Influence of the H1 Antihistamine Mepyramine on the Antibacterial Effect of Florfenicol in Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Protocol
2.3. Isolation and Screening of E. coli from Faeces
2.4. Checkerboard Experiments
2.5. Statistical Analysis
3. Results
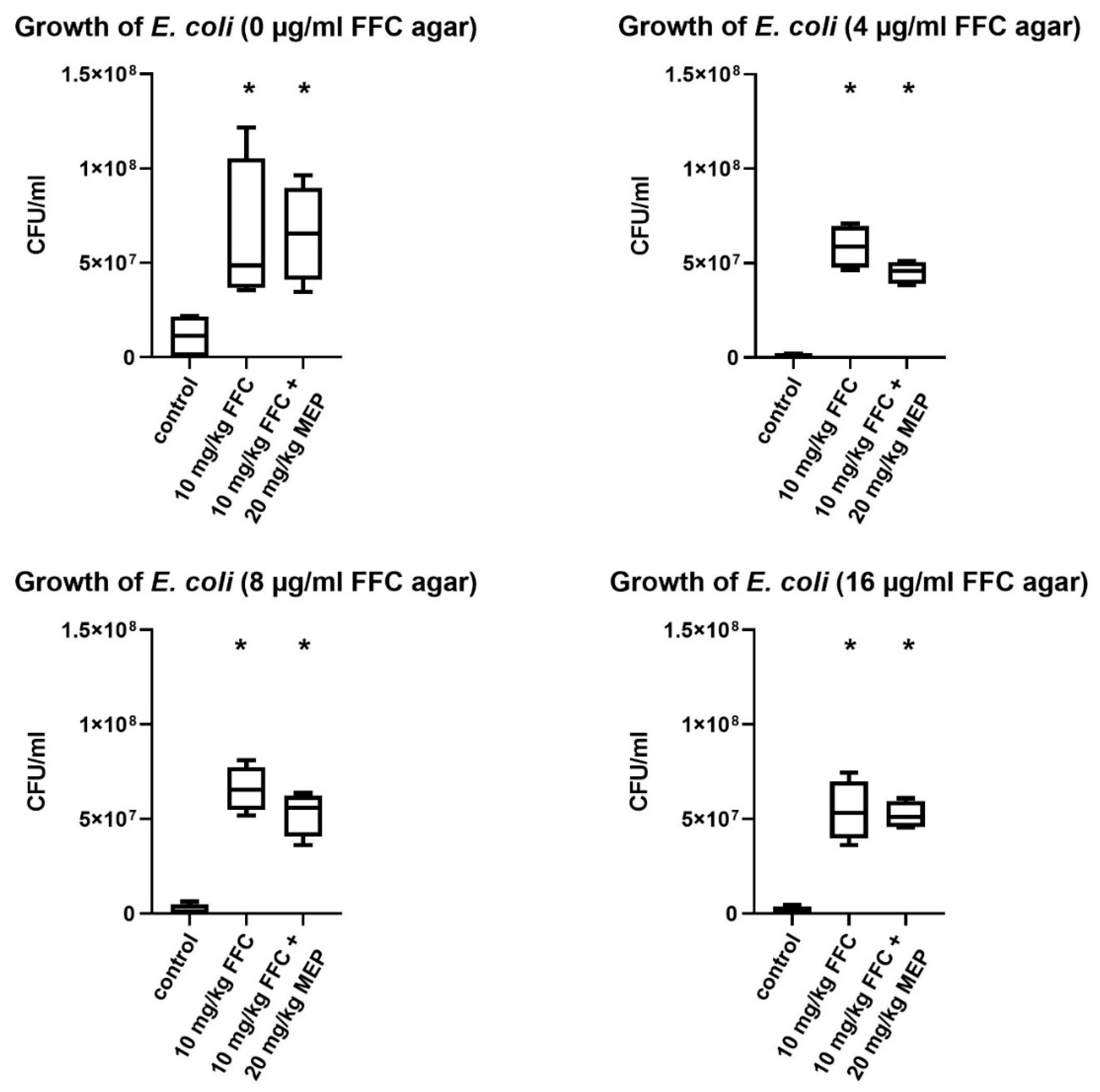
3.1. In Vivo Study
3.2. Checkerboard Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- Bruer, G.G.; Hagedorn, P.; Kietzmann, M.; Tohamy, A.F.; Filor, V.; Schultz, E.; Mielke-Kuschow, S.; Meissner, J. Histamine H1 receptor antagonists enhance the efficacy of antibacterials against Escherichia coli. BMC Vet. Res. 2019, 15, 55. [Google Scholar] [CrossRef] [Green Version]
- Merk, H.F. Standard treatment: The role of antihistamines. In Journal of Investigative Dermatology Symposium Proceedings; Elsevier: Amsterdam, The Netherlands, 2001; Volume 6, pp. 153–156. [Google Scholar] [CrossRef] [Green Version]
- Pan, I.C.; Chen, H.C.; Morter, R.L. Treatment of edema disease of swine. Can. J. Comp. Med. Rev. Can. Med. Comp. 1970, 34, 148–154. [Google Scholar]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef] [Green Version]
- Syriopoulou, V.P.; Harding, A.L.; Goldmann, D.A.; Smith, A.L. In vitro antibacterial activity of fluorinated analogs of chloramphenicol and thiamphenicol. Antimicrob. Agents Chemother. 1981, 19, 294–297. [Google Scholar] [CrossRef] [Green Version]
- Van den Bogaard, A.E.; Stobberingh, E.E. Epidemiology of resistance to antibiotics: Links between animals and humans. Int. J. Antimicrob. Agents 2000, 14, 327–335. [Google Scholar] [CrossRef]
- Brinkac, L.; Voorhies, A.; Gomez, A.; Nelson, K.E. The Threat of Antimicrobial Resistance on the Human Microbiome. Microb. Ecol. 2017, 74, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Fantin, B.; Duval, X.; Massias, L.; Alavoine, L.; Chau, F.; Retout, S.; Andremont, A.; Mentré, F. Ciprofloxacin Dosage and Emergence of Resistance in Human Commensal Bacteria. J. Infect. Dis. 2009, 200, 390–398. [Google Scholar] [CrossRef]
- WHO. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://appswhoint/iris/bitstream/handle/10665/193736/9789241509763_engpdf?sequence=1&isAllowed=y (accessed on 14 May 2020).
- OIE. The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. 2016. Available online: http://wwwoieint/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategypdf (accessed on 14 May 2020).
- Leopoldina, A.D.W.H. Antibiotika-Forschung: Probleme und Perspektiven; De Gruyter: Berlin, Germany, 2013; Volume 2. [Google Scholar] [CrossRef]
- Römer, A.; Scherz, G.; Reupke, S.; Meißner, J.; Wallmann, J.; Kietzmann, M.; Kaspar, H. Effects of intramuscularly administered enrofloxacin on the susceptibility of commensal intestinal Escherichia coli in pigs (sus scrofa domestica). BMC Vet. Res. 2017, 13, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Atlas, R.M.; Snyder, J.W. Handbook of Media for Clinical Microbiology; CRC Press: Boca Raton, FL, USA, 2006; pp. 190–191. [Google Scholar] [CrossRef]
- De Smet, J.; Boyen, F.; Croubels, S.; Rasschaert, G.; Haesebrouck, F.; De Backer, P.; Devreese, M. Similar Gastro-Intestinal Exposure to Florfenicol After Oral or Intramuscular Administration in Pigs, Leading to Resistance Selection in Commensal Escherichia coli. Front. Pharmacol. 2018, 9, 1265. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST): MIC distributions and ECOFFs. Available online: http://www.eucast.org/mic_distributions_and_ecoffs/ (accessed on 19 April 2019).
- Corry, J.; Curtis, G.D.W.; Baird, R.M. Handbook of Culture Media for Food and Water Microbiology; Royal Society of Chemistry: Cambridge, UK, 2012; p. 801. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Saenz, J.S.; Marques, T.V.; Barone, R.S.C.; Cyrino, J.E.P.; Kublik, S.; Nesme, J.; Schloter, M.; Rath, S.; Vestergaard, G. Oral administration of antibiotics increased the potential mobility of bacterial resistance genes in the gut of the fish Piaractus mesopotamicus. Microbiome 2019, 7, 24. [Google Scholar] [CrossRef]
- Mouton, J.W.; Vinks, A.A. Pharmacokinetic/pharmacodynamic modelling of antibacterials in vitro and in vivo using bacterial growth and kill kinetics: The minimum inhibitory concentration versus stationary concentration. Clin. Pharmacokinet. 2005, 44, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Weese, J.S. Understanding the Intestinal Microbiome in Health and Disease. Vet. Clin. Equine Pract. 2018, 34, 1–12. [Google Scholar] [CrossRef]
- Tuddenham, S.; Sears, C.L. The intestinal microbiome and health. Curr. Opin. Infect. Dis. 2015, 28, 464–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdes, A.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherz, G.; Stahl, J.; Glünder, G.; Kietzmann, M. Effects of carry-over of fluoroquinolones on the susceptibility of commensal Escherichia coli in the intestinal microbiota of poultry. Berliner und Munchener tierarztliche Wochenschrift 2015, 127, 478–485. [Google Scholar]
- El-Banna, T.; Sonbol, F.; El-Aziz, A.; Al-Fakharany, O. Modulation of antibiotic efficacy against Klebsiella pneumoniae by antihistaminic drugs. J. Med. Microb. Diagn. 2016, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Attwood, D.; Florence, A.T. Physicochemical Principles of Pharmacy, 6th ed.; Pharmaceutical Press: London, UK, 2016; pp. 193–246. [Google Scholar]
- Guth, P.S.; Spirtes, M.A. The Phenothiazinetranquilizers: Biochemical and Biophysical Actions. Int. Rev. Neurobiol. 1964, 7, 231–278. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.; Kiräly, J.; Mándi, Y. The antibacterial action and R-factor-inhibiting activity by chlorpromazine. Cell. Mol. Life Sci. 1975, 31, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Hagmar, P.; Pierrou, S.; Nielsen, P.; Nordén, B.; Kubista, M. Ionic Strength Dependence of the Binding of Methylene Blue to Chromatin and Calf Thymus DNA. J. Biomol. Struct. Dyn. 1992, 9, 667–679. [Google Scholar] [CrossRef] [PubMed]
| FFC in Combination with MEP | MIC (µg/mL) | DRI Median | DRI Range | |||
|---|---|---|---|---|---|---|
| Alone | Combined | |||||
| Median | Range | Median | Range | |||
| P. multocida 1117/1/19 | 0.5 | 0.5–1 | 0.25 * | 0.125–0.5 | 2 | 2–4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruer, G.G.; Gödecke, D.; Kietzmann, M.; Meißner, J. Influence of the H1 Antihistamine Mepyramine on the Antibacterial Effect of Florfenicol in Pigs. Vet. Sci. 2021, 8, 197. https://doi.org/10.3390/vetsci8090197
Bruer GG, Gödecke D, Kietzmann M, Meißner J. Influence of the H1 Antihistamine Mepyramine on the Antibacterial Effect of Florfenicol in Pigs. Veterinary Sciences. 2021; 8(9):197. https://doi.org/10.3390/vetsci8090197
Chicago/Turabian StyleBruer, Gustav G., Daria Gödecke, Manfred Kietzmann, and Jessica Meißner. 2021. "Influence of the H1 Antihistamine Mepyramine on the Antibacterial Effect of Florfenicol in Pigs" Veterinary Sciences 8, no. 9: 197. https://doi.org/10.3390/vetsci8090197
APA StyleBruer, G. G., Gödecke, D., Kietzmann, M., & Meißner, J. (2021). Influence of the H1 Antihistamine Mepyramine on the Antibacterial Effect of Florfenicol in Pigs. Veterinary Sciences, 8(9), 197. https://doi.org/10.3390/vetsci8090197






