Comparison of Protein Carbonyl (PCO), Paraoxonase-1 (PON1) and C-Reactive Protein (CRP) as Diagnostic and Prognostic Markers of Septic Inflammation in Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Caseload
- -
- Group A, clinically healthy: 35 dogs (12 males, 7 castrated males, 10 females, 6 neutered females; median age 24 months, age range: 6 months–13 years) that were considered healthy on the basis of normal physical examination, history and blood test results;
- -
- Group B, septic: 34 dogs (12 males, 2 castrated males, 12 females, 8 neutered females; median age 60 months; age range: 1 month–15 years) that were considered septic as a result of the presence of symptoms such as abnormal mentation, fever or hypothermia, tachycardia, tachypnoea and of appropriate tests on the basis of the suspected diagnosis (e.g., inflammatory leukogram, cytology consistent with presence of intracellular bacteria, abdomen ultrasound examination, thorax X-rays, positive blood culture) [46];
- -
- Group C, non-septic inflammation: 23 dogs (13 males, 1 castrated male, 7 females, 2 neutered females; median age 24 months; age range: 3 months–15 years) polytraumatized after motor vehicle accidents (n = 15) or falls (n = 3) or blunt trauma of unknown origin (n = 5): in these dogs, sepsis was excluded based on history and collateral tests reported above.
2.2. Measurement of PCO, PON1 and CRP
2.3. Statistical Analysis
3. Results
3.1. Group Composition
3.2. Group Comparison and ROC Curve Analyses for the Diagnosis of Sepsis
3.3. Group Comparison and ROC Curve Analyses Based on the Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthiesen, D.T.; Mullen, H.S. Problems and complications associated with endocrine surgery in the dog and cat. Probl. Vet. Med. 1990, 2, 627–667. [Google Scholar] [PubMed]
- Badalov, N.; Baradarian, R.; Iswara, K.; Li, J.; Steinberg, W.; Tenner, S. Drug-induced acute pancreatitis: An evidence-based review. Clin. Gastroenterol. Hepatol. 2007, 5, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Lem, K.; Fosgate, G.; Norby, B.; Steiner, J.M. Associations between dietary factors and pancreatitis in dogs. J. Am. Vet. Med. Assoc. 2008, 233, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Xenoulis, P.G.; Suchodolski, S.J.; Ruaux, C.G.; Steiner, J.M. Association between serum triglyceride and canine pancreatic lipase immunoreactivity concentrations in miniature schnauzers. J. Am. Anim. Hosp. Assoc. 2010, 46, 229–234. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Dowd, S.E.; Westermarck, E.; Steiner, J.M.; Wolcott, R.D.; Spillmann, T.; Harmoinen, J.A. The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rRNA gene sequencing. BMC Microbiol. 2009, 9, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Werner, M.; Suchodolski, J.S.; Straubinger, R.K.; Wolf, G.; Steiner, J.M.; Lidbury, J.A.; Neurer, F.; Hartmann, K.; Unterer, S. Effect of Amoxicillin-Clavulanic acid on clinical scores, intestinal microbiome, and Amoxicillin-resistant Escherichia Coli in dogs with uncomplicated acute diarrhea. J. Vet. Intern. Med. 2020, 34, 1166–1176. [Google Scholar] [CrossRef] [Green Version]
- Soulountsi, V.; Schizodimos, T. Use of antibiotics in acute pancreatitis: Ten major concerns. Scand. J. Gastroenterol. 2020, 1–8. [Google Scholar] [CrossRef]
- Giunti, M.; Grossi, G.; Troìa, R.; Fracassi, F.; Dondi, F. Evaluation of serum apolipoprotein A1 in canine sepsis. Front. Vet. Sci. 2020, 7, 263. [Google Scholar] [CrossRef]
- Torrente, C.; Manzanilla, E.G.; Bosch, L.; Villaverde, C.; Pastor, J.; Ruiz de Gopegui, R.; Tvarijonaviciute, A. Adiponectin as a sepsis biomarker in dogs: Diagnostic and prognostic value. Vet. Clin. Pathol. 2020, 49, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Troìa, R.; Ciuffoli, E.; Vasylyeva, K.; Foglia, A.; Dondi, F.; Giunti, M. Circulating methemoglobin fraction in dogs with sepsis. Front. Vet. Sci. 2020, 7, 341. [Google Scholar] [CrossRef]
- Marik, P.E.; Stephenson, E. The ability of procalcitonin, lactate, white blood cell count and neutrophil-lymphocyte count ratio to predict blood stream infection. Analysis of a large database. J. Crit. Care 2020, 60, 135–139. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Liaskou-Antoniou, L.; Adamis, G.; Panagaki, A.; Melachroinopoulos, N.; Drakou, E.; Marousis, K.; Chrysos, G.; Spyrou, A.; Alexiou, N.; et al. Procalcitonin to reduce long-term infection-associated adverse events in sepsis: A randomized trial. Am. J. Respir. Crit. Care Med. 2021, 203, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Floras, A.N.K.; Holowaychuk, M.K.; Hodgins, D.C.; Marr, H.S.; Birkenheuer, A.; Sharif, S.; Bersenas, A.M.E.; Bienzle, D. Investigation of a commercial ELISA for the detection of canine procalcitonin. J. Vet. Intern. Med. 2014, 28, 599–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easley, F.; Holowaychuk, M.K.; Lashnits, E.W.; Nordone, S.K.; Marr, H.; Birkenheuer, A.J. Serum procalcitonin concentrations in dogs with induced endotoxiemia. J. Vet. Intern. Med. 2020, 34, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Goggs, R.; Milloway, M.; Troia, R.; Giunti, M. Plasma procalcitonin concentrations are increased in dogs with sepsis. Vet. Rec. Open 2018, 5, e000255. [Google Scholar] [CrossRef] [Green Version]
- Troìa, R.; Giunti, M.; Goggs, R. Plasma procalcitonin concentrations predict organ dysfunction and outcome in dogs with sepsis. BMC Vet. Res. 2018, 14, 111. [Google Scholar] [CrossRef]
- Cerón, J.J.; Eckersall, P.D.; Martínez-Subiela, S. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Fransson, B.A.; Lagerstedt, A.S.; Bergsttrom, A.; Hagman, R.; Park, J.S.; Chew, B.P.; Evans, M.A.; Ragle, C.A. C-Reactive protein, tumor necrosis factor a, and interleukin-6 in dogs with pyometra and SIRS. J. Vet. Emerg. Crit. Care 2007, 17, 373–381. [Google Scholar] [CrossRef]
- Gebhardt, C.; Hirschberger, J.; Rau, S.; Arndt, G.; Krainer, K.; Schweigert, F.J.; Brunnberg, L.; Kaspers, B.; Kohn, B. Use of C-reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J. Vet. Emerg. Crit. Care 2009, 19, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Schreiner, C.A.; Frank, D.E.; Niyo, Y.; Ahrens, F.E.; Eckersall, P.D.; Benson, T.J.; Evans, R. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 2003, 17, 291–297. [Google Scholar] [CrossRef]
- Tecles, F.; Spiranelli, E.; Bonfanti, U.; Cerón, J.J.; Paltrinieri, S. Preliminary studies of serum acute-phase protein concentrations in hematologic and neoplastic diseases of dog. J. Vet. Intern. Med. 2005, 19, 965–970. [Google Scholar] [CrossRef]
- Raila, J.; Schweigert, F.J.; Kohn, B. C-Reactive protein concentrations in serum of dogs with naturally occurring renal disease. J. Vet. Diagn. Investig. 2011, 23, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Polizopoulou, Z.S.; Koutinas, C.K.; Cerón, J.J.; Tvarijonaviciute, A.; Martínez-Subiela, S.; Dasopoulou, A.; York, M.J.; Roman, F.; Gandhi, M.; Patel, S.; et al. Correlation of serum cardiac troponin I and acute phase protein concentrations with clinical staging in dogs with degenerative mitral valve disease. Vet. Clin. Pathol. 2015, 44, 397–404. [Google Scholar] [CrossRef]
- Fazio, F.; Casella, S.; Giannetto, C.; Giudice, E.; Piccione, G. Characterization of acute phase proteins and oxidative stress response to road transportation in the dog. Exp. Anim. 2015, 64, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindenberg, S.; Bauer, N.; Moritz, A. Extremely high canine C-reactive protein concentrations > 100 mg/L—Prevalence, etiology and prognostic significance. BMC Vet. Res. 2020, 16, 147. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, K.; Shimada, Y.; Amano, M.; Sakai, T.; Okada, T.; Yoshiya, I. Plasma lipid peroxides and alpha-tocopherol in critically ill patients. Crit. Care Med. 1984, 12, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Goode, H.F.; Cowley, H.C.; Walker, B.E.; Howdle, P.D.; Webster, N.R. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit. Care Med. 1995, 23, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Galley, H.F.; Webster, N.R. Oxidative stress and gene expression in sepsis. Br. J. Anaesth. 2003, 90, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Bosmann, M.; Ward, P. The inflammatory response in sepsis. Trends Immunol. 2013, 34, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Amorim de Oliveira, Y.P.A.; Pontes-de-Carvalho, L.C.; Couto, R.D.; Noronha-Dutra, A.A. Oxidative stress in sepsis. Possible production of free radicals through an erythrocyte-mediated positive feedback mechanism. Braz. J. Infect. Dis. 2017, 21, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Memon, R.A.; Moser, A.H.; Grunfeld, C. Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis 1998, 139, 307–315. [Google Scholar] [CrossRef]
- Rossi, G.; Giordano, A.; Pezzia, F.; Kjelgaard-Hansen, M.; Paltrinieri, S. Serum paraoxonase 1 activity in dogs: Preanalytical and analytical factors and correlation with C-reactive protein and alpha-2-globulin. Vet. Clin. Pathol. 2013, 42, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Carretón, E.; Cerón, J.J.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Caro-Vadillo, A.; Montoya-Alonso, J.A. Acute phase proteins and markers of oxidative stress to assess the severity of the pulmonary hypertension in heartworm-infected dogs. Parasit. Vectors 2017, 10 (Suppl. 2), 477. [Google Scholar] [CrossRef] [Green Version]
- Ibba, F.; Rossi, G.; Meazzi, S.; Giordano, A.; Paltrinieri, S. Serum concentration of high density lipoproteins (HDLs) in leishmaniotic dogs. Res. Vet. Sci. 2015, 98, 89–91. [Google Scholar] [CrossRef]
- Rossi, G.; Kuleš, J.; Barić Rafaj, R.; Mriljak, V.; Lauzi, S.; Giordano, A.; Paltrinieri, S. Relationship between paraoxonase 1 activity and high density lipoprotein concentration during naturally occurring babesiosis in dogs. Res. Vet. Sci. 2014, 97, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Daza González, M.A.; Fragío Arnold, C.; Fermín Rodríguez, M.; Checa, R.; Montoya, A.; Portero Fuentes, M.; Rupérez Noguer, C.; Martínez Subiela, S.; Cerón, J.J.; Mirò, G. Effect of two treatments on changes in serum acute phase protein concentrations in dogs with clinical leishmaniosis. Vet. J. 2019, 245, 22–28. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Scaloni, A.; Giustarini, D.; Cavarra, E.; Tell, G.; Lungarella, G.; Colombo, R.; Rossi, R.; Milzani, A. Proteins as biological markers of oxidative/nitrosative stress in diseases. The contribution of redox-proteomics. Mass Spectrom. Rev. 2005, 24, 55–99. [Google Scholar] [CrossRef]
- Abu-Zidan, F.M.; Plank, L.D.; Windsor, J.A. Proteolysis in severe sepsis is related to oxidation of plasma protein. Eur. J. Surg. 2002, 168, 119–123. [Google Scholar] [CrossRef]
- Ruggerone, B.; Colombo, G.; Paltrinieri, S. Identification of Protein Carbonyls (PCOs) in canine serum by Western Blot Techinque and preliminary evaluation of PCO concentration in dogs with systemic inflammation. Front. Vet. Sci. 2020, 7, 566402. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, C.I.; Jordao, A.A.; Vannucchi, H. Antioxidant compounds and oxidative stress in female dogs during pregnancy. Res. Vet. Sci. 2007, 83, 188–193. [Google Scholar] [CrossRef]
- Escobar, T.A.; Dowich, G.; Pacheco dos Santos, T.; Zuravski, L.; Duarte, C.A.; Lübeck, I.; Manfredini, V. Assessment of Leishmania infantum infection in equine populations in a canine visceral leishmaniosis transmission area. BMC Vet. Res. 2019, 15, 381. [Google Scholar] [CrossRef]
- Zini, E.; Gabbai, G.; Salesov, E.; Gerardi, G.; Da Dalt, L.; Lutz, T.A.; Reusch, C. Oxidative status of erythrocytes, hyperglycemia, and hyperlipidemia in diabetic cats. J. Vet. Intern. Med. 2020, 34, 616–625. [Google Scholar] [CrossRef]
- Hauptman, J.G.; Walshaw, R.; Olivier, N.B. Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Vet. Surg. 1997, 26, 393–397. [Google Scholar] [CrossRef]
- Rossi, G.; Ibba, F.; Meazzi, S.; Giordano, A.; Paltrinieri, S. Paraoxonase activity as a tool for clinical monitoring of dogs treated for canine leishmaniasis. Vet. J. 2014, 199, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Gardner, I.A.; Greiner, M. Receiver-operating characteristic curves and likelihood ratios: Improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Vet. Clin. Pathol. 2006, 35, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Christenson, R.H. Evidence-based laboratory medicine—A guide for critical evaluation of in vitro laboratory testing. Ann. Clin. Biochem. 2007, 44, 1367–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruopp, M.D.; Perkins, N.J.; Whitcomb, B.W.; Schisterman, E.F. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom. J. 2008, 50, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Cray, C.; Zaias, J.; Altman, N.H. Acute phase response in animals: A review. Comp. Med. 2009, 59, 517–526. [Google Scholar]
- Tothova, C.; Nagy, O.; Kovac, G. Serum proteins and their diagnostic utility in veterinary medicine: A review. Vet. Med. 2016, 61, 475–496. [Google Scholar] [CrossRef] [Green Version]
- Cabana, V.G.; Reardon, C.A.; Feng, N.; Neath, S.; Lukens, J.; Getz, G.S. Serum paraoxonase: Effect of the apolipoprotein composition of HDL and the acute phase response. J. Lipid Res. 2003, 44, 780–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, F.; Vavrova, L.; Kodydkova, J.; Novak Sr, F.; Hynkova, M.; Zak, A.; Novakova, O. Decreased paraoxonase activity in critically ill patients with sepsis. Clin. Exp. Med. 2010, 10, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Shida, T.; Okimura, T.; Otabe, K.; Honda, M.; Ashida, Y.; Furukawa, E.; Sarikaputi, M.; Naiki, M. Determination of C-reactive protein in serum and plasma from healthy dogs and dogs with pneumonia by ELISA and slide reversed passive latex agglutination test. Vet. Quart. 1994, 16, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Subiela, S.; Tecles, F.; Eckersall, P.D.; Cerón, J.J. Serum concentrations of acute phase proteins in dogs with leishmaniasis. Vet. Rec. 2002, 150, 241–244. [Google Scholar] [CrossRef]
- Fransson, B.A.; Karlstam, E.; Bergstrom, A.; Lagerstedt, A.S.; Park, J.S.; Evans, M.A.; Ragle, C.A. C-reactive protein in the differentiation of pyometra from cystic endometrial hyperplasia/mucometra in dogs. J. Am. Anim. Hosp. Assoc. 2004, 40, 391–399. [Google Scholar] [CrossRef]
- Jitpean, S.; Holst, B.S.; Hoglund, O.V.; Petterson, A.; Olsson, U.; Strage, E.; Södersten, F.; Hagman, R. Serum insulin-like growth factor-1, iron, C-reactive protein, and Serum Amyloid A for prediction of outcome in dogs with pyometra. Theriogenology 2014, 82, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Viitanen, S.J.; Laurilla, H.P.; Lilija-Maula, L.I. Serum C-reactive protein as a diagnostic biomarker in dogs with bacterial respiratory diseases. J. Vet. Intern. Med. 2014, 28, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Torrente, C.; Manzanilla, E.G.; Bosch, L.; Villaverde, C.; Pastor, J.; de Gopegui, R.R.; Tvarijonaviciute, A. The diagnostic and prognostic value of paraoxonase-1 and butyrylcholinesterase activities compared with acute-phase proteins in septic dogs and stratified by the acute patient physiologic and laboratory evaluation score. Vet. Clin. Pathol. 2019, 48, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Bavunoglu, I.; Genc, H.; Konukoglu, D.; Cicecki, H.; Sozer, V.; Gelisgen, R.; Uzun, H. Oxidative stress parameters and inflammatory and immune mediators as markers of the severity of sepsis. J. Infect. Dev. Ctries 2016, 10, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Camps, J.; Iftimie, S.; García-Heredia, A.; Castro, A.; Joven, J. Paraoxonases and infectious diseases. Clin. Biochem. 2017, 50, 804–811. [Google Scholar] [CrossRef]
- Bednarz-Misa, I.; Mierzchala-Pasierb, M.; Lesnik, P.; Placzkowska, S.; Kedzior, K.; Gamina, A.; Krystek-Korpacka, M. Cardiovascular insufficiency, abdominal sepsis, and patients’ age are associated with decreased paraoxonase-1 (PON-1) activity in critically ill patients with multiple organ dysfunction syndrome (MODS). Dis. Markers 2019, 2019, 1314623. [Google Scholar] [CrossRef] [Green Version]
- Meisner, M.; Adina, H.; Schmidt, J. Correlation of procalcitonin and C-reactive protein to inflammation, complications, and outcome during the intensive care unit course of multiple-trauma patients. Crit. Care 2006, 10, R1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjelgaard-Hansen, M.; Kristensen, A.T.; Jensen, A.L. Evaluation of a commercially available enzyme-linked immunosorbent assay (ELISA) for the determination of C-reactive protein in canine serum. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2003, 50, 164–168. [Google Scholar] [CrossRef]
- Da Silva, A.S.; Munhoz, T.D.; Faria, J.L.; Vargas-Hérnandez, G.; Machado, R.Z.; Almeida, T.C.; Moresco, R.N.; Stefani, L.M.; Tinucci-Costa, M. Increase nitric oxide and oxidative stress in dogs expermentally infected by Ehrlichia canis: Effect on the pathogenesis of the disease. Vet. Microbiol. 2013, 164, 366–369. [Google Scholar] [CrossRef]
- Bottari, N.B.; Munhoz, T.D.; Torbitz, V.D.; Tonin, A.A.; Anai, L.A.; Semolin, L.M.S.; Jark, P.C.; Bollicj, Y.S.; Moresco, R.N.; França, R.T.; et al. Oxidative stress in dogs with multicentric lymphoma: Effect of chemotherapy on oxidative and antioxidant biomarkers. Redox Rep. 2015, 20, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Póvoa, P. Serum markers in community-acquired pneumonia and ventilator-associated pneumonia. Curr. Opin. Infect. Dis. 2008, 21, 157–162. [Google Scholar] [CrossRef]
- Lisboa, T.; Seligman, R.; Diaz, E.; Rodriguez, A.; Teixera, P.J.; Rello, J. C-reactive protein correlates with bacterial load and appropriate antibiotic therapy in suspected ventilator-associated pneumonia. Crit. Care Med. 2008, 36, 166–171. [Google Scholar] [CrossRef]
- Cals, J.W.; Butler, C.C.; Hopstaken, R.M.; Hook, K.; Dinant, G.J. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: Cluster randomized trial. BMJ 2009, 338, b1374. [Google Scholar] [CrossRef] [Green Version]
- Póvoa, P.; Teixera-Pinto, A.M.; Carneiro, A.H.; Portuguese Community-Acquired Sepsis Study Group SACiUCI. C-reactive protein, an early biomarker of community-acquired sepsis resolution: A multi-center prospective observational study. Crit. Care 2011, 15, R169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teggert, A.; Datta, H.; Ali, Z. Biomarkers for Point-of-Care Diagnosis of Sepsis. Micromachines 2020, 11, 286. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, L.; Toft, N.; Eckersall, P.D.; Mellor, D.J.; Morris, J.S. Serum C-reactive protein concentration as an indicator of remission status in dogs with multicentric lymphoma. J. Vet. Intern. Med. 2007, 21, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, I.; Tuli, R.; Ractliffe, S.C.; Tappin, S.W.; Foale, R.D.; Roos, A.; Slater, K.J. Utility of a multiple serum biomarker test to monitor remission status and relapse in dogs with lymphoma undergoing treatment with chemotherapy. Vet. Comp. Oncol. 2017, 15, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Kanno, N.; Hayakawa, N.; Suzuki, S.; Harada, Y.; Yogo, T.; Hara, Y. Changes in canine C-reactive protein levels following orthopaedic surgery: A prospective study. Acta Vet. Scand. 2019, 61, 33. [Google Scholar] [CrossRef] [PubMed]
- Thames, B.E.; Barr, J.W.; Suchodolski, J.S.; Steiner, S.M.; Heilmann, R.M. Prospective evaluation of S100A12 and S100A8/A9 (calprotectin) in dogs with sepsis or the systemic inflammatory response syndrome. J. Vet. Diagn. Investig. 2019, 31, 645–651. [Google Scholar] [CrossRef] [PubMed]
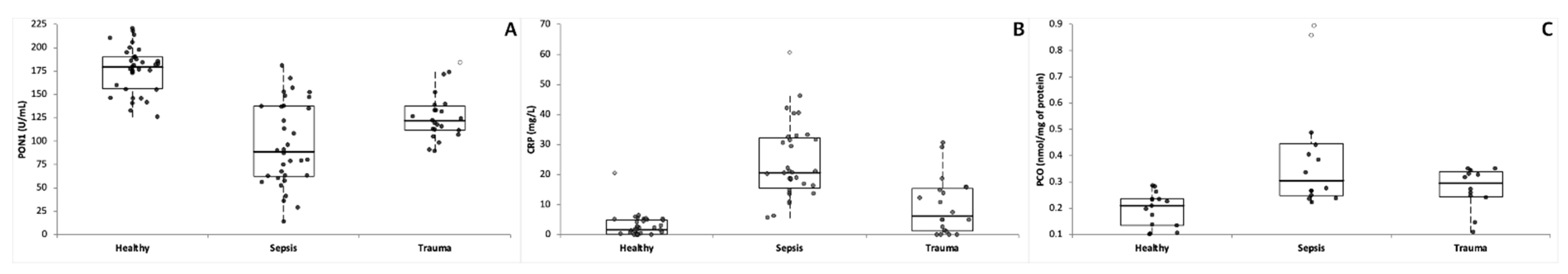


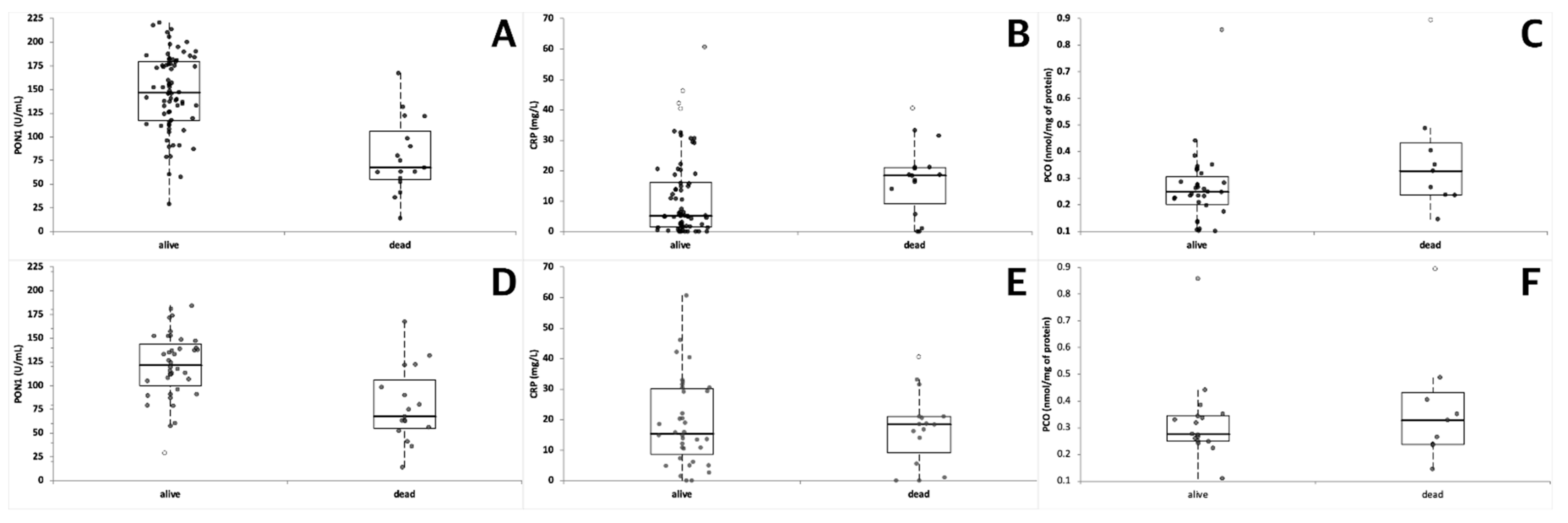

| Dogs | PON1 (U/mL) | CRP (mg/L) | PCO (nmol/mg of Protein) |
|---|---|---|---|
| Sepsis | 96.4 ± 44.4 (88.7) *** ††† (‡) 14.1–180.9 | 24.22 ± 12.47 (20.60) *** ††† (‡‡‡) 5.66–60.70 | 0.40 ± 0.22 (0.31) *** 0.22–0.89 |
| Trauma | 126.6 ± 25.1 (111.5) *** 89.6–184.4 | 9.46 ± 9.36 (6.17) * 0.00–30.60 | 0.27 ± 0.08 (0.30) ** 0.11–0.35 |
| Clinically healthy | 176.8 ± 24.4 (179.5) 126.0–220.6 | 2.88 ± 3.76 (1.72) 0.00–20.50 | 0.20 ± 0.06 (0.21) 0.10–0.29 |
| Statistical Data | Whole Population | Only Sick Dogs | ||||
|---|---|---|---|---|---|---|
| PON1 (U/mL) | CRP (mg/L) | PCO (nmol/mg of Protein) | PON1 (U/mL) | CRP (mg/L) | PCO (nmol/mg of Protein) | |
| AUC (%) | 95 (87–104) * | 95 (91–100) * | 78 (63–93) | 90 (76–104) * | 90 (79–100) * | 63 (41–86) |
| Youden index | 98.6 (Y: 0.554) | 5.64 (Y: 0.764) | 0.23 (Y: 0.447) | 91.5 (Y: 0.515) | 15.90 (Y: 0.600) | 0.35 (Y:0.429) |
| Max LR+ | 90.9 (LR+ 30.7) | 29.20 (LR+ 20.6) | 0.35 (LR+ 11.6) | 91.1 (LR+ 12.8) | 29.20 (LR+ 7.5) | 0.35 (LR+ 5.1) |
| 100% Sp | 89.6 | 30.60 | 0.35 | 89.6 | 30.30 | 0.35 |
| Dogs | PON1 (U/mL) | CRP (mg/L) | PCO (nmol/mg of Protein) |
|---|---|---|---|
| Dead | 79.1 ± 39.0 (67.6) *** ††† 14.1–167.4; n = 17 | 17.36 ± 11.70 (18.50) * 0.00–40.60; n = 16 | 0.37 ± 0.22 (0.33) 0.15–0.89; n = 9 |
| Alive (all the groups) | 146.6 ± 40.9 (146.7) 28.9–220.6; n = 74 | 11.25 ± 13.24 (5.20) 0.00–60.70; n = 70 | 0.26 ± 0.14 (0.25) 0.10–0.86; n = 32 |
| Alive (only dogs with sepsis or trauma) | 121.1 ± 34.3 (121.7) 14.1–106.4; n = 40 | 19.07 ± 14.22 (15.35) 0.00–60.70; n = 36 | 0.32 ± 0.16 (0.28) 0.11–0.86; n = 17 |
| Statistical Data | Whole Population | Only Sick Dogs | ||||
|---|---|---|---|---|---|---|
| PON1 (U/mL) | CRP (mg/L) | PCO (nmol/mg of Protein) | PON1 (U/mL) | CRP (mg/L) | PCO (nmol/mg of Protein) | |
| AUC (%) | 90 *** (82–99) | 54 (38–70) | 70 (48–90) | 82 *** (68–96) | 39 (22–57) | 54 (27–82) |
| Youden index | 132.7 (Y:0.635) | 13.90 (Y:0.454) | 0.35 (Y: 0.351) | 90.9 (Y: 0.531) | 15.90 (Y:0.243) | 0.35 (Y:0.268) |
| Max LR+ | 57.7 (LR+ 22.1) | 15.90 (LR+ 2.9) | 0.44 (LR+ 7.1) | 57.7 (LR+ 11.8) | 15.90 (LR+ 1.5) | 0.44 (LR+ 3.8) |
| 100% Sp | 28.9 | 60.70 | 0.86 | 28.9 | 60.70 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggerone, B.; Scavone, D.; Troìa, R.; Giunti, M.; Dondi, F.; Paltrinieri, S. Comparison of Protein Carbonyl (PCO), Paraoxonase-1 (PON1) and C-Reactive Protein (CRP) as Diagnostic and Prognostic Markers of Septic Inflammation in Dogs. Vet. Sci. 2021, 8, 93. https://doi.org/10.3390/vetsci8060093
Ruggerone B, Scavone D, Troìa R, Giunti M, Dondi F, Paltrinieri S. Comparison of Protein Carbonyl (PCO), Paraoxonase-1 (PON1) and C-Reactive Protein (CRP) as Diagnostic and Prognostic Markers of Septic Inflammation in Dogs. Veterinary Sciences. 2021; 8(6):93. https://doi.org/10.3390/vetsci8060093
Chicago/Turabian StyleRuggerone, Beatrice, Donatella Scavone, Roberta Troìa, Massimo Giunti, Francesco Dondi, and Saverio Paltrinieri. 2021. "Comparison of Protein Carbonyl (PCO), Paraoxonase-1 (PON1) and C-Reactive Protein (CRP) as Diagnostic and Prognostic Markers of Septic Inflammation in Dogs" Veterinary Sciences 8, no. 6: 93. https://doi.org/10.3390/vetsci8060093
APA StyleRuggerone, B., Scavone, D., Troìa, R., Giunti, M., Dondi, F., & Paltrinieri, S. (2021). Comparison of Protein Carbonyl (PCO), Paraoxonase-1 (PON1) and C-Reactive Protein (CRP) as Diagnostic and Prognostic Markers of Septic Inflammation in Dogs. Veterinary Sciences, 8(6), 93. https://doi.org/10.3390/vetsci8060093





