Mycobacterium bovis or Mycobacterium avium: Susceptibility, pathogenesis and immune response. J. Comp. Pathol. 2000, 123, 15–21. [Google Scholar] [CrossRef]
- Sergeant, E. EpiTools Epidemiological Calculators. AusVet Animal Health Services, Australia. 2017. Available online: http://epitools.ausvet.com.au/content.php?page=home (accessed on 1 October 2019).
- Aranaz, A.; De Juan, L.; Montero, N.; Sánchez, C.; Galka, M.; Delso, C.; Álvarez, J.; Romero, B.; Bezos, J.; Vela, A.I.; et al. Bovine tuberculosis (Mycobacterium bovis) in wildlife in Spain. J. Clin. Microbiol. 2004, 42, 2602–2608. [Google Scholar] [CrossRef]
- Wobeser, G.A. Investigation and Management of Disease in Wild Animals; Plenum Press: New York, NY, USA, 1994. [Google Scholar]



| Organ System | No. of Mongooses (%) | Type of Gross Pathology |
|---|---|---|
| Lung | 14 (16.5%) | Consolidation, nodules, pallor |
| Mesenteric lymph node | 10 (11.8%) | Lymphadenomegaly |
| Kidney | 5 (5.9%) | Atrophy, pallor, nodules |
| Skin | 4 (4.7%) | Abscess, wound |
| Liver | 2 (4.7%) | Nodules, pallor, mineralization |
| Submandibular lymph node | 1 (1.2%) | Lymphadenomegaly |
| Bronchial lymph node | 1 (1.2%) | Lymphadenomegaly |
| Prescapular lymph node | 1 (1.2%) | Lymphadenomegaly |
| Spleen | 1 (1.2%) | Nodule |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayton, P.J.; Whittington, R.J.; Wakelin, C.; Colville, P.; Reid, A.; Borja, L.; Toribio, J.-A. Mongoose (Herpestes auropunctatus) May Not Be Reservoir Hosts for Mycobacterium bovis in Fiji Despite High Population Density and Direct Contact with Cattle. Vet. Sci. 2019, 6, 85. https://doi.org/10.3390/vetsci6040085
Hayton PJ, Whittington RJ, Wakelin C, Colville P, Reid A, Borja L, Toribio J-A. Mongoose (Herpestes auropunctatus) May Not Be Reservoir Hosts for Mycobacterium bovis in Fiji Despite High Population Density and Direct Contact with Cattle. Veterinary Sciences. 2019; 6(4):85. https://doi.org/10.3390/vetsci6040085
Chicago/Turabian StyleHayton, Philip J., Richard J. Whittington, Colin Wakelin, Paul Colville, Aoife Reid, Leo Borja, and Jenny-Ann Toribio. 2019. "Mongoose (Herpestes auropunctatus) May Not Be Reservoir Hosts for Mycobacterium bovis in Fiji Despite High Population Density and Direct Contact with Cattle" Veterinary Sciences 6, no. 4: 85. https://doi.org/10.3390/vetsci6040085





