Clinical Characteristics, Serum Biochemical Changes, and Expression Profile of Serum Cfa-miRNAs in Dogs Confirmed to Have Congenital Portosystemic Shunts Accompanied by Liver Pathologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Animals and Study Design
2.3. Sample Collection and Handling
2.4. Serum Biochemical Parameters Assays
2.5. Histopathological Investigations
2.6. RNA Isolation
2.7. Reverse Transcription and Quantification of miRNAs by real-Time Polymerase Chain Reaction (RT-PCR)
2.8. Statistical Analysis
3. Results
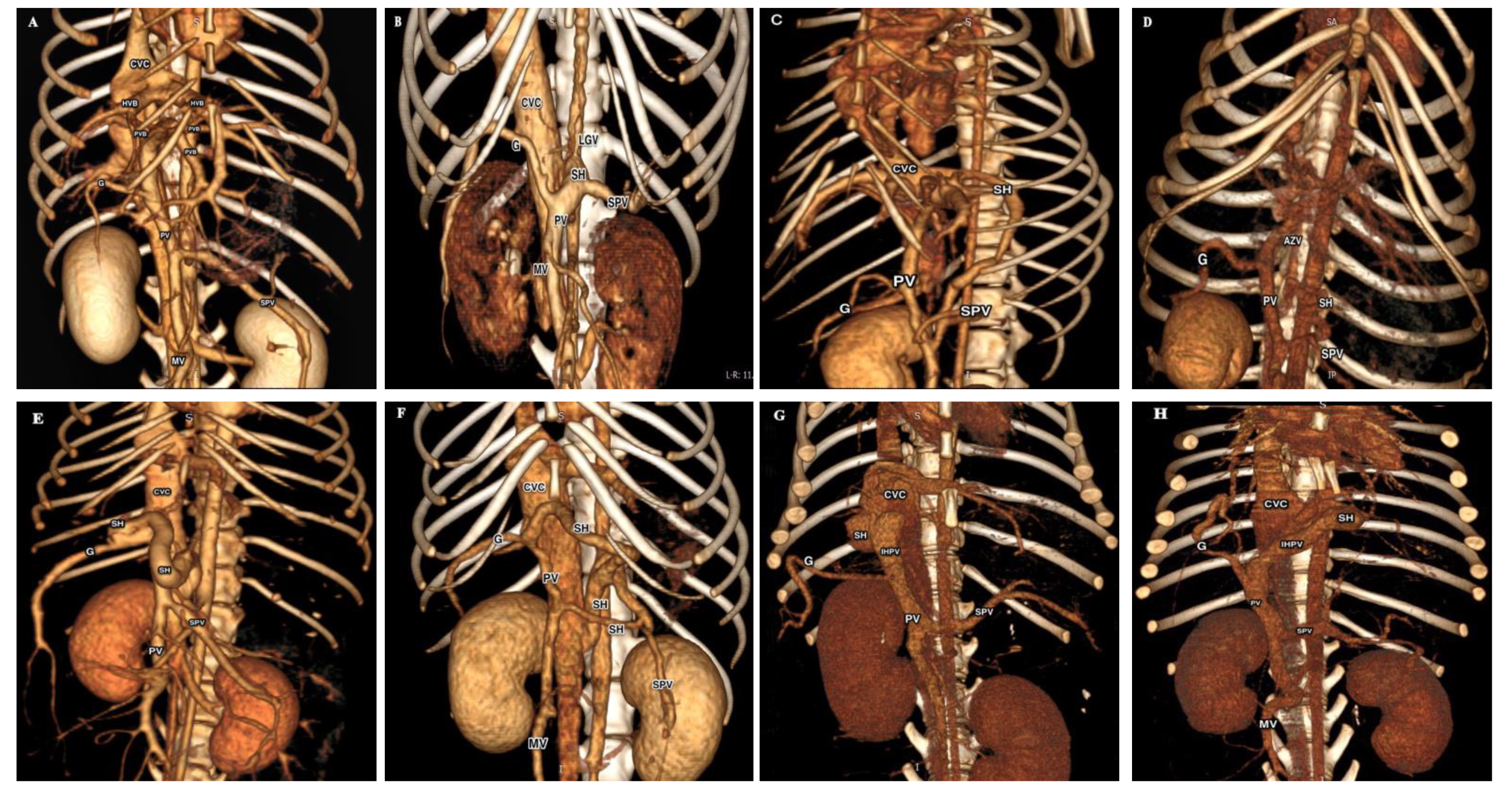
3.1. Characteristics of Dogs
3.2. Biochemical Results
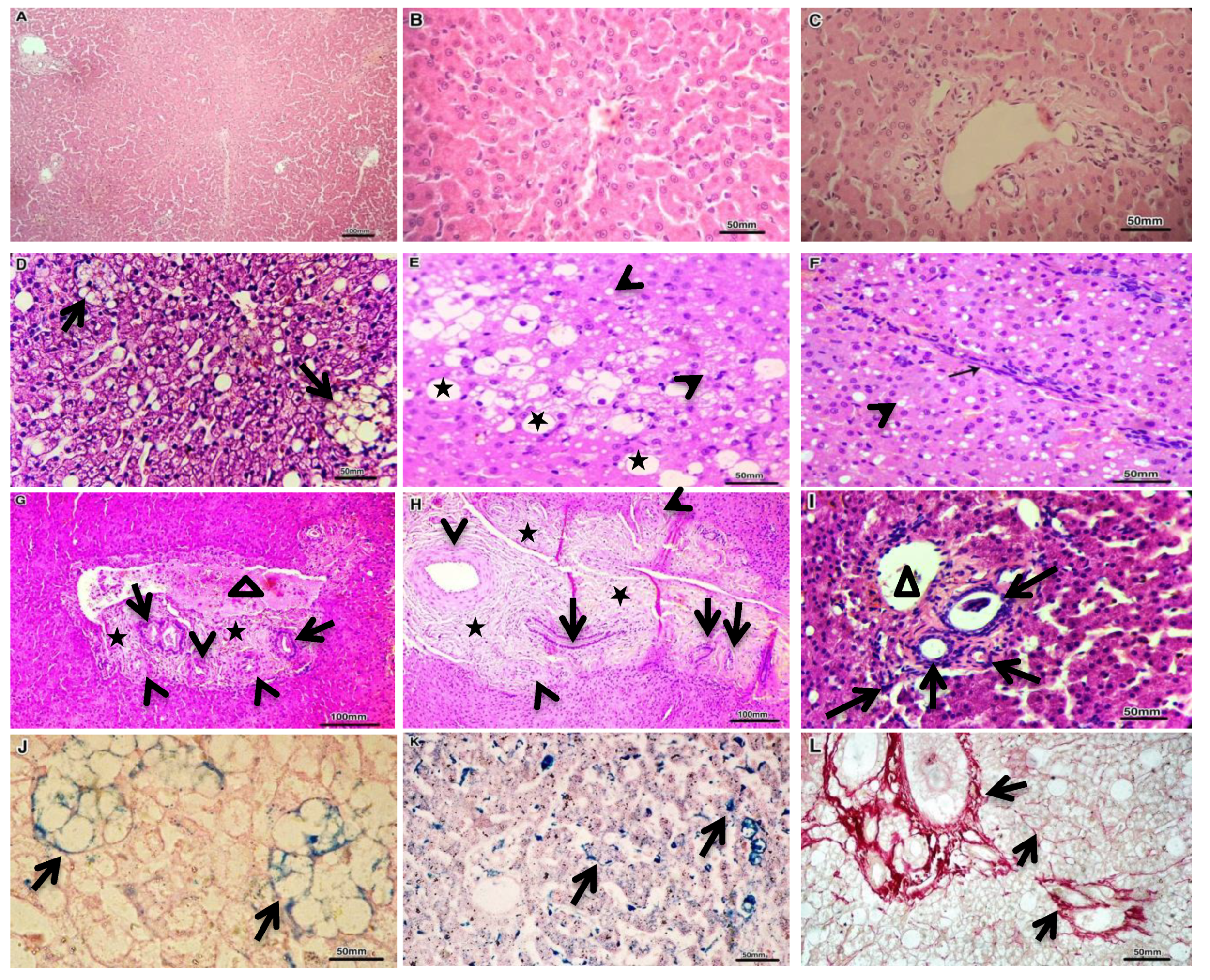
3.3. Histopathological Results
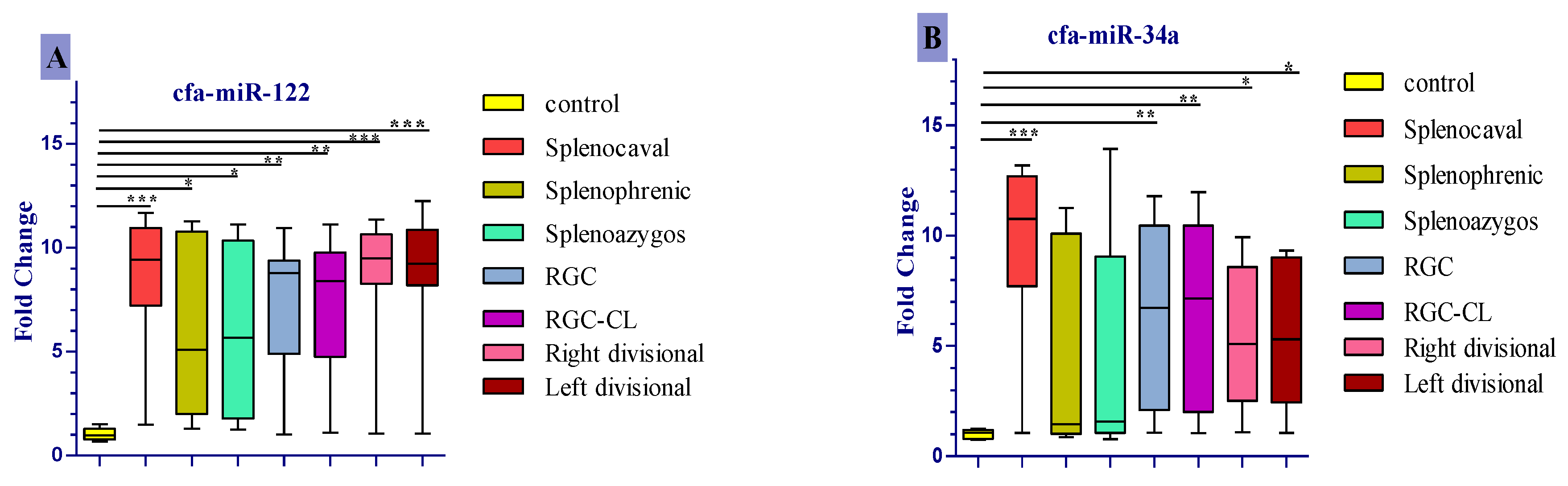
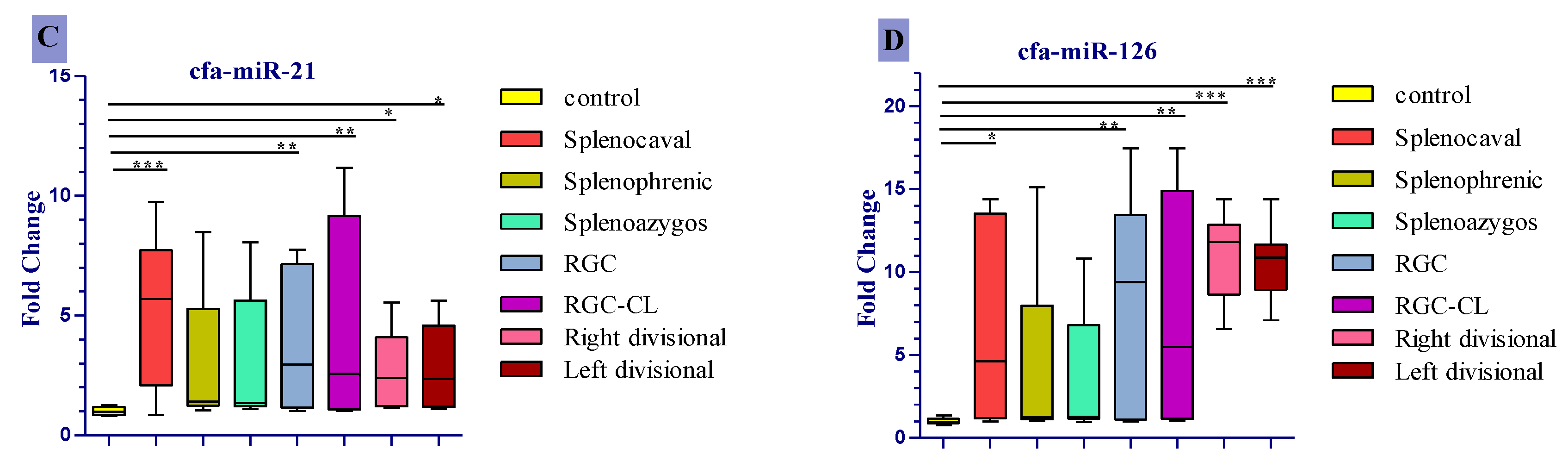
3.4. Expression Profile of the Studied Cfa-Mirnas in the Investigated Groups Compared to the Control Group
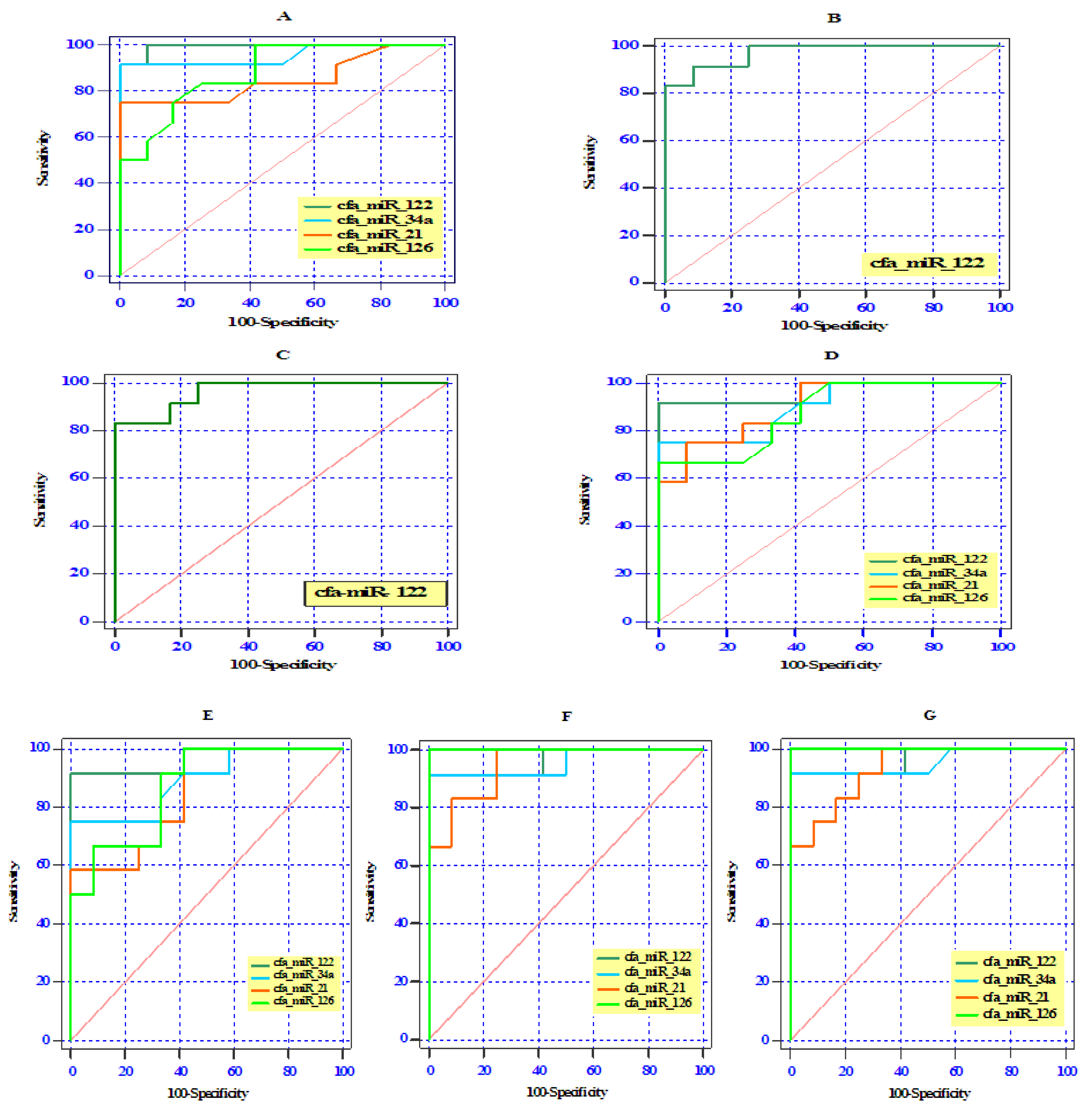
3.5. ROC Curve Analysis of Differentially Expressed Serum miRNAs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stalker, M.J.; Hayes, M.A. Developmental anomalies. Liver and biliary system. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals; Maxie, M.G., Saunders, W.B., Eds.; Elsevier: Philadelphia, PA, USA, 2007; pp. 301–304. [Google Scholar]
- Cavalcanti, E.B.O.; Baioto, G.C.; Pereira, C.M.; de Souza, M.C.C.; Rassele, A.C.; dos Santos, H.R. Congenital Extrahepatic Portosystemic Deviation in a Mixed-Breed Dog. Acta Sci. Vet. 2019, 47. [Google Scholar] [CrossRef]
- Sobczak-Filipiak, M.; Szarek, J.; Badurek, I.; Padmanabhan, J.; Trębacz, P.; Januchta-Kurmin, M.; Galanty, M. Retrospective liver histomorphological analysis in dogs in instances of clinical suspicion of congenital portosystemic shunt. J. Vet. Res. 2019, 63, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Van Straten, G.; van Dalen, D.; Mesu, S.J.; Rothuizen, J.; Teske, E.; Spee, B.; van Geijlswijk, I.M. Efficacy of orally administered sodium benzoate and sodium phenylbutyrate in dogs with congenital portosystemic shunts. J. Vet. Intern. Med. 2019, 33, 1331–1335. [Google Scholar] [CrossRef]
- Greenhalgh, S.N.; Dunning, M.D.; McKinley, T.J.; Goodfellow, M.R.; Kelman, K.R.; Freitag, T.; Jeffery, N.D. Comparison of survival after surgical or medical treatment in dogs with a congenital portosystemic shunt. J. Am. Vet. Med. Assoc. 2010, 236, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G. Anomalies of the Portal Venous System in Dogs and Cats as Seen on Multidetector-Row Computed Tomography: An Overview and Systematization Proposal. Vet. Sci. 2019, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavien, P.A.; Emond, J.; Vauthey, J.N.; Belghiti, J.; Chari, R.S.; Strasberg, S.M. Protection of the liver during hepatic surgery. J. Gastrointest. Surg. 2004, 8, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Mathews, K.G.; Bunch, S.K. Vascular liver diseases. In Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 6th ed.; Ettinger, S.J., Feldman, E.C., Eds.; Elsevier Saunders: St Louis, MO, USA, 2005; pp. 1453–1464. [Google Scholar]
- Parker, J.S.; Monnet, E.; Powers, B.E.; Twedt, D.C. Histologic examination of hepatic biopsy samples as a prognostic indicator in dogs undergoing surgical correction of congenital portosystemic shunts: 64 cases (1997–2005). J. Am. Vet. Med. Assoc. 2008, 232, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Bala, S. MicroRNAs in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 542–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geyik, M.; Nazik, M.; Ucler, N.; Erkutlu, I.; Alptekin, M.; Atadag, A.; Nehir, A. Comparison of serum and tissue values of miRNAs related to autophagy in glial brain tumors and metastases other than lymphoma. Ann. Med. Res. 2019, 26, 309–313. [Google Scholar] [CrossRef]
- Dirksen, K.; Verzijl, T.; van den Ingh, T.S.G.A.M.; Vernooij, J.C.M.; van der Laan, L.J.W.; Burgener, I.A.; Fieten, H. Hepatocyte-derived microRNAs as sensitive serum biomarkers of hepatocellular injury in Labrador retrievers. Vet. J. 2016, 211, 75–81. [Google Scholar] [CrossRef]
- Hayes, C.; Chayama, K. MicroRNAs as biomarkers for liver disease and hepatocellular carcinoma. Int. J. Mol. Sci. 2016, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Schueller, F.; Roy, S.; Vucur, M.; Trautwein, C.; Luedde, T.; Roderburg, C. The role of miRNAs in the pathophysiology of liver diseases and toxicity. Int. J. Mol. Sci. 2018, 19, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.E.; Simpson, J.W.; Williams, D.A. BSAVA Manual of Canine and Feline Gastroenterology, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005; pp. 188–190. [Google Scholar]
- Zwingenberger, A. CT diagnosis of portosystemic shunts. Vet. Clin. Small Anim. 2009, 9, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Kraun, M.B.; Nelson, L.L.; Hauptman, J.G.; Nelson, N.C. Analysis of the relationship of extrahepatic portosystemic shunt morphology with clinical variables in dogs: 53 cases (2009–2012). J. Am. Vet. Med. Assoc. 2014, 245, 540–549. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue processing and hematoxylin and eosin staining. In Histopathology; Humana Press: New York, NY, USA, 2014; pp. 31–43. [Google Scholar] [CrossRef]
- Lillie, R.D. Histopathologic Technic and Practical Histochemistry; McGraw-Hill: New York, NY, USA, 1954. [Google Scholar]
- Constantine, V.S. A combined tissue stain for the selective staining of collagen, elastic fibers and acidic carbohydrates. J. Investig. Dermatol. 1969, 52, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Cullen, J.M.; van den Ingh, T.S.; Bunch, S.E.; Rothuizen, J.; Washabau, R.J.; Desmet, V.J. Morphological classification of circulatory disorders of the canine and feline liver. In WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Disease; Elsevier: Philadelphia, PA, USA; Edinburgh, UK, 2006; pp. 41–59. [Google Scholar]
- Kirschner, M.B.; Kao, S.C.; Edelman, J.J.; Armstrong, N.J.; Vallely, M.P.; van Zandwijk, N.; Reid, G. Haemolysis during sample preparation alters microRNA content of plasma. PLoS ONE 2011, 6, e24145. [Google Scholar] [CrossRef]
- Lin, M.S.; Chen, W.C.; Huang, J.X.; Gao, H.J.; Sheng, H.H. Aberrant expression of microRNAs in serum may identify individuals with pancreatic cancer. Int. J. Clin. Exp. Med. 2014, 7, 5226–5234. [Google Scholar]
- Mizuno, H.; Nakamura, A.; Aoki, Y.; Ito, N.; Kishi, S.; Yamamoto, K.; Hashido, K. Identification of muscle-specific microRNAs in serum of muscular dystrophy animal models: Promising novel blood-based markers for muscular dystrophy. PLoS ONE 2011, 6, e18388. [Google Scholar] [CrossRef] [Green Version]
- Sourvinou, I.S.; Markou, A.; Lianidou, E.S. Quantification of circulating miRNAs in plasma: Effect of preanalytical and analytical parameters on their isolation and stability. J. Mol. Diagn. 2013, 15, 827–834. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kirkwood, B.R.; Sterne, J. Essential Medical Statistics, 2nd ed.; Blackwell: Malden, MA, USA, 2003. [Google Scholar]
- Fukushima, K.; Kanemoto, H.; Ohno, K.; Takahashi, M.; Fujiwara, R.; Nishimura, R.; Tsujimoto, H. Computed tomographic morphology and clinical features of extrahepatic portosystemic shunts in 172 dogs in Japan. Vet. J. 2014, 199, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Van Steenbeek, F.G.; Van den Bossche, L.; Grinwis, G.C.; Kummeling, A.; van Gils, I.H.; Koerkamp, M.J.G.; Leegwater, P.A. Aberrant gene expression in dogs with portosystemic shunts. PLoS ONE 2013, 8, e57662. [Google Scholar] [CrossRef]
- Tobias, K.M.; Rohrbach, B.W. Association of breed with the diagnosis of congenital portosystemic shunts in dogs: 2400 cases (1980–2002). J. Am. Vet. Med. Assoc. 2003, 223, 1636–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, R.N.; Burton, C.A.; McEvoy, F.J. Surgical treatment of intrahepatic portosystemic shunts in 45 dogs. Vet. Rec. 1998, 142, 358–365. [Google Scholar] [CrossRef]
- Papazoglou, L.G.; Monnet, E.; Seim, H.B., III. Survival and prognostic indicators for dogs with intrahepatic portosystemic shunts: 32 cases (1990–2000). Vet. Surg. 2002, 31, 561–570. [Google Scholar] [CrossRef]
- Paepe, D.; Haers, H.; Vermote, K.; Saunders, J.; Risselada, M.; Daminet, S. Portosystemic shunts in dogs and cats: Laboratory diagnosis of congenital portosystemic shunts. Vlaams Diergeneeskd. Tijdschr. 2007, 76, 241–248. [Google Scholar]
- Winkler, J.T.; Bohling, M.W.; Tillson, D.M.; Wright, J.C.; Ballagas, A.J. Portosystemic shunts: Diagnosis, prognosis, and treatment of 64 cases (1993–2001). J. Am. Anim. Hosp. Assoc. 2003, 39, 169–185. [Google Scholar] [CrossRef]
- Ramaiah, S.K. Preclinical safety assessment: Current gaps, challenges, and approaches in identifying translatable biomarkers of drug-induced liver injury. Clin. Lab. Med. 2011, 31, 161–172. [Google Scholar] [CrossRef]
- Dirksen, K.; Verzijl, T.; Grinwis, G.C.; Favier, R.P.; Penning, L.C.; Burgener, I.A.; Spee, B. Use of Serum Micro RNAs as Biomarker for Hepatobiliary Diseases in Dogs. J. Vet. Intern. Med. 2016, 30, 1816–1823. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, E.S.; Kubesy, A.A.; Barak, T.A.; Torad, F.A.; Salem, S.I.; Salem, N.Y. Expression of blood hepatocyte-derived microRNA-122 in canine multicentric lymphoma with hepatic involvement. Vet. Res. Commun. 2019, 43, 1–8. [Google Scholar] [CrossRef]
- Lee, K.C.; Winstanley, A.; House, J.V.; Lipscomb, V.; Lamb, C.; Gregory, S.; Brockman, D.J. Association between hepatic histopathologic lesions and clinical findings in dogs undergoing surgical attenuation of a congenital portosystemic shunt: 38 cases (2000–2004). J. Am. Vet. Med. Assoc. 2011, 239, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.B.; Luff, J.A.; Daniel, L.; Bergh, R.V.D. Evaluation of hepatic steatosis in dogs with congenital portosystemic shunts using Oil Red O staining. Vet. Pathol. 2013, 50, 1109–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isobe, K.; Nakayama, H.; Uetsuka, K. Relation between lipogranuloma formation and fibrosis, and the origin of brown pigments in lipogranuloma of the canine liver. Comp. Hepatol. 2008, 7, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Sobczak-Filipiak, M.; Męcik-Kronenberg, T.; Czopowicz, M.; Galanty, M.; Trębacz, P.; Frymus, J.; Szarek, J. Lipogranulomas and pigment granulomas in livers of dogs with portosystemic shunt. Pol. J. Vet. Sci. 2018, 21, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Iravani, F.; Hosseini, N.; Mojarrad, M. Role of MicroRNAs in Pathophysiology of Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis. Middle East J. Dig. Dis. 2018, 10, 213–219. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Ochiya, T. Extracellular microRNAs and oxidative stress in liver injury: A systematic mini review. J. Clin. Biochem. Nutr. 2018, 63, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Oosthuyzen, W.; Ten Berg, P.W.L.; Francis, B.; Campbell, S.; Macklin, V.; Milne, E.; Dear, J.W. Sensitivity and specificity of microRNA122 for liver disease in dogs. J. Vet. Intern. Med. 2018, 32, 1637–1644. [Google Scholar] [CrossRef] [Green Version]
- Pirola, C.J.; Gianotti, T.F.; Castaño, G.O.; Mallardi, P.; San Martino, J.; Ledesma, M.M.G.L.; Sookoian, S. Circulating microRNA signature in non-alcoholic fatty liver disease: From serum non-coding RNAs to liver histology and disease pathogenesis. Gut 2015, 64, 800–812. [Google Scholar] [CrossRef] [Green Version]
- Muangpaisarn, P.; Jampoka, K.; Payungporn, S.; Wisedopas, N.; Bunchorntavakul, C.; Tangkijvanich, P.; Treeprasertsuk, S. Serum microRNA-34a is potential biomarker for inflammation in nonalcoholic fatty liver disease. Asian Biomed. 2017, 10, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Baade, S.; Aupperle, H.; Grevel, V.; Schoon, H.A. Histopathological and immunohistochemical investigations of hepatic lesions associated with congenital portosystemic shunt in dogs. J. Comp. Pathol. 2006, 134, 80–90. [Google Scholar] [CrossRef]
- Lautt, W.W. Regulatory processes interacting to maintain hepatic blood flow constancy: Vascular compliance, hepatic arterial buffer response, hepatorenal reflex, liver regeneration, and escape from vasoconstriction. Hepatol. Res. 2007, 37, 891–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roskams, T.A.; Theise, N.D.; Balabaud, C.; Bhagat, G.; Bhathal, P.S.; Bioulac-Sage, P.; Finegold, M.J. Nomenclature of the finer branches of the biliary tree: Canals, ductules, and ductular reactions in human livers. Hepatology 2004, 39, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, S.A.; Kim, C.H.; Park, S.Y.; Kim, J.S.; Kim, D.K.; Sheen, Y.Y. TGF-β type I receptor kinase inhibitor EW-7197 suppresses Cholestatic liver fibrosis by inhibiting HIF1α-induced epithelial mesenchymal transition. Cell. Physiol. Biochem. 2016, 38, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.J.; Xu, C.Z.; Wang, J.T.; Li, X.J.; Wang, M.M.; Gu, Y.H.; Liang, Z.G. miR-21 promotes proliferation and inhibits apoptosis of hepatic stellate cells through targeting PTEN/PI3K/AKT pathway. J. Recept. Signal Transduct. Res. 2018, 38, 455–461. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, X.; Bai, F.; Nie, J.; Wen, S.; Wei, Y.; Lin, X. Methyl helicterte ameliorates liver fibrosis by regulating miR-21-mediated ERK and TGF-β1/Smads pathways. Int. Immunopharmacol. 2019, 66, 41–51. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, N.; Wu, K.; Dai, W.; Ye, C.; Shi, J.; Lin, Y. MiR-21 simultaneously regulates ERK1 signaling in HSC activation and hepatocyte EMT in hepatic fibrosis. PLoS ONE 2014, 9, e108005. [Google Scholar] [CrossRef] [Green Version]
- Alique, M.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramírez, R. MicroRNA-126 regulates Hypoxia-Inducible Factor-1α which inhibited migration, proliferation, and angiogenesis in replicative endothelial senescence. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.F.; Wythe, J.D.; Srivastava, D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zheng, Y.; Wang, M.; Yan, M.; Jiang, J.; Li, Z. Exosomes derived miR-126 attenuates oxidative stress and apoptosis from ischemia and reperfusion injury by targeting ERRFI1. Gene 2019, 690, 75–80. [Google Scholar] [CrossRef]
- Heishima, K.; Ichikawa, Y.; Yoshida, K.; Iwasaki, R.; Sakai, H.; Nakagawa, T.; Maruo, K. Circulating microRNA-214 and-126 as potential biomarkers for canine neoplastic disease. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Orso, F.; Quirico, L.; Dettori, D.; Coppo, R.; Virga, F.; Ferreira, L.C.; Taverna, D. Role of miRNAs in tumor and endothelial cell interactions during tumor progression. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
| Groups. | EHPSS | IHPSS | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Control (n = 12) | Splenocaval (n = 12) | Splenophrenic (n = 12) | Splenoazygos (n = 12) | RGC (n = 12) | RGC-CL (n = 12) | Right Divisional (n = 12) | Left Divisional (n = 12) |
| Breed (NO.) | ||||||||
| Yorkshire terrier | (3) | (4) | (2) | (4) | (5) | (4) | (0) | (0) |
| Miniature dachshund | (1) | (3) | (2) | (1) | (1) | (2) | (0) | (0) |
| Miniature pincher | (1) | (1) | (0) | (2) | (0) | (0) | (0) | (0) |
| Russian toy terrier | (2) | (0) | (5) | (0) | (0) | (1) | (0) | (0) |
| Papillon | (1) | (0) | (1) | (1) | (2) | (2) | (0) | (0) |
| Shih Tzu | (0) | (1) | (1) | (2) | (2) | (2) | (0) | (0) |
| Pug | (0) | (1) | (0) | (1) | (1) | (0) | (0) | (0) |
| Jack Russell terrier | (1) | (2) | (1) | (1) | (1) | (1) | (0) | (0) |
| Golden Retriever | (2) | (0) | (0) | (0) | (0) | (0) | (8) | (9) |
| Labrador retriever | (1) | (0) | (0) | (0) | (0) | (0) | (4) | (3) |
| Clinical symptoms (NO.) | ||||||||
| Neurologic signs after feeding | (0) | (11) | (2) | (1) | (9) | (9) | (11) | (12) |
| Intermittent vomition | (0) | (12) | (9) | (9) | (10) | (8) | (12) | (12) |
| Stranguria and hematuria | (0) | (9) | (1) | (2) | (8) | (10) | (9) | (11) |
| Gender (NO.) | F(3), FS(1), M(5), MN(3) | F(2), FS(1), M(8), MN(1) | F(1), FS(2), M(7), MN(2) | F(2), FS(0), M(7), MN(3) | F(4), FS(0), M(7), MN(1) | F(4), FS(1), M(6), MN(1) | F(1), FS(1), M(9), MN(1) | F(2), FS(0), M(9), MN(1) |
| Age (months), Mean ± SD | 28.95 ± 10.72 b | 22.85 ± 6.47 b | 39.83 ± 9.31 a | 40.58 ± 13.05 a | 22.58 ± 7.34 b | 20.33 ± 6.30 b | 6.58 ± 1.78 c | 6.67 ± 1.92 c |
| Body weight (kg), Mean ± SD | 5.12 ± 0.93 a | 3.69 ± 0.67 b | 4.98 ± 0.80 a | 5.01 ± 0.98 a | 3.78 ± 1.01 b | 3.70 ± 0.91 b | 5.12 ± 1.07 a | 5.29 ± 1.02 a |
| Gene Name | Accession Number | Mature Sequence 5′–3′ | Primer Sequence 5′–3′ |
|---|---|---|---|
| cfa-miR-122-5p | MIMAT0006619 | UGGAGUGUGACAAUGGUGUUUG | CAAACACCATTGTCACACTCCA |
| cfa-miR-34a-5p | MIMAT0006690 | UGGCAGUGUCUUAGCUGGUUGU | ACAACCAGCTAAGACACTGCCA |
| cfa-miR-21-5p | MIMAT0006741 | UAGCUUAUCAGACUGAUGUUGA | TCAACATCAGTCTGATAAGCTA |
| cfa-miR-126-5p | MIMAT0006730 | CAUUAUUACUUUUGGUACGCG | CGCGTACCAAAAGTAATAATG |
| cfa-miR-16-5p | MIMAT0006648 | UAGCAGCACGUAAAUAUUGGCG | CGCCAATATTTACGTGCTGCTA |
| Group | Control | EHPSS | IHPSS | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | Splenocaval | Splenophrenic | Splenoazygos | RGC | RGC-CL | Right Divisional | Left Divisional | |
| ALT (U/L) | 44.59 ± 8.35 b | 134.16 ± 21.57 a | 68.98 ± 12.80 b | 66.28 ± 14.41 b | 120.16 ± 18.14 a | 123.52 ± 23.33 a | 143.64 ± 31.94 a | 139.32 ± 20.73 a |
| AST (U/L) | 34.07 ± 4.76 b | 88.37 ±8.95 a | 46.28 ± 7.44 b | 45.24 ± 6.53 b | 77.22 ± 14.60 a | 81.91 ± 17.12 a | 92.05 ± 8.71 a | 90.03 ± 7.55 a |
| ALP (U/L) | 76.15 ± 11.31 b | 292.1 ± 55.45 a | 100.65 ± 19.83 b | 102.25 ±18.22 b | 271.21 ± 30.15 a | 275.13 ± 26.24 a | 307.93 ± 31.22 a | 304.59 ± 30.17 a |
| GGT (U/L) | 7.20 ± 1.70 b | 12.07 ± 1.57 a | 8.06 ± 1.36 b | 8.24 ± 1.18 b | 10.54 ± 1.88 a | 10.73 ± 1.49 a | 12.28 ± 1.58 a | 12.16 ± 1.35 a |
| TP(g/dL) | 6.47 ± 0.72 a | 3.73 ± 0.82 b | 5.70 ± 0.59 a | 5.74 ± 0.70 a | 3.94 ± 0.46 b | 3.85 ± 0.43 b | 3.32 ±0.45 b | 3.20 ± 0.68 b |
| ALB (g/dL) | 3.00 ± 0.58 a | 1.98 ± 0.40 b | 2.78 ± 0.43 a | 2.72 ± 0.70 a | 2.09 ± 0.37 b | 2.03 ± 0.29 b | 1.64 ± 0.38 b | 1.60 ± 0.45 b |
| Globulin (g/dL) | 3.46 ± 0.42 a | 1.75 ± 0.63 b | 2.92 ± 0.69 a | 2.99 ± 0.36 a | 1.84 ± 0.33 b | 1.82 ± 0.40 b | 1.69 ± 0.56 b | 1.59 ± 0.47 b |
| A/G ratio | 0.88 ± 0.21 a | 1.26 ± 0.46 a | 1.01 ± 0.32 a | 0.94 ± 0.28 a | 1.18 ± 0.32 a | 1.17 ± 0.36 a | 1.14 ± 0.69 a | 1.10 ± 0.53 a |
| T.BIL (μmol/L) | 4.64 ± 0.98 a | 5.27 ± 0.85 a | 4.82 ±0.81 a | 4.74 ± 0.72 a | 5.24 ± 0.75 a | 5.31 ±0.67 a | 5.32 ± 0.88 a | 5.36 ± 0.83 a |
| BUN (μmol/L) | 5.78 ± 0.77 a | 2.49 ± 0.40 b | 5.40 ± 1.68 a | 5.51 ± 1.57 a | 2.98 ± 1.05 b | 2.88 ± 1.43 b | 2.25 ± 0.59 b | 2.29 ± 0.37 b |
| Ammonia (μmol/L) | 25.31 ± 6.23 b | 230.80 ± 67.83 a | 76.29 ± 14.44 b | 70.32 ± 17.25 b | 205.70 ± 29.28 a | 211.45 ± 29.85 a | 253.48 ± 56.67 a | 260.26 ± 63.04 a |
| TC (μmol/L) | 5.02 ± 1.02 a | 2.51 ± 0.50 b | 4.02 ± 1.07 a | 4.15 ± 1.17 a | 2.73 ± 0.81 b | 2.81 ± 0.77 b | 2.44 ± 0.46 b | 2.26 ± 0.51 b |
| TG (μmol/L) | 0.75 ± 0.23 a | 0.33 ± 0.16 b | 0.66 ± 0.22 a | 0.68 ± 0.20 a | 0.37 ± 0.13 b | 0.38 ± 0.11 b | 0.30 ± 0.14 b | 0.28 ± 0.09 b |
| GLU (mg/dl) | 4.20 ±0.93 a | 2.35 ± 0.77 b | 3.68 ± 0.49 a | 3.57 ± 0.56 a | 2.41 ± 0.61 b | 2.46 ± 0.55 b | 2.24 ± 0.66 b | 2.34 ± 0.57 b |
| CREA (μmol/L) | 49.33 ± 5.93 a | 48.67 ± 5.09 a | 46.53 ± 4.24 a | 47.68 ± 5.23 a | 48.88 ± 2.38 a | 46.43 ± 6.13 a | 50.34 ± 5.56 a | 48.54 ± 5.66 a |
| LIPA (U/L) | 131.75 ± 31.84 a | 128.25 ± 24.61 a | 129.67 ± 26.02 a | 130.42 ±25.66 a | 133.00 ± 31.87 a | 131.42 ± 37.64 a | 125.83 ± 34.56 a | 129.33 ± 27.49 a |
| AMYL (U/L) | 174.99 ± 59.44 a | 169.27 ± 97.28 a | 181.44 ± 54.76 a | 177.50 ± 93.67 a | 167.56 ± 60.19 a | 173.32 ± 60.51 a | 183.62 ± 78.73 a | 179.96 ± 68.96 a |
| Group | Control | EHPSS | IHPSS | |||||
|---|---|---|---|---|---|---|---|---|
| Histopathologic Feature (s) | Splenocaval | Splenophrenic | Splenoazygos | RGC | RGC–CL | Right Divisional | Left Divisional | |
| Microvesicular steatosis | 0 (0%) | 12 (100%) | 8 (66.67%) | 9 (75%) | 10 (83.33%) | 9 (75%) | 12 (100%) | 12 (100%) |
| Macrovesicular steatosis | 0 (0%) | 10 (83.33%) | 0 (0%) | 1 (8.33%) | 9 (75%) | 8 (66.67%) | 4 (33.33%) | 3 (25%) |
| Lipid granuloma | 0 (0%) | 9 (75%) | 0 (0%) | 0 (0%) | 7 (66.67%) | 8 (66.67%) | 2 (16.67%) | 1 (8.33%) |
| Hemosiderin within lipid granuloma or Kupffer cells | 0 (0%) | 11 (91.67%) | 1 (8.33%) | 2 (16.67%) | 10 (83.33%) | 10 (83.33%) | 11 (91.67%) | 12 (100%) |
| Portal veins absence or hypoplasia | 0 (0%) | 12 (100%) | 3 (25%) | 3 (16.67%) | 12 (100%) | 12 (100%) | 12 (100%) | 12 (100%) |
| Arteriolar hyperplasia | 0 (0%) | 7 (58.33%) | 2 (16.67%) | 3 (25%) | 9 (75%) | 8 (66.67%) | 12 (100%) | 12 (100%) |
| Biliary hyperplasia | 0 (0%) | 7 (58.33%) | 2 (16.67%) | 3 (25%) | 9 (75%) | 8 (66.67%) | 12 (100%) | 12 (100%) |
| Fibrosis (portal, periportal and parenchymal) | 0 (0%) | 7 (58.33%) | 2 (16.67%) | 2 (16.67%) | 6 (50%) | 7 (58.33%) | 3 (25%) | 4 (33.33%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. El-Sebaey, A.; N. Abramov, P.; M. Abdelhamid, F. Clinical Characteristics, Serum Biochemical Changes, and Expression Profile of Serum Cfa-miRNAs in Dogs Confirmed to Have Congenital Portosystemic Shunts Accompanied by Liver Pathologies. Vet. Sci. 2020, 7, 35. https://doi.org/10.3390/vetsci7020035
M. El-Sebaey A, N. Abramov P, M. Abdelhamid F. Clinical Characteristics, Serum Biochemical Changes, and Expression Profile of Serum Cfa-miRNAs in Dogs Confirmed to Have Congenital Portosystemic Shunts Accompanied by Liver Pathologies. Veterinary Sciences. 2020; 7(2):35. https://doi.org/10.3390/vetsci7020035
Chicago/Turabian StyleM. El-Sebaey, Ahmed, Pavel N. Abramov, and Fatma M. Abdelhamid. 2020. "Clinical Characteristics, Serum Biochemical Changes, and Expression Profile of Serum Cfa-miRNAs in Dogs Confirmed to Have Congenital Portosystemic Shunts Accompanied by Liver Pathologies" Veterinary Sciences 7, no. 2: 35. https://doi.org/10.3390/vetsci7020035
APA StyleM. El-Sebaey, A., N. Abramov, P., & M. Abdelhamid, F. (2020). Clinical Characteristics, Serum Biochemical Changes, and Expression Profile of Serum Cfa-miRNAs in Dogs Confirmed to Have Congenital Portosystemic Shunts Accompanied by Liver Pathologies. Veterinary Sciences, 7(2), 35. https://doi.org/10.3390/vetsci7020035





