Cardiorespiratory Effects of Three Infusion Doses of Adenosine in Conscious Goats: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
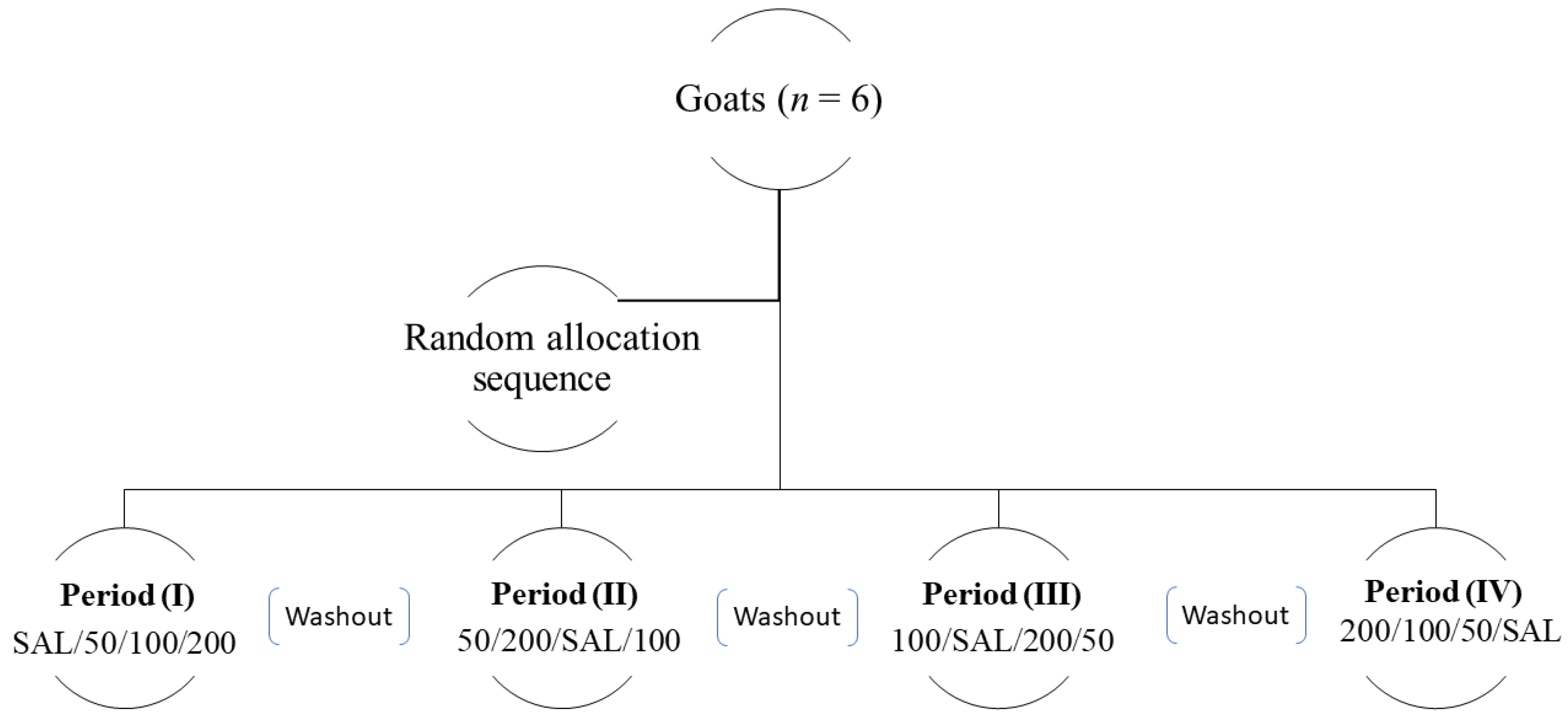
2.2. Study Design
2.2.1. Cardiorespiratory Parameters and Rectal Temperature
2.2.2. Echocardiographic Parameters
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, F.D.; Dobson, J.G., Jr. Adenosine modulates β-adrenergic signal transduction in guinea-pig heart ventricular membranes. J. Mol. Cell Cardiol. 1990, 22, 1359–1370. [Google Scholar] [CrossRef]
- Belardinelli, L.; Linden, J.; Berne, R.M. The cardiac effects of adenosine. Prog. Cardiovasc. Dis. 1989, 32, 73–97. [Google Scholar] [CrossRef]
- Hansen, P.B.; Schnermann, J. Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am. J. Physiol. Renal. Physiol. 2003, 285, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Reiss, A.B.; Grossfeld, D.; Kasselman, L.J.; Renna, H.A.; Vernice, N.A.; Drewes, W.; Konig, J.; Carsons, S.E.; DeLeon, J. Adenosine and the cardiovascular system. Am. J. Cardiovasc. Drugs 2019, 19, 449–464. [Google Scholar] [CrossRef]
- Chandrasekera, P.C.; McIntosh, V.J.; Cao, F.X.; Lasley, R.D. Differential effects of adenosine A2a and A2b receptors on cardiac contractility. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, 2082–2089. [Google Scholar] [CrossRef] [Green Version]
- Biaggioni, I.; Olafsson, B.; Robertson, R.M.; Hollister, A.S.; Robertson, D. Cardiovascular and respiratory effects of adenosine in conscious man. Evidence for chemoreceptor activation. Circ. Res. 1987, 61, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Biaggioni, I.; Killian, T.J.; Mosqueda-Garcia, R.; Robertson, R.M.; Robertson, D. Adenosine increases sympathetic nerve traffic in humans. Circulation 1991, 83, 1668–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dibner-Dunlap, M.E.; Kinugawa, T.; Thames, M.D. Activation of cardiac sympathetic afferents: Effects of exogenous adenosine and adenosine analogues. Am. J. Physiol. Circ. Physiol. 1993, 265, 395–400. [Google Scholar] [CrossRef]
- Engelstein, E.D.; Lerman, B.B.; Somers, V.K.; Rea, R.F. Role of arterial chemoreceptors in mediating the effects of endogenous adenosine on sympathetic nerve activity. Circulation 1994, 90, 2919–2926. [Google Scholar] [CrossRef] [Green Version]
- Timmers, H.J.; Rongen, G.A.; Karemaker, J.M.; Wieling, W.; Marres, H.A.; Lenders, J.W. The role of carotid chemoreceptors in the sympathetic activation by adenosine in humans. Clin. Sci. 2004, 106, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Reddy, B.S.; Venkatasivakumar, R.; Sivajothi, S.; Reddy, Y.P. Electrocardiographic abnormalities in young healthy sheep and goats. Int. J. Biol. Res. 2014, 2, 21–22. [Google Scholar]
- Kotelko, D.M.; Shnider, S.M.; Dailey, P.A.; Brizgys, R.V.; Levinson, G.; Shapiro, W.A.; Koike, M.; Rosen, M.A. Bupivacaine-induced cardiac arrhythmias in sheep. Anesthesiology 1984, 60, 10–18. [Google Scholar] [CrossRef]
- Camm, A.J.; Garratt, C.J. Adenosine and supraventricular tachycardia. N. Engl. J. Med. 1991, 325, 1621–1629. [Google Scholar] [CrossRef]
- Bailey, A.M.; Baum, R.A.; Rose, J.; Humphries, R.L. High-dose adenosine for treatment of refractory supraventricular tachycardia in an emergency department of an academic medical center: A case report and literature review. J. Emerg. Med. 2016, 50, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, M.; Vairo, D.; Marlinge, M.; Gaubert, M.; Guiol, C.; Mottola, G.; Gariboldi, V.; Deharo, P.; Sadrin, S.; Maixent, J.M.; et al. Adenosine and Its Receptors: An Expected Tool for the Diagnosis and Treatment of Coronary Artery and Ischemic Heart Diseases. Int. J. Mol. Sci. 2020, 21, 5321. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, A.F.; Morgan, P.J.; Kikuta, Y.; Taniguchi, Y.; Lippmann, M.; Hsu, D. Cardiovascular changes during adenosine induced hypotension for major orthopedic and cerebral aneurysm surgery. Anesth. Analg. 1990, 70, 117. [Google Scholar] [CrossRef]
- Alaa, A.M.; Bose, G.; Hunt, K.; Toma, A.K. Adenosine-assisted neurovascular surgery: Initial case series and review of literature. Neurosurg. Rev. 2019, 42, 15–22. [Google Scholar]
- Hayashida, M.; Fukunaga, A.; Fukuda, K.; Sakurai, S.; Mamiya, H.; Ichinohe, T.; Kaneko, Y.; Hanaoka, K. The characteristics of intravenous adenosine-induced antinociception in a rabbit model of acute nociceptive pain: A comparative study with remifentanil. Anesth. Analg. 2006, 103, 1004–1010. [Google Scholar] [CrossRef]
- Rauck, R.L.; North, J.; Eisenach, J.C. Intrathecal clonidine and adenosine: Effects on pain and sensory processing in patients with chronic regional pain syndrome. Pain 2015, 156, 88–95. [Google Scholar] [CrossRef]
- Goldman, N.; Chen, M.; Fujita, T.; Xu, Q.; Peng, W.; Liu, W.; Jensen, T.K.; Pei, Y.; Wang, F.; Han, X.; et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat. Neurosci. 2010, 13, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Granfeldt, A.; Letson, H.L.; Dobson, G.P.; Shi, W.; Vinten-Johansen, J.; Tønnesen, E. Adenosine, lidocaine and Mg2+ improves cardiac and pulmonary function, induces reversible hypotension and exerts anti-inflammatory effects in an endotoxemic porcine model. Crit. Care 2014, 18, 682. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.S.; Gabriel-Costa, D.; Sudo, R.T.; Wang, H.; Groban, L.; Ferraz, E.B.; Nascimento, J.H.; Fraga, C.A.; Barreiro, E.J.; Zapata-Sudo, G. Adenosine A2A receptor agonist prevents cardiac remodeling and dysfunction in spontaneously hypertensive male rats after myocardial infarction. Drug Des. Devel. Ther. 2017, 11, 553–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joerger, F.B.; Dennler, M.; Meira, C.; Mosing, M.; Richter, H.; Ringer, S.K. Cardiovascular effects of two adenosine constant rate infusions in anaesthetized dogs. Vet. Anaesth. Analg. 2019, 46, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Fuller, R.W.; Maxwell, D.L.; Conradson, T.B.; Dixon, C.M.; Barnes, P.J. Circulatory and respiratory effects of infused adenosine in conscious man. Br. J. Clin. Pharmacol. 1987, 24, 306–317. [Google Scholar] [CrossRef] [Green Version]
- DiMarco, J.P.; Sellers, T.D.; Berne, R.M.; West, G.A.; Belardinelli, L. Adenosine: Electrophysiologic effects and therapeutic use for terminating paroxysmal supraventricular tachycardia. Circulation 1983, 68, 1254–1263. [Google Scholar] [CrossRef] [Green Version]
- Mason, B.A.; Ogunyemi, D.; Punla, O.; Koos, B.J. Maternal and fetal cardiorespiratory responses to adenosine in sheep. Am. J. Obstet. Gynecol. 1993, 168, 1558–1561. [Google Scholar] [CrossRef]
- Sollevi, A.; Lagerkranser, M.; Irestedt, L.; Gordon, E.; Lindquist, C. Controlled hypotension with adenosine in cerebral aneurysm surgery. Anesthesiology 1984, 61, 400–405. [Google Scholar] [CrossRef]
- Watt, A.H.; Routledge, P.A. Adenosine stimulates respiration in man. Br. J. Clinc. Pharmacol. 1985, 20, 503–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, P.G.; Watt, A.H.; Routledge, P.A.; Smith, A.P. Intravenous infusion of adenosine but not inosine stimulates respiration in man. Br. J. Clin. Pharmacol. 1987, 23, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Linke, A.; Xu, X.; Ochoa, M.; Belloni, F.; Belardinelli, L.; Hintze, T.H. Comparative profile of vasodilation by CVT-3146, a novel A2A receptor agonist, and adenosine in conscious dogs. J. Pharmacol. Exp. Ther. 2003, 307, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Rosete, J.; Kohler, G.; Huang, B.L.; Blackburn, B.; Belardinelli, L. Negative chronotropic effect of CVT-510 in anesthetized and awake rats. Drug Dev. Res. 2001, 52, 424–430. [Google Scholar] [CrossRef]
- Belloni, F.L.; Brown, I.; Hintze, T.H. Mechanism of the apparent parasympathetic inhibition of adenosine induced heart rate slowing in the dog. Cardiovasc. Res. 1989, 23, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Biaggioni, I.; Onrot, J.; Hollister, A.S.; Robertson, D. Cardiovascular effects of adenosine infusion in man and their modulation by dipyridamole. Life Sci. 1986, 39, 2229–2236. [Google Scholar] [CrossRef]
- Vatner, S.F.; McRitchie, R.J. Interaction of the chemoreflex and the pulmonary inflation reflex in the regulation of coronary circulation in conscious dogs. Circ. Res. 1975, 37, 664–673. [Google Scholar] [CrossRef] [Green Version]
- Ogilby, J.D.; Iskandrian, A.S.; Untereker, W.J.; Heo, J.; Nguyen, T.N.; Mercuro, J. Effect of intravenous adenosine infusion on myocardial perfusion and function. Hemodynamic/angiographic and scintigraphic study. Circulation 1992, 86, 887–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]

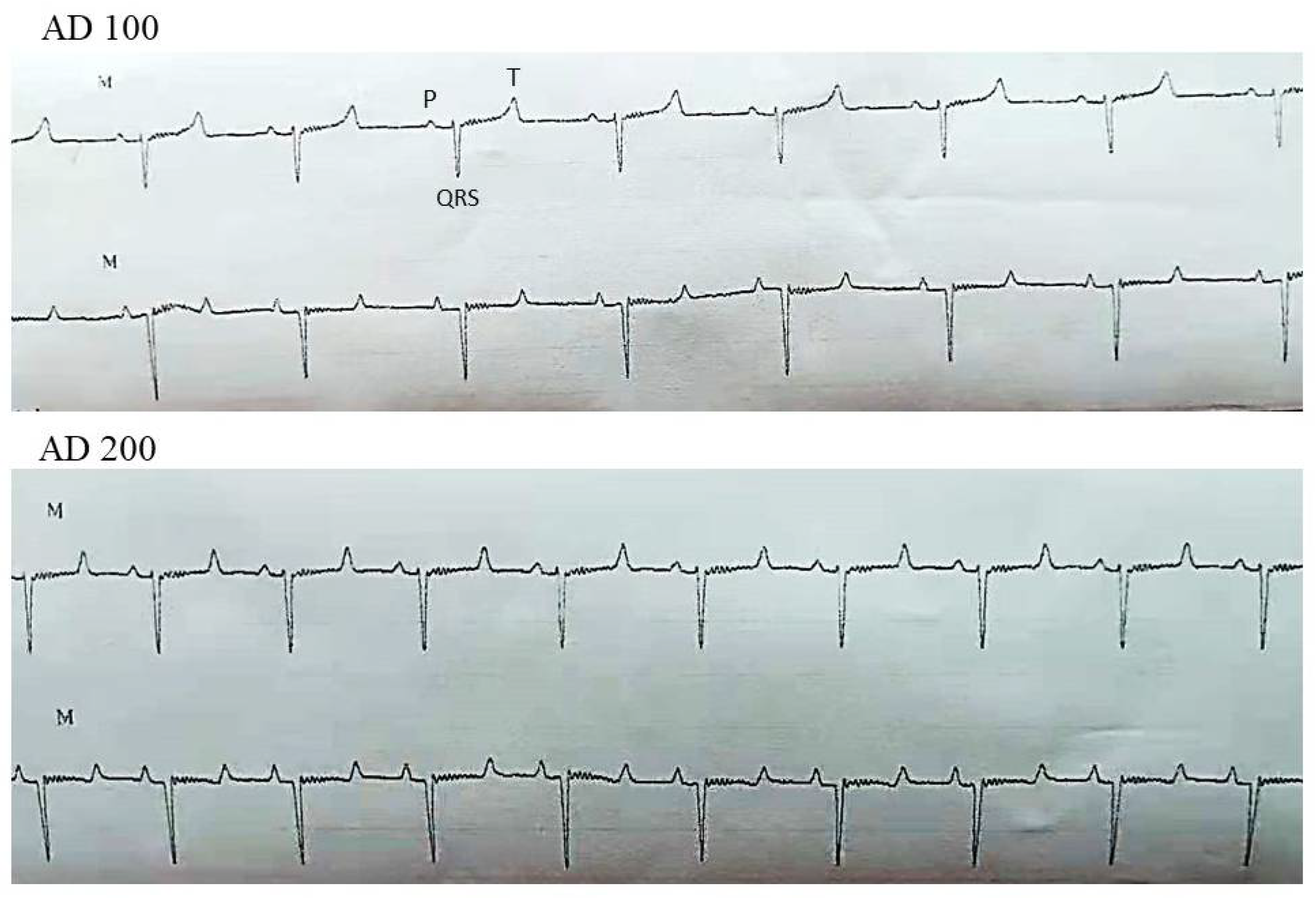
| Parameters | Group | Time Points (Minutes) | |||||
|---|---|---|---|---|---|---|---|
| B | dInf | 1 | 3 | 5 | 10 | ||
| HR (beats/min) | SAL | 108.3 ± 5.2 | 98.1 ±14.2 | 91.5 ± 10.5 | 98.5 ± 10.4 | 109 ± 11.6 | 100.5 ± 12.5 |
| AD 50 | 108.7 ± 6.8 | 98.1 ± 6.8 | 101.8 ± 11.6 | 99.3 ± 9.1 | 102.3 ± 11 | 93.1 ± 6.9 | |
| AD 100 | 103.3 ± 6 | 113.7 ± 4.2 | 94.6 ± 9.1 | 97.3 ± 9.9 | 99.6 ± 10.3 | 96.5 ± 13 | |
| p = 0.001 ٭ | |||||||
| AD 200 | 105.3 ± 8.6 | 131 ± 7.1 ٭ | 121.8 ± 6.4 ٭ | 106 ± 7 | 102 ± 7.8 | 105.2 ± 12.2 | |
| p = 0.006 | p = 0.035 | ||||||
| RR (breaths/min) | SAL | 20.1 ± 3.7 | 21.5 ± 2.8 | 21 ± 5.8 | 22 ± 5.1 | 22.5 ± 4 | 21.3 ± 3.5 |
| AD 50 | 23.8 ± 3 | 22.6 ± 4.1 | 20 ± 3.2 | 21.6 ± 2.9 | 22.5 ± 4.4 | 21.3 ± 5.2 | |
| AD 100 | 23 ± 5 | 22.1 ± 3.6 | 20.1 ± 2 | 20.6 ± 4.2 | 22.8 ± 4.9 | 23.8 ± 2 | |
| AD 200 | 22.5 ± 3.3 | 41.1 ± 3٭ | 22.8 ± 3 | 21 ± 3 | 20.5 ± 3.5 | 23.3 ± 3 | |
| p < 0.001 | |||||||
| SpO2 (%) | SAL | 94.8 ± 3 | 93.8 ± 2.2 | 94 ± 2 | 93.5 ± 2.5 | 93.6 ± 2 | 94 ± 2.6 |
| AD 50 | 95.2 ± 3 | 94 ± 2 | 93.8 ± 2.9 | 94 ± 2.8 | 94 ± 2.6 | 94.6 ± 2.6 | |
| AD 100 | 95.1 ± 3.2 | 95.3 ± 3 | 94.6 ± 3.6 | 95 ± 2.9 | 94.6 ± 3.5 | 94.5 ± 3.4 | |
| AD 200 | 95.2 ± 2.9 | 94.8 ± 2.4 | 95.3 ± 2.8 | 95 ± 3.4 | 94.2 ± 2.5 | 94.1 ± 1.6 | |
| RT (°C) | SAL | 39.4 ± 0.16 | 39.5 ± 0.29 | 39.5 ± 0.19 | 39.5 ± 0.17 | 39.5 ± 0.24 | 39.15 ± 0.19 |
| AD 50 | 39.4 ± 0.23 | 39.5 ± 0.21 | 39.5 ± 0.27 | 39.4 ± 0.23 | 39.5 ± 0.13 | 39.5 ± 0.10 | |
| AD 100 | 39.4 ± 0.22 | 39.4 ± 0.24 | 39.5 ± 0.15 | 39.4 ± 0.27 | 39.4 ± 0.14 | 39.6 ± 0.12 | |
| AD 200 | 39.5 ± 0.24 | 39.4 ± 0.17 | 39.5 ± 0.24 | 39.4 ± 0.30 | 39.5 ± 0.21 | 39.6 ± 0.20 | |
| Parameters | Group | Time Points (Minutes) | |||||
|---|---|---|---|---|---|---|---|
| B | dInf | 1 | 3 | 5 | 10 | ||
| EF (%) | SAL | 72.1 ± 5.9 | 71.8 ± 4.2 | 72.6 ± 5.7 | 71.6 ± 4.8 | 75.5 ± 4.4 | 74.5 ± 4 |
| AD 50 | 71.5 ± 2.8 | 72.8 ± 2.4 | 72.1 ± 4.8 | 71.8 ± 3.4 | 73 ± 2.9 | 73.5 ± 3 | |
| AD 100 | 73 ± 4 | 74 ± 3.8 | 74.3 ± 3.3 | 73.5 ± 3.2 | 72.8 ± 4.7 | 72.5 ± 3.7 | |
| AD 200 | 72.8 ± 2.7 | 75 ± 3.3 | 73.6 ± 4.2 | 73 ± 4.1 | 72.8 ± 4.4 | 73.8 ± 3.9 | |
| FS (%) | SAL | 38.5 ± 3 | 39.3 ± 3.2 | 37.3 ± 4.6 | 38.3 ± 3.5 | 36.5 ± 2.5 | 38.1 ± 4 |
| AD 50 | 38.3 ± 2.7 | 37.6 ± 2.2 | 37.3 ± 2.7 | 36.6 ± 2.5 | 37.3 ± 2.1 | 36.8 ± 2.5 | |
| AD 100 | 38 ± 3 | 38.3 ± 1.6 | 36.8 ± 1.4 | 37.3 ± 2.8 | 37 ± 2.6 | 36.6 ± 1.8 | |
| AD 200 | 37 ± 2.3 | 38.3 ± 2.3 | 37 ± 2 | 36.6 ± 2.2 | 37 ± 1.7 | 36.5 ± 2 | |
| CO (L/min) | SAL | 2.5 ± 0.3 | 2.3 ± 0.5 | 2.6 ± 0.3 | 2.4 ± 0.3 | 2.5 ± 0.4 | 2.6 ± 0.2 |
| AD 50 | 2.4 ± 0.28 | 2.4 ± 0.29 | 2.3 ± 0.33 | 2.3 ± 0.23 | 2.3 ± 0.30 | 2.5 ± 0.27 | |
| AD 100 | 2.4 ± 0.36 | 2.5 ± 0.35 | 2.3 ± 0.32 | 2.2 ± 0.31 | 2.2 ± 0.21 | 2.3 ± 0.30 | |
| AD 200 | 2.4 ± 0.35 | 2.5 ± 0.42 | 2.2 ± 0.32 | 2.1 ± 0.30 | 2.2 ± 0.21 | 2.3 ± 0.29 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salah, E.; Abouelfetouh, M.M.; Englar, R.E.; Ding, M.; Ding, Y. Cardiorespiratory Effects of Three Infusion Doses of Adenosine in Conscious Goats: A Preliminary Study. Vet. Sci. 2021, 8, 158. https://doi.org/10.3390/vetsci8080158
Salah E, Abouelfetouh MM, Englar RE, Ding M, Ding Y. Cardiorespiratory Effects of Three Infusion Doses of Adenosine in Conscious Goats: A Preliminary Study. Veterinary Sciences. 2021; 8(8):158. https://doi.org/10.3390/vetsci8080158
Chicago/Turabian StyleSalah, Eman, Mahmoud M. Abouelfetouh, Ryane E. Englar, Mingxing Ding, and Yi Ding. 2021. "Cardiorespiratory Effects of Three Infusion Doses of Adenosine in Conscious Goats: A Preliminary Study" Veterinary Sciences 8, no. 8: 158. https://doi.org/10.3390/vetsci8080158
APA StyleSalah, E., Abouelfetouh, M. M., Englar, R. E., Ding, M., & Ding, Y. (2021). Cardiorespiratory Effects of Three Infusion Doses of Adenosine in Conscious Goats: A Preliminary Study. Veterinary Sciences, 8(8), 158. https://doi.org/10.3390/vetsci8080158







