Escherichia coli: Physiological Clues Which Turn On the Synthesis of Antimicrobial Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains: Cultures, Media and Culture Conditions
2.2. Effect of Carbon Source on Molecule Production
- (a)
- Molecule Extraction Assay:
- (b)
- Omelette method:
2.3. Effect of Bile Salts on Molecule Production
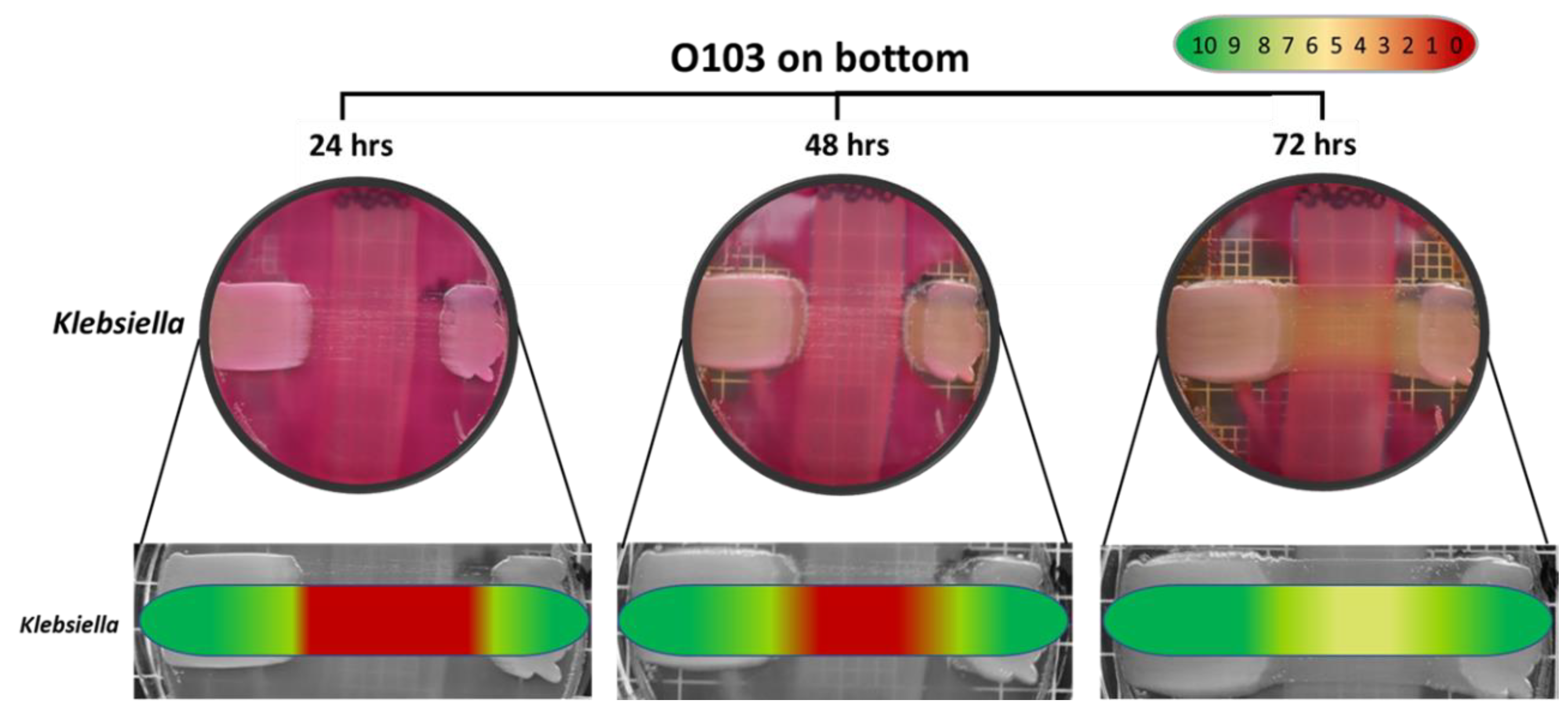
2.4. Effect of E. coli O103F Growth Stage on Molecule Production
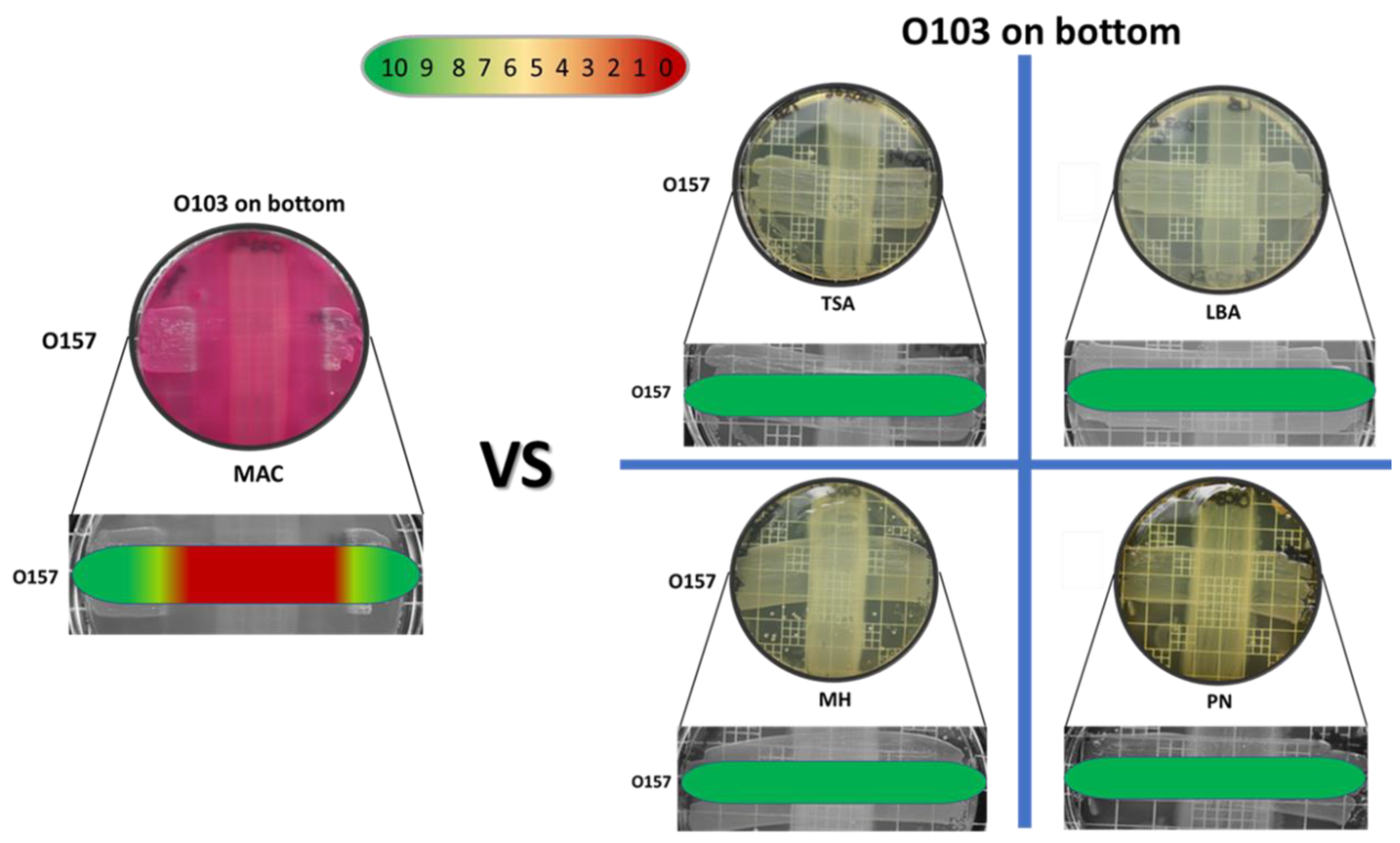
2.5. Specificity of the E. coli O103F Molecule
2.6. Statistical Analysis
3. Results
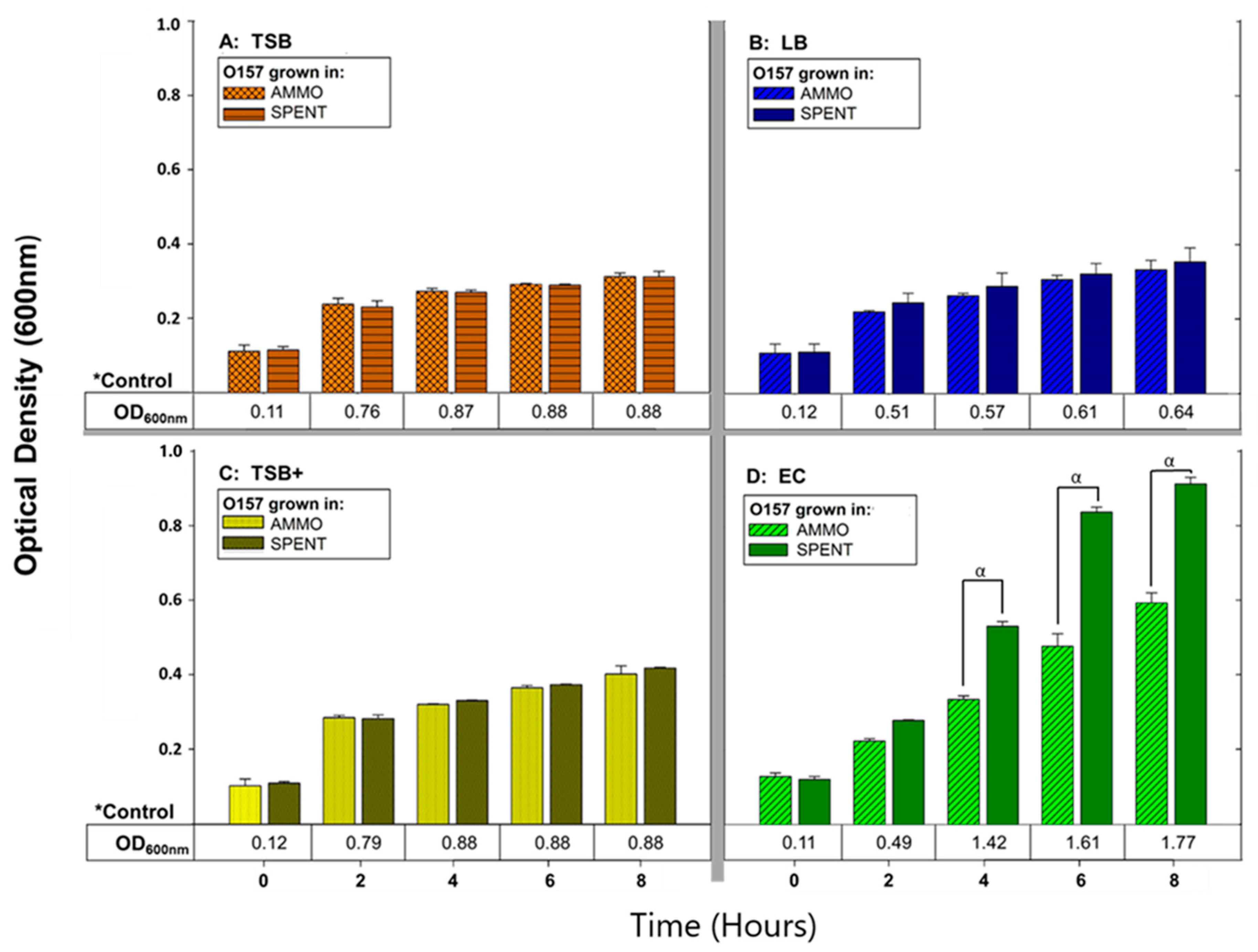
3.1. Effect of Carbon Source on Molecule Production
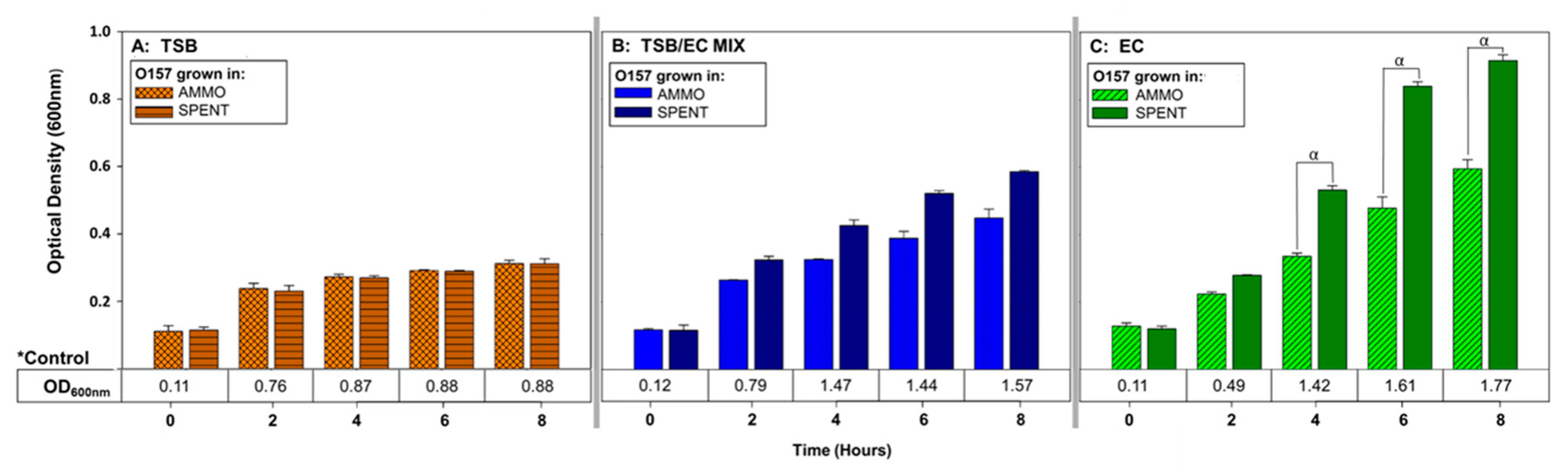
3.1.1. Molecule Isolation Assay—Molecule Production in Different Liquid Media
3.1.2. Omelette Method—Molecule Production on Different Solid Media
3.2. Effect of Bile on Molecule Production
3.3. Effect of E. coli O103F Growth Stage on Molecule Production
3.4. Specificity of the E. coli O103F Molecule
4. Discussion
4.1. Effect of Carbon Source on Molecule Production
4.2. Effect of Bile on Molecule Production
4.3. Effect of E. coli O103F Growth Stage on Molecule Production
4.4. E. coli O103F Molecule Specificity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biology Online: Physiological Adaptation. Available online: https://www.biology-online.com/dictionary/Physiological_adaptation (accessed on 27 May 2020).
- Pantin, C.F.A. Physiological adaptation. Zool. J. Linn. Soc. 1932, 37, 705–711. [Google Scholar] [CrossRef]
- Feugeas, J.-P.; Tourret, J.; Launay, A.; Bouvet, O.; Hoede, C.; Denamur, E.; Tenaillon, O. Links between transcription, environmental adaptation and gene variability in Escherichia coli: Correlations between gene expression and gene variability reflect growth efficiencies. Mol. Biol. Evol. 2016, 33, 2515–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sistrunk, J.R.; Nickerson, K.P.; Chanin, R.B.; Rasko, D.A.; Faherty, C.S. Survival of the fittest: How bacterial pathogens utilize bile to enhance infection. Clin. Microbiol. Rev. 2016, 29, 819–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Hur, H.-G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Walk, S.T.; Gordon, D.M.; Feldgarden, M.; Tiedje, J.M.; Konstantinidis, K.T. Genome sequencing of environmental Escherichia coli expands understanding of the ecology and speciation of the model bacterial species. Proc. Natl. Acad. Sci. USA 2011, 108, 7200–7205. [Google Scholar] [CrossRef] [Green Version]
- Conway, T.; Cohen, P.S. Commensal and pathogenic Escherichia coli metabolism in the gut. Microbiol. Spectr. 2015, 3, 343–362. [Google Scholar]
- Nguyen, Y.; Sperandio, V. Enterohemorrhagic E. coli (EHEC) pathogenesis. Front. Cell Infect. Microbiol. 2012, 2, 90. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.; Wu, Y.E.; Fu, X.; Chang, Z. Chaperone-dependent mechanisms for acid resistance in enteric bacteria. Cell 2012, 20, 328–335. [Google Scholar] [CrossRef]
- Merritt, M.E.; Donaldson, J.R. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J. Med. Micobiol. 2009, 58, 1533–1541. [Google Scholar] [CrossRef] [Green Version]
- Cremers, C.M.; Knoefler, D.; Vitvitsky, V.; Banerjee, R.; Jakob, U. Bile salts act as effective protein-unfolding agents and instigators of disulfide stress in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, E1610–E1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamner, S.; Mcinnerney, K.; Williamson, K.; Franklin, M.J.; Ford, T.E. Bile salts affect expression of Escherichia coli O157:H7 genes for virulence and iron acquisition, and promote growth under iron limiting conditions. PLoS ONE. 2013, 8, e74647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabich, A.J.; Jones, S.A.; Chowdhury, F.Z.; Cernosek, A.; Anderson, A.; Smalley, D.; McHargue, J.W.; Hightower, G.A.; Smith, J.T.; Autieri, S.M.; et al. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect. Immun. 2008, 76, 1143–1152. [Google Scholar] [CrossRef] [Green Version]
- Askari, N.; Ghanbarpour, R. Molecular investigation of the colicinogenic Escherichia coli strains that are capable of inhibiting E. coli O157:H7 in vitro. BMC Vet. Res. 2019, 15, 14. [Google Scholar] [CrossRef]
- Gillor, O.; Etzion, A.; Riley, M.A. The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 2008, 81, 591–606. [Google Scholar] [CrossRef] [Green Version]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef] [Green Version]
- Paquette, S.-J.; Zaheer, R.; Stanford, K.; Thomas, J.; Reuter, T. Competition among Escherichia coli Strains for Space and Resources. Vet. Sci. 2018, 5, 93. [Google Scholar] [CrossRef] [Green Version]
- Paquette, S.-J.; Reuter, T. Properties of an antimicrobial molecule produced by an Escherichia coli champion. Antibiotics 2019, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Cameron, A.; Zaheer, R.; Adator, E.H.; Barbieri, R.; Reuter, T.; McAllister, T.A. Bacteriocin occurrence and activity in Escherichia coli isolated from bovines and wastewater. Toxins 2019, 11, 475. [Google Scholar] [CrossRef] [Green Version]
- Urdaneta, V.; Casadesús, J. Interactions between bacteria and bile salts in the gastrointestinal and hepatobiliary tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, J.W.; Nguyen, Y.; Curtis, M.M.; Moreira, C.G.; Sperandio, V. Virulence meets metabolism: Cra and KdpE gene regulation in enterohemorrhagic Escherichia coli. MBio 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremling, A.; Geiselmann, J.; Ropers, D.; De Jong, H. Understanding carbon catabolite repression in Escherichia coli using quantitative models. Trends Microbiol. 2015, 23, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, T.; Wu, H. The transport and mediation mechanisms of the common sugars in Escherichia coli. Biotechnol. Adv. 2014, 32, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Görke, B.; Stülke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.B.; Uknalis, J.; Tu, S.-I. Effects of sugar addition in Luria Bertani (LB) media on Escherichia coli O157:H7. J. Food Saf. 2011, 31, 386–394. [Google Scholar] [CrossRef]
- Duquesne, S.; Destoumieux-Garzon, D.; Peduzzi, J.; Rebuffat, S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat. Prod. Rep. 2007, 24, 708–734. [Google Scholar] [CrossRef]
- Fang, A.; Demain, A.L. Influence of aeration and carbon source on production of microcin B17 by Escherichia coli ZK650. Appl. Microbiol. Biotechnol. 1997, 47, 547–553. [Google Scholar] [CrossRef]
- Salomón, R.A.; Farías, R.N. Microcin 25, a novel antimicrobial peptide produced by Escherichia coli. J. Bacteriol. 1992, 174, 7428–7435. [Google Scholar] [CrossRef] [Green Version]
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef] [Green Version]
- Vassiliadis, G.; Destoumieux-Garzón, D.; Peduzzi, J. Class II microcins. In Prokaryotic Antimicrobial Peptides; Drider, D., Rebuffat, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 309–332. [Google Scholar]
- Duquesne, S.; Petit, V.; Peduzzi, J.; Rebuffat, S. Structural and functional diversity of microcins, gene-encoded antibacterial peptides from enterobacteria. J. Mol. Microbiol. Biotechnol. 2007, 13, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Corsini, G.; Baeza, M.; Monasterio, O.; Lagos, R. The expression of genes involved in microcin maturation regulates the production of active microcin E492. Biochimie 2002, 84, 539–544. [Google Scholar] [CrossRef]
- García-Bayona, L.; Comstock, L.E. Bacterial antagonism in host-associated microbial communities. Science 2018, 361, eaat2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleanthous, C. Swimming against the tide: Progress and challenges in our understanding of colicin translocation. Nat. Rev. Microbiol. 2010, 8, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L. Resistance in Gram-negative bacteria: Enterobacteriaceae. Am. J. Infect. Control 2006, 34, S20–S28. [Google Scholar] [CrossRef]
- Naimi, S.; Zirah, S.; Hammami, R.; Fernandez, B.; Rebuffat, S.; Fliss, I. Fate and biological activity of the antimicrobial lasso peptide microcin J25 under gastrointestinal tract conditions. Front. Microbiol. 2018, 9, 1764. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, A.R.; Sperandio, V. Shiga toxin in enterohemorrhagic E. coli: Regulation and novel anti-virulence strategies. Front. Cell Infect. Microbiol. 2012, 2, 81. [Google Scholar] [CrossRef] [Green Version]
- Sperandio, V.; Mellies, J.L.; Nguyen, W.; Shin, S.; Kaper, J.B. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 1999, 96, 15196–15201. [Google Scholar] [CrossRef] [Green Version]
- Moritz, R.L.; Welch, R.A. The Escherichia coli argW-dsdCXA genetic island is highly variable, and E. coli K1 strains commonly possess two copies of dsdCXA. J. Clin. Microbiol. 2006, 44, 4038–4048. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.L.; Fratamico, P.M.; Gunther, N.W. Chapter three—Shiga toxin-producing Escherichia coli. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 145–197. [Google Scholar]
- Jubelin, G.; Desvaux, M.; Schüller, S.; Etienne-Mesmin, L.; Muniesa, M.; Blanquet-Diot, S. Modulation of enterohaemorrhagic Escherichia coli survival and virulence in the human gastrointestinal tract. Microorganisms 2018, 6, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanford, K.; Hannon, S.; Booker, C.W.; Jim, G.K. Variable efficacy of a vaccine and direct-fed microbial for controlling Escherichia coli O157:H7 in feces and on hides of feedlot cattle. Foodborne Pathog. Dis. 2014, 11, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Stephens, T.P.; Stanford, K.; Rode, L.M.; Booker, C.W.; Vogstad, A.R.; Schunicht, O.C.; Jim, G.K.; Wildman, B.K.; Perrett, T.; McAllister, T.A. Effect of a direct-fed microbial on animal performance, carcass characteristics and the shedding of Escherichia coli O157 by feedlot cattle. Ani. Feed Sci. Technol. 2010, 158, 65–72. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Y.; Iwaasa, A.D.; Li, Y.; Xu, Z.; Schellenberg, M.P.; Liu, X.L.; McAllister, T.A.; Stanford, K. Purple Prairie Clover (Dalea purpurea Vent) reduces fecal shedding of Escherichia coli in pastured cattle. J. Food Prot. 2015, 78, 1434–1441. [Google Scholar] [CrossRef]
- Sassone-Corsi, M.; Nuccio, S.-P.; Liu, H.; Hernandez, D.; Vu, C.T.; Takahashi, A.A.; Edwards, R.A.; Raffatellu, M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 2016, 540, 280–283. [Google Scholar] [CrossRef]


| Media | Lab Code | Modification | Bile | Sugar | Supplier |
|---|---|---|---|---|---|
| E. coli | EC | none | 1.5 g/L | lactose | 1 |
| Luria-Bertani (Miller) | LB | none | none | 2 | |
| Luria-Bertani agar | LBA | none | none | 2 | |
| MacConkey | MAC | none | 1.5 g/L | lactose | 2 |
| Mueller-Hinton II | MH | none | none | starch | 2 |
| Nutrient agar | PN | none | none | 3 | |
| Tryptic soy broth | TSB | none | none | glucose | 1 |
| Tryptic soy agar | TSA | none | none | 1 | |
| Modified | TSB/EC | 50:50 w:w | 50% EC | ||
| Modified | TSB+ | 5.0 g/L lactose | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paquette, S.-J.; Reuter, T. Escherichia coli: Physiological Clues Which Turn On the Synthesis of Antimicrobial Molecules. Vet. Sci. 2020, 7, 184. https://doi.org/10.3390/vetsci7040184
Paquette S-J, Reuter T. Escherichia coli: Physiological Clues Which Turn On the Synthesis of Antimicrobial Molecules. Veterinary Sciences. 2020; 7(4):184. https://doi.org/10.3390/vetsci7040184
Chicago/Turabian StylePaquette, Sarah-Jo, and Tim Reuter. 2020. "Escherichia coli: Physiological Clues Which Turn On the Synthesis of Antimicrobial Molecules" Veterinary Sciences 7, no. 4: 184. https://doi.org/10.3390/vetsci7040184
APA StylePaquette, S. -J., & Reuter, T. (2020). Escherichia coli: Physiological Clues Which Turn On the Synthesis of Antimicrobial Molecules. Veterinary Sciences, 7(4), 184. https://doi.org/10.3390/vetsci7040184





