Effect of Leukoreduction by Pre-Storage Filtration on Coagulation Activity of Canine Plasma Collected for Transfusion
Abstract
:1. Introduction
2. Materials and Methods
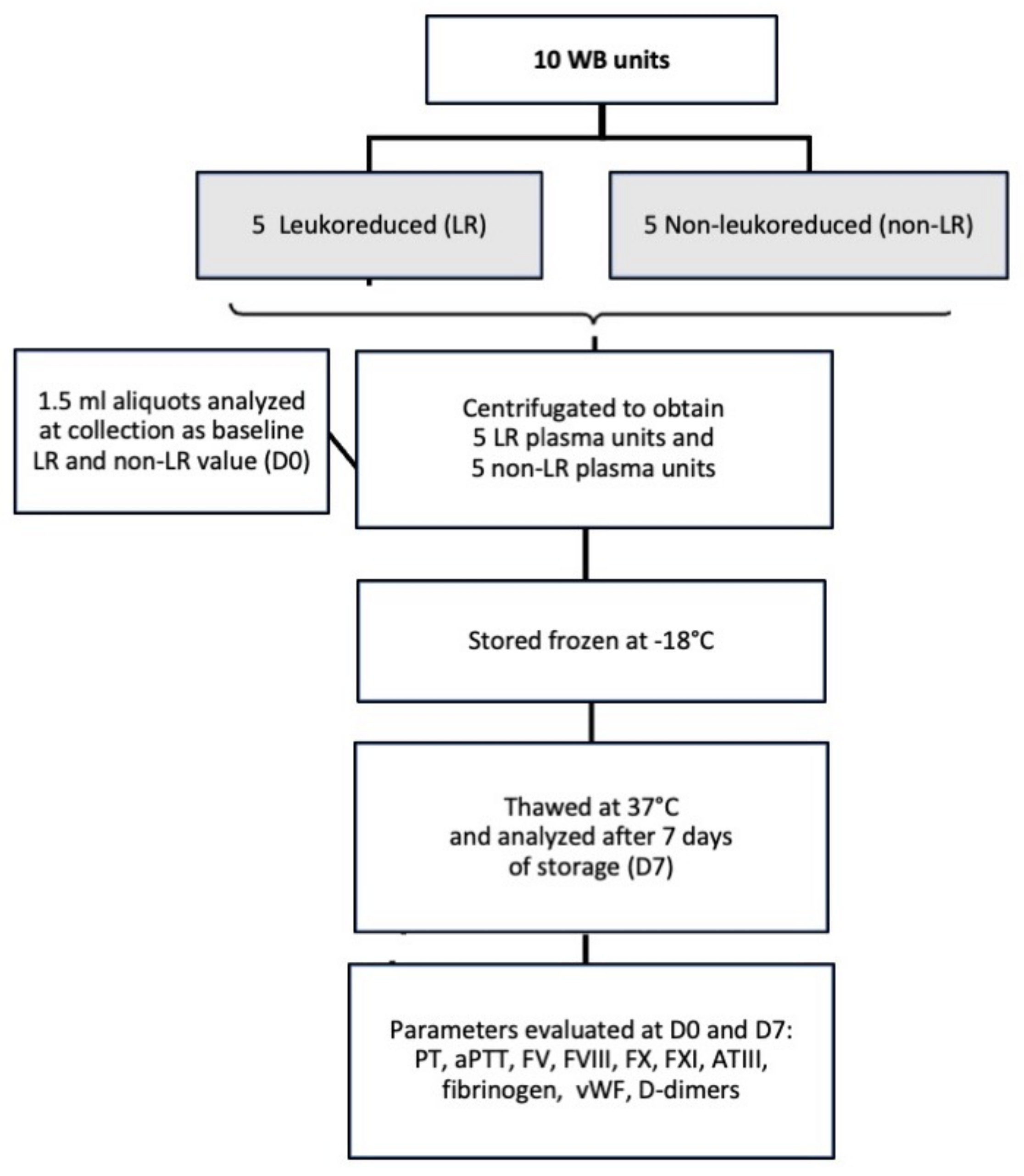
2.1. Blood Donors, Sample Collection and Processing
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snow, S.J.; Ari Jutkowitz, L.; Brown, A.J. Trends in plasma transfusion at a veterinary teaching hospital: 308 patients (1996–1998 and 2006–2008). J. Vet. Emerg. Crit. Care 2010, 20, 441–445. [Google Scholar] [CrossRef]
- Santo-Domingo, N.E.; Lewis, D. Indications for use and complications associated with canine plasma products in 170 patients. J. Vet. Emerg. Crit. Care 2021, 31, 263–268. [Google Scholar] [CrossRef]
- Logan, J.C.; Callan, M.B.; Drew, K.; Marryott, K.; Oakley, D.A.; Jefferies, L.; Giger, U. Clinical indications for use of fresh frozen plasma in dogs: 74 dogs (October through December 1999). J. Am. Vet. Med. Assoc. 2001, 218, 1449–1455. [Google Scholar] [CrossRef]
- Aggeler, P.M. Physiological basis for transfusion therapy in hemorrhagic disorders: A critical review. Transfusion 1961, 1, 71–86. [Google Scholar] [CrossRef]
- Roback, J.D.; Caldwell, S.; Carson, J.; Davenport, R.; Drew, M.J.; Eder, A.; Fung, M.; Hamilton, M.; Hess, J.R.; Luban, N.; et al. Evidence-based practice guidelines for plasma transfusion. Transfusion 2010, 50, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Alhumaidan, H.S.; Cheves, T.A.; Holme, S.; Sweeney, J.D. The effect of filtration on residual levels of coagulation factors in plasma. Am. J. Clin. Pathol. 2013, 139, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretzschmar, E.; Kruse, F.; Greiss, O.; Paunovic, D.; Kallweit, T.; Trobisch, H. Effects of extended storage of whole blood before leucocyte depletion on coagulation factors in plasma. Vox Sang. 2004, 87, 156–164. [Google Scholar] [CrossRef]
- Chan, K.S.K.; Sparrow, R.L. Microparticle profile and procoagulant activity of fresh frozen plasma is affected by whole blood-leukocyte depletion rather than 24-h room temperature-hold. Transfusion 2014, 54, 1935–1944. [Google Scholar] [CrossRef] [Green Version]
- Cardigan, R.; Sutherland, J.; Garwood, M.; Krailadsiri, P.; Seghatchian, J.; Beard, M.; Beckman, N.; Williamson, L.M. The effect of leucocyte depletion on the quality of fresh-frozen plasma. Br. J. Haematol. 2001, 114, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Riggert, J.; Simson, G.; Dittmann, J.; Koehler, M. Prestorage leukocyte depletion with in-line filtration of whole blood in comparison with blood component depletion. Vox Sang. 1995, 69, 201–205. [Google Scholar] [CrossRef]
- Rapaille, A.; Moore, G.; Siquet, J.; Flament, J.; Sondag-Thull, D. Prestorage leukocyte reduction with in-line filtration of whole blood: Evaluation of red cells and plasma storage. Vox Sang. 1997, 73, 28–35. [Google Scholar] [CrossRef]
- Riggert, J.; Schwartz, D.W.; Wieding, J.U.; Mayr, W.R.; Kohler, M. Prestorage inline filtration of whole blood for obtaining white cell-reduced blood components. Transfusion 1997, 37, 1039–1044. [Google Scholar] [CrossRef]
- Williamson, L.M.; Rider, J.R.; Swann, I.D.; Winter, M.A.; Ali, F.; Pamphilon, D.H. Evaluation of plasma and red cells obtained after leucocyte depletion of whole blood. Transfus. Med. 1999, 9, 51–61. [Google Scholar] [CrossRef]
- Heiden, M.; Salge, U.; Henschler, R.; Pfeiffer, H.-U.; Volkers, P.; Hesse, J.; Sireis, W.; Seitz, R. Plasma quality after whole-blood filtration depends on storage temperature and filter type. Transfus. Med. 2004, 14, 297–304. [Google Scholar] [CrossRef]
- Runkel, S.; Bach, J.; Haubelt, H.; Anders, C.; Hitzler, W.; Hellstern, P. The impact of two whole blood inline filters on markers of coagulation, complement and cell activation. Vox Sang. 2005, 88, 17–21. [Google Scholar] [CrossRef]
- Kazatchkine, M.D.; Carreno, M.P. Activation of the complement system at the interface between blood and artificial surfaces. Biomaterials 1988, 9, 30–35. [Google Scholar] [CrossRef]
- Solheim, B.G.; Flesland, O.; Brosstad, F.; Mollnes, T.E.; Seghatchian, J. Improved preservation of coagulation factors after pre-storage leukocyte depletion of whole blood. Transfus. Apher. Sci. 2003, 29, 133–139. [Google Scholar] [CrossRef]
- McMichael, M.; Smith, S.; Galligan, A.; Swanson, K.; Fan, T. Effect of Leukoreduction on Transfusion-Induced Inflammation in Dogs. J. Vet. Intern. Med. 2010, 24, 1131–1137. [Google Scholar] [CrossRef]
- Herring, J.M.; Smith, S.A.; McMichael, M.A.; O’Brien, M.; Ngwenyama, T.R.; Corsi, R.; Galligan, A.; Beloshapka, A.N.; Deng, P.; Swanson, K. Microparticles in stored canine RBC concentrates. Vet. Clin. Pathol. 2013, 42, 163–169. [Google Scholar] [CrossRef]
- Graf, C.; Raila, J.; Schweigert, F.J.; Kohn, B. Effect of leukoreduction treatment on vascular endothelial growth factor concentration in stored canine blood transfusion products. Am. J. Vet. Res. 2012, 73, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Avenick, D.; Kidd, L.; Istvan, S.; Dong, F.; Richter, K.; Edwards, N.; Hisada, Y.; Posma, J.J.; Massih, C.A.; Mackman, N. Effects of storage and leukocyte reduction on the concentration and procoagulant activity of extracellular vesicles in canine packed red cells. J. Vet. Emerg. Crit. Care 2021. [Google Scholar] [CrossRef]
- Brownlee, L.; Wardrop, K.J.; Sellon, R.K.; Meyers, K.M. Use of a prestorage leukoreduction filter effectively removes leukocytes from canine whole blood while preserving red blood cell viability. J. Vet. Intern. Med. 2000, 14, 412–417. [Google Scholar] [CrossRef]
- Muro, S.M.; Lee, J.H.; Stokes, J.V.; Ross, M.K.; Archer, T.M.; Wills, R.W.; Mackin, A.J.; Thomason, J.M. Effects of leukoreduction and storage on erythrocyte phosphatidylserine expression and eicosanoid concentrations in units of canine packed red blood cells. J. Vet. Intern. Med. 2017, 31, 410–418. [Google Scholar] [CrossRef]
- Stefani, A.; Capello, K.; Carminato, A.; Wurzburger, W.; Furlanello, T.; Bertazzo, V.; Marsilio, E.; Albertin, E.; La Pietra, G.; Bozzato, E.; et al. Effects of leukoreduction on storage lesions in whole blood and blood components of dogs. J. Vet. Intern. Med. 2021, 35, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Davidow, B.; Blois, S.; Goy-Thollot, I.; Harris, L.; Humm, K.; Musulin, S.; Nash, K.; Odunayo, A.; Sharp, C.; Spada, E.; et al. Association of Veterinary Hematology and Transfusion Medicine (AVHTM) transfusion Reaction Small Animal Consensus Statement (TRACS) Part 2: Prevention and Monitoring. J. Vet. Emerg. Crit. Care 2021, 31, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Foote, M.L.; Brooks, M.B.; Archer, T.M.; Wills, R.W.; Mackin, A.J.; Thomason, J.M. Coagulation factor activity in units of leukoreduced and nonleukoreduced canine fresh-frozen plasma. Am. J. Vet. Res. 2019, 80, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, M.T.; Marenzoni, M.L.; Misia, A.L.; Avellini, L.; Chiaradia, E.; Gavazza, A.; Miglio, A. Effect of leukoreduction on hematobiochemical parameters and storage hemolysis in canine whole blood units. Animals 2021, 11, 925. [Google Scholar] [CrossRef]
- Purcell, S.L.; Claus, M.; Hosgood, G.; Smart, L. Effect of leukoreduction on concentrations of interleukin-8, interleukin-1β, and tumor necrosis factor-α in canine packed red blood cells during storage. Am. J. Vet. Res. 2015, 76, 969–974. [Google Scholar] [CrossRef]
- Yang, H.; Kim, W.; Bae, J.; Kim, H.; Kim, S.; Choi, J.; Park, J.; Jung, D.-I.; Koh, H.; Yu, D. Effects of irradiation and leukoreduction on down-regulation of CXCL-8 and storage lesion in stored canine whole blood. J. Vet. Sci. 2019, 20, 72–78. [Google Scholar] [CrossRef]
- Ergül Ekiz, E.; Arslan, M.; Akyazi, I.; Uygur, E.E.; Gültekin, G.I.; Özcan, M. The effects of prestorage leukoreduction and storage duration on the in vitro quality of canine packed red blood cells. Turkish J. Vet. Anim. Sci. 2012, 36, 711–717. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare, Council of Europe. Guide to the Preparation, Use and Quality Assurance of Blood Components: CD-P-TS; European Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM): Strasbourg, France, 2020; Chapter 5, Part D; p. 250. [Google Scholar]
- AABB Standards for Blood Banks and Transfusion Services, 30th ed.; AABB Press: Bethesda, MD, USA, 2016.
- Brooks, M.B.; De Laforcade, A. Acquired Coagulopathies. In Schalm’s Veterinary Hematology, 6th ed.; Douglass, J.W., Wardrop, K.J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010; Chapter 85; pp. 654–660. [Google Scholar]
- Shooshtari, M.M.; Hosseini, K.M. Evaluation of the plasma quality after filtration. Daru 2010, 18, 114–117. [Google Scholar]
- Smith, S.A. The cell-based model of coagulation. J. Vet. Emerg. Crit. Care 2009, 19, 3–10. [Google Scholar] [CrossRef]
- Hardy, J.F.; Moerloose, P.D.; Samama, M. Massive transfusion and coagulopathy: Pathophysiology and implications for clinical management. Can. J. Anaesth. 2004, 51, 293–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter (Reference Range) | Time | Non-Leuko-Reduced (Non-LR) Plasma | Leuko-Reduced (LR) Plasma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Median | 25th–75th Percentiles | Min | Max | Median | 25th–75th Percentiles | ||
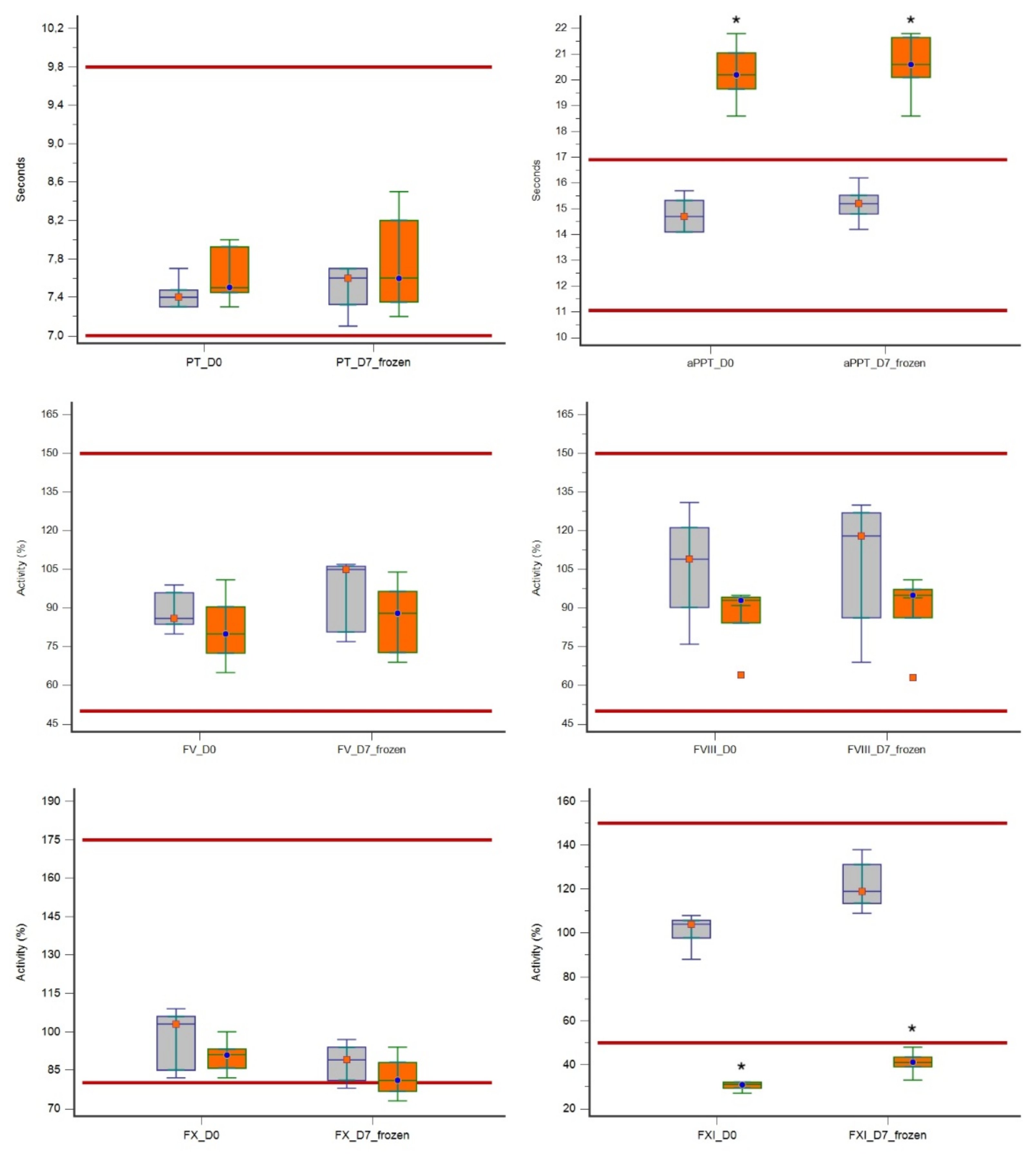
| PT (7–9.8 s) | D0 | 7.3 | 7.7 | 7.4 | 7.3–7.4 | 7.3 | 8.0 | 7.5 | 7.4–7.9 |
| D7 | 7.1 | 7.7 | 7.6 | 7.3–7.7 | 7.2 | 8.5 | 7.6 | 7.3–8.2 | |
| aPPT (11–16.9 s) | D0 | 14.1 | 15.7 | 14.7 | 14.1–15.3 | 18.6 | 21.8 | 20.2 | 19.6–21.0 |
| D7 | 14.2 | 16.2 | 15.2 | 14.8–15.5 | 18.6 | 21.8 | 20.6 | 20.1–21.6 | |
| FV (50–150%) | D0 | 80.0 | 99.0 | 86.0 | 83.7–96.0 | 65.0 | 101.0 | 80.0 | 72.5–90.5 |
| D7 | 77.0 | 107.0 | 105.0 | 80.7–106.2 | 69.0 | 104.0 | 88.0 | 72.7–96.5 | |
| FVIII (50–150%) | D0 | 76.0 | 131.0 | 109.0 | 90.2–121.2 | 64.0 | 95.0 | 93.0 | 84.2–94.2 |
| D7 | 69.0 | 130.0 | 118.0 | 86.2–127.0 | 63.0 | 101.0 | 95.0 | 86.2–97.2 | |
| FX (80–175%) | D0 | 82.0 | 109.0 | 103.0 | 85.0–106.0 | 82.0 | 100.0 | 91.0 | 85.7–93.2 |
| D7 | 78.0 | 97.0 | 89.0 | 81.0–94.0 | 73.0 | 94.0 | 81.0 | 76.7–88.0 | |
| FXI (50–150%) | D0 | 88.0 | 108.0 | 104.0 | 97.7–105.7 | 27.0 | 32.0 | 31.0 | 29.2–32.0 |
| D7 | 109.0 | 138.0 | 119.0 | 113.5–131.2 | 33.0 | 48.0 | 41.0 | 39.0–43.5 | |
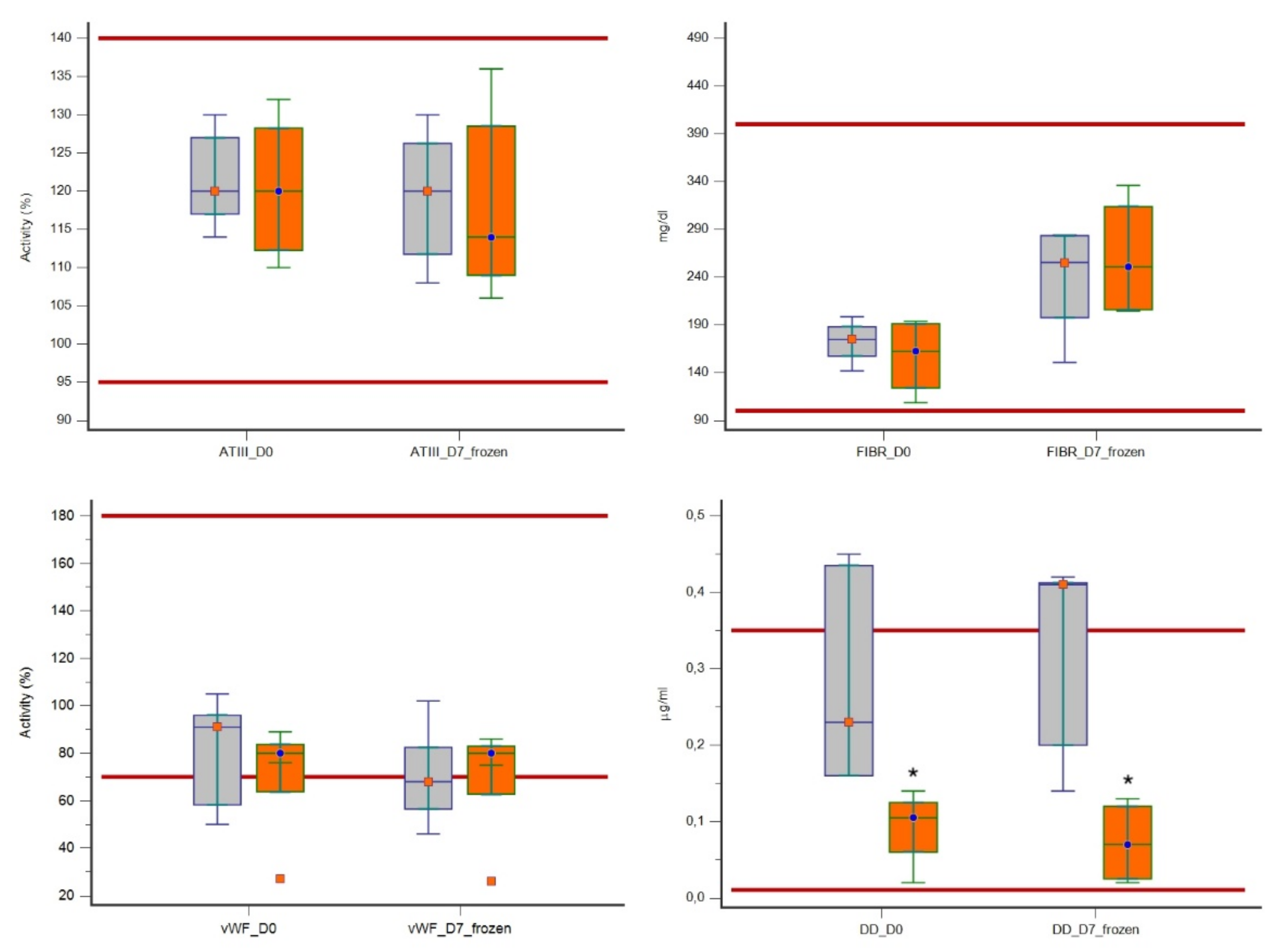
| ATIII (95–140%) | D0 | 114.0 | 130.0 | 120.0 | 117.0–127.0 | 110.0 | 132.0 | 120.0 | 112.2–128.2 |
| D7 | 108.0 | 130.0 | 120.0 | 111.7–126.2 | 106.0 | 136.0 | 114.0 | 109.0–128.5 | |
| Fibrinogen (100–400 mg/mL) | D0 | 141.8 | 198.6 | 174.6 | 157.3–188.0 | 108.5 | 193.7 | 162.3 | 123.9–191.2 |
| D7 | 150.7 | 284.2 | 255.3 | 197.5–283.4 | 204.6 | 336.1 | 250.8 | 205.8–313.7 | |
| vWF (70–180%) | D0 | 50.0 | 105.0 | 91.0 | 58.2–96.0 | 27.0 | 89.0 | 80.0 | 63.7–83.7 |
| D7 | 46.0 | 102.0 | 68.0 | 56.5–82.5 | 26.0 | 86.0 | 80.0 | 62.7–83.0 | |
| D-dimers (0.01–0.35 μg/mL) | D0 | 0.16 | 0.45 | 0.23 | 0.16–0.43 | 0.02 | 0.14 | 0.10 | 0.06–0.12 |
| D7 | 0.14 | 0.42 | 0.41 | 0.20–0.41 | 0.02 | 0.13 | 0.07 | 0.02–0.12 | |
| Variable (Reference Range) | Time Point | Non-Leuko-Reduced (Non-LR) Plasma Units | Leuko-Reduced (LR) Plasma Units | p-Value for Comparison Non-LR and LR Plasma Units | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Average Rank | Paired Difference D0 vs. D7 | Median | Average Rank | Paired Difference D0 vs. D7 | |||||
| Median | p-Value | Median | p-Value | |||||||
| PT (7–9.8 s) | D0 | 7.4 | 4.2 | 0.0 | - | 7.5 | 6.8 | 0.10 | 0.4375 | 0.1666 |
| D7 | 7.6 | 5.0 | 7.6 | 6.0 | 0.5982 | |||||
| aPTT (11–16.9 s) | D0 | 14.7 | 3.0 | 0.5 | 0.1250 | 20.2 | 8.0 | 0.4 | - | 0.0088 |
| D7 | 15.2 | 3.0 | 20.6 | 8.0 | 0.0088 | |||||
| FV (50–150%) | D0 | 86.0 | 6.3 | 8.0 | 0.3125 | 80.0 | 4.7 | 4.0 | 0.3125 | 0.4020 |
| D7 | 105.0 | 6.8 | 88.0 | 4.2 | 0.1745 | |||||
| FVIII (50–150%) | D0 | 109.0 | 7.1 | −1.0 | 0.8125 | 93.0 | 3.9 | 1.0 | 0.1875 | 0.0937 |
| D7 | 118.0 | 6.4 | 95.0 | 4.6 | 0.3472 | |||||
| FX (80–175%) | D0 | 103.0 | 6.3 | −8.0 | 0.0625 | 91.0 | 4.7 | −9.0 | 0.0625 | 0.4005 |
| D7 | 89.0 | 6.5 | 81.0 | 4.5 | 0.2948 | |||||
| FXI (50–150%) | D0 | 104.0 | 8.0 | 21.0 | 0.0625 | 31.0 | 3.0 | 9.0 | 0.0625 | 0.0088 |
| D7 | 119.0 | 8.0 | 41.0 | 3.0 | 0.0088 | |||||
| ATIII (95–140%) | D0 | 120.0 | 5.7 | −1.0 | - | 120.0 | 5.3 | −3.0 | 0.3125 | 0.8340 |
| D7 | 120.0 | 5.6 | 114.0 | 5.4 | 0.9168 | |||||
| Fibrinogen (100–400 mg/dl) | D0 | 174.6 | 6.2 | 80.7 | 0.125 | 162.3 | 4.8 | 97.8 | 0.0625 | 0.4647 |
| D7 | 255.3 | 5.2 | 250.8 | 5.8 | 0.7540 | |||||
| vWF (70–180%) | D0 | 91.0 | 6.4 | −4.0 | 0.0625 | 80.0 | 4.6 | −1.0 | - | 0.3472 |
| D7 | 68.0 | 5.0 | 80.0 | 6.0 | 0.6015 | |||||
| D-Dimers (0.01–0.35 μg/mL) | D0 | 0.23 | 7.00 | −0.01 | 0.8125 | 0.10 | 2.50 | −0.01 | - | 0.0139 |
| D7 | 0.41 | 7.00 | 0.07 | 2.50 | 0.0139 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spada, E.; Perego, R.; Baggiani, L.; Proverbio, D. Effect of Leukoreduction by Pre-Storage Filtration on Coagulation Activity of Canine Plasma Collected for Transfusion. Vet. Sci. 2021, 8, 157. https://doi.org/10.3390/vetsci8080157
Spada E, Perego R, Baggiani L, Proverbio D. Effect of Leukoreduction by Pre-Storage Filtration on Coagulation Activity of Canine Plasma Collected for Transfusion. Veterinary Sciences. 2021; 8(8):157. https://doi.org/10.3390/vetsci8080157
Chicago/Turabian StyleSpada, Eva, Roberta Perego, Luciana Baggiani, and Daniela Proverbio. 2021. "Effect of Leukoreduction by Pre-Storage Filtration on Coagulation Activity of Canine Plasma Collected for Transfusion" Veterinary Sciences 8, no. 8: 157. https://doi.org/10.3390/vetsci8080157








