Feline and Canine Cutaneous Lymphocytosis: Reactive Process or Indolent Neoplastic Disease?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Histopathology
2.3. Immunohistochemistry
2.4. PARR
3. Results
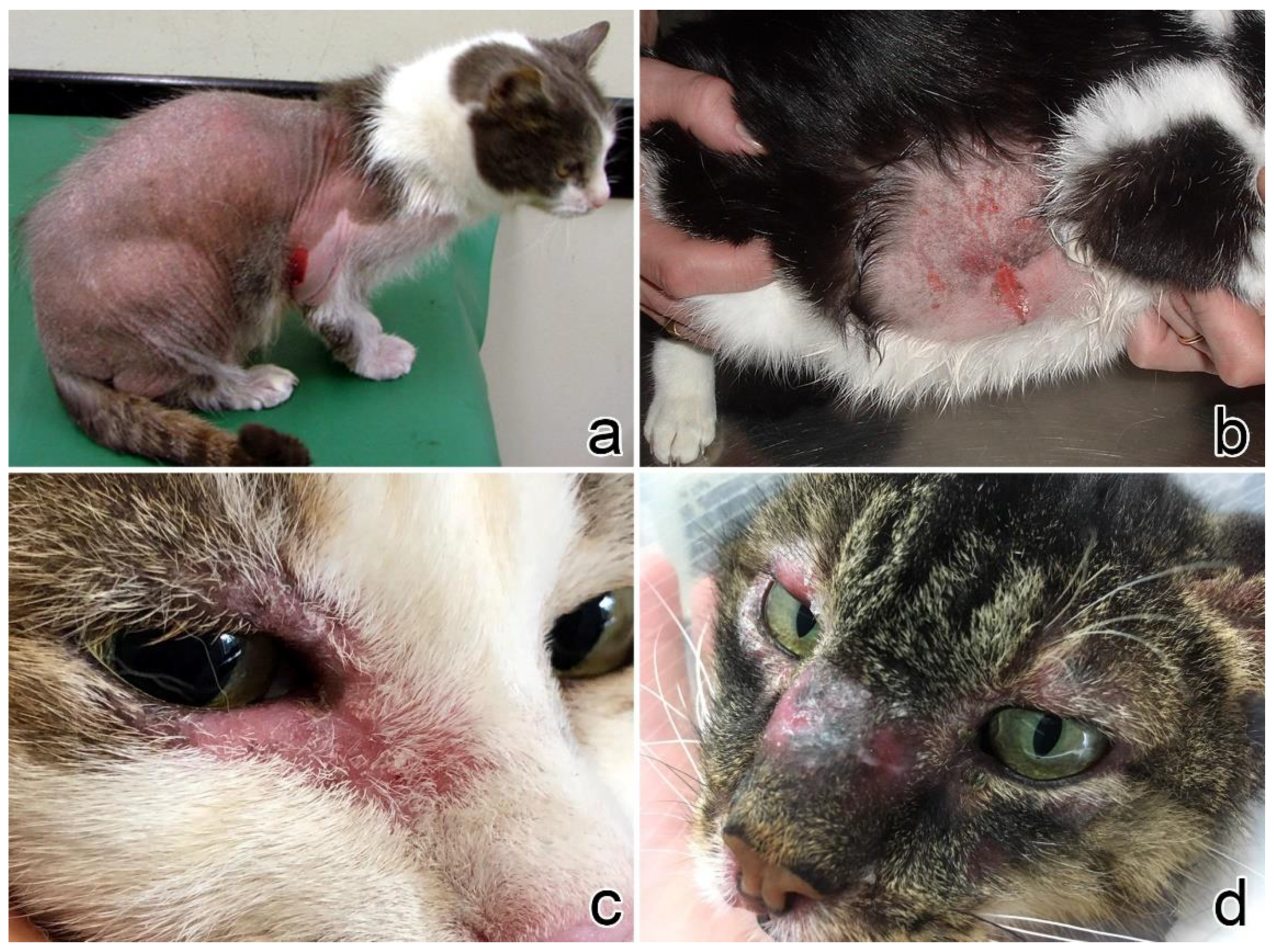
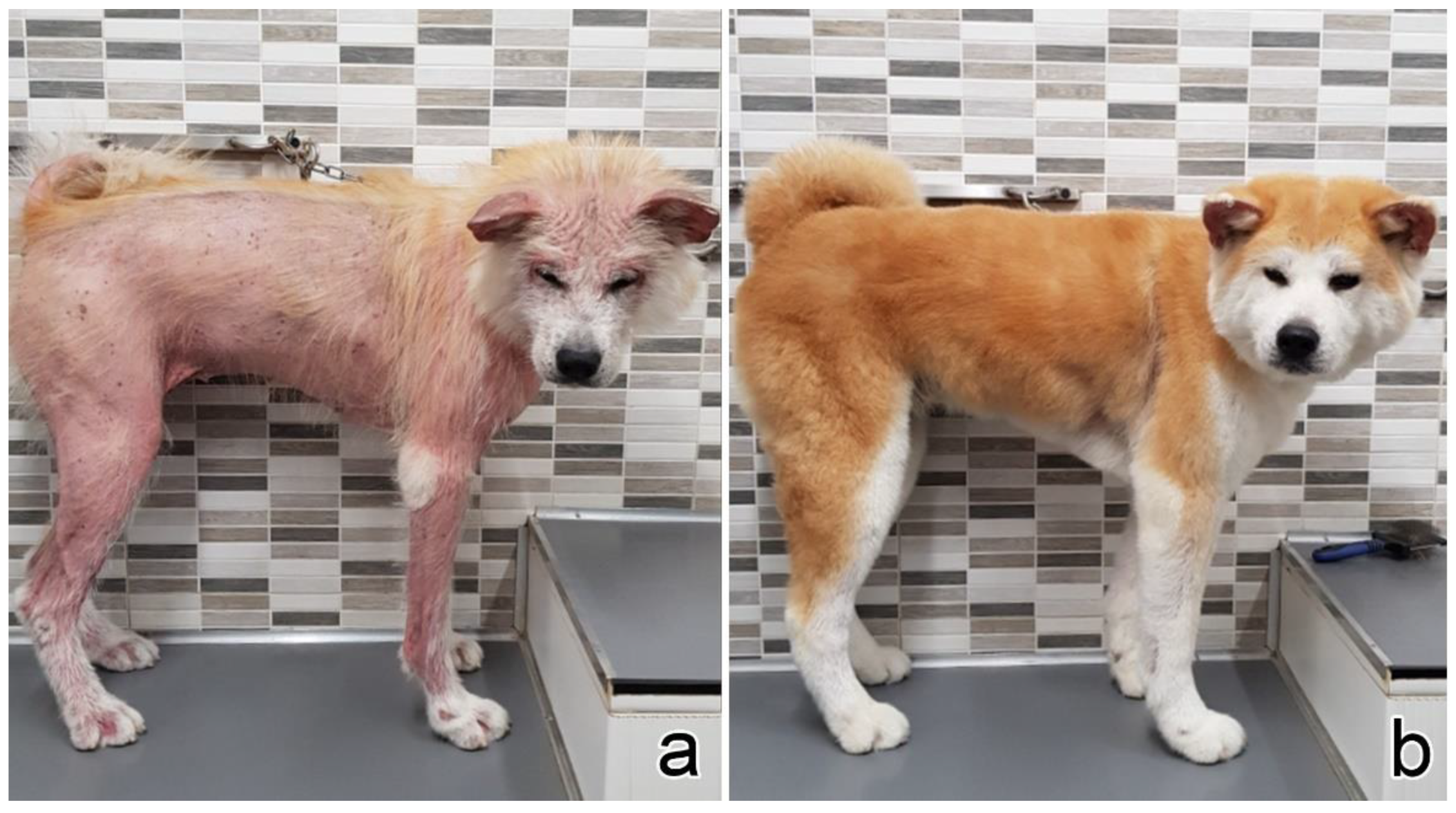
3.1. Clinical Presentation
3.2. Histopathology
3.3. Immunophenotyping
3.4. Clonality Test
3.5. Staging and Clinical Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ploysangam, T.; Breneman, D.L.; Mutasim, D.F. Cutaneous pseudolymphomas. J. Am. Acad. Dermatol. 1998, 38, 877–898. [Google Scholar] [CrossRef]
- Bergman, R. Pseudolymphoma and cutaneous lymphoma: Facts and controversies. Clin. Dermatol. 2010, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, A.C.; Wood, G.S. Cutaneous lymphoid hyperplasias. Semin. Cutan. Med. Surg. 2000, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.; Affolter, V.K.; Gross, T.L.; Moore, P.F.; Ihrke, P.J. Clinical, morphological and immunohistochemical characterization of cutaneous lymphocytosis in 23 cats. Vet. Dermatol. 2004, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Affolter, V.K.; Gross, T.L.; Moore, P.F. Indolent cutaneous T-cell lymphoma presenting as cutaneous lymphocytosis in dogs. Vet. Dermatol. 2009, 20, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Pariser, M.S.; Gram, D.W. Feline cutaneous lymphocytosis: Case report and summary of the literature. J. Feline Med. Surg. 2014, 16, 758–763. [Google Scholar] [CrossRef]
- Gross, T.L. Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis; Blackwell Science: Ames, IA, USA, 2005; ISBN 978-0-632-06452-6. [Google Scholar]
- Bagg, A. Immunoglobulin and T-cell receptor gene rearrangements: Minding your B’s and T’s in assessing lineage and clonality in neoplastic lymphoproliferative disorders. J. Mol. Diagn. 2006, 8, 426–429. [Google Scholar] [CrossRef] [Green Version]
- Waugh, E.M.; Gallagher, A.; Haining, H.; Johnston, P.E.J.; Marchesi, F.; Jarrett, R.F.; Morris, J.S. Optimisation and validation of a PCR for antigen receptor rearrangement (PARR) assay to detect clonality in canine lymphoid malignancies. Vet. Immunol. Immunopathol. 2016, 182, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Ehrhart, E.J.; Wong, S.; Richter, K.; Zismann, V.; Grimes, C.; Hendricks, W.; Khanna, C. Polymerase chain reaction for antigen receptor rearrangement: Benchmarking performance of a lymphoid clonality assay in diverse canine sample types. J. Vet. Intern. Med. 2019, 33, 1392–1402. [Google Scholar] [CrossRef] [Green Version]
- Hristov, A.C.; Tejasvi, T.; Wilcox, R.A. Mycosis fungoides and Sézary syndrome: 2019 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2019, 94, 1027–1041. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, S.; Affolter, V.K.; Schmidt, P.; Kosten, S.; Kramme, P.M.; Gross, T.L.; Ihrke, P.; Moore, P.F. Clonality studies of feline cutaneous lymphocytosis. Vet. Dermatol. 2004, 15, 24. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A.; Kiupel, M.; Baszler, T.; Bliven, L.; Brodersen, B.; Chelack, B.; West, K.; Czub, S.; Del Piero, F.; Dial, S.; et al. Suggested Guidelines for Immunohistochemical Techniques in Veterinary Diagnostic Laboratories. J. Vet. Diagn. Investig. 2008, 20, 393–413. [Google Scholar] [CrossRef] [Green Version]
- Burnett, R.C.; Vernau, W.; Modiano, J.F.; Olver, C.S.; Moore, P.F.; Avery, A.C. Diagnosis of Canine Lymphoid Neoplasia Using Clonal Rearrangements of Antigen Receptor Genes. Vet. Pathol. 2003, 40, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Nakamura, K.; Sato, H.; Goto-Koshino, Y.; Sato, M.; Takahashi, M.; Fujino, Y.; Ohno, K.; Uchida, K.; Nakayama, H.; et al. Multiplex PCR and Genescan analysis to detect immunoglobulin heavy chain gene rearrangement in feline B-cell neoplasms. Vet. Immunol. Immunopathol. 2011, 143, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.F.; Woo, J.C.; Vernau, W.; Kosten, S.; Graham, P.S. Characterization of feline T cell receptor gamma (TCRG) variable region genes for the molecular diagnosis of feline intestinal T cell lymphoma. Vet. Immunol. Immunopathol. 2005, 106, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.M.; Vernau, W.; Moore, P.F. Clonality Testing in Veterinary Medicine: A Review With Diagnostic Guidelines. Vet. Pathol. 2016, 53, 711–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rook, K.A. Canine and Feline Cutaneous Epitheliotropic Lymphoma and Cutaneous Lymphocytosis. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Siewert, J.; Pellin, M.A.; Husbands, B.D.; Curran, K.M.; Scavelli, D.; Sampene, E. Feline cutaneous lymphoma: An evaluation of disease presentation and factors affecting response to treatment. J. Feline Med. Surg. 2021, 1098612X211028837. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.; Heimann, M.; Day, M.J. Cutaneous epitheliotropic T-cell lymphoma in the cat: A review of the literature and five new cases. Vet. Dermatol. 2011, 22, 454–461. [Google Scholar] [CrossRef]
- Mitteldorf, C.; Kempf, W. Cutaneous pseudolymphoma—A review on the spectrum and a proposal for a new classification. J. Cutan. Pathol. 2020, 47, 76–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laprais, A.; Olivry, T. Is CCNU (lomustine) valuable for treatment of cutaneous epitheliotropic lymphoma in dogs? A critically appraised topic. BMC Vet. Res. 2017, 13, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Case No. | Signalment | Clinical Features of the Lesions | Other Clinical Findings | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breed | Sex | Age (Years) | Site | Longest Diameter (cm) | Distribution | Duration (Months) | U | Pr | A | Er | Ex | Pl | Additional Features | ||
| 1 | DSH | F | 12 | Head, neck, forelimb (shoulder) | 1.5 | Multifocal | 3 | X | Nodules | None | |||||
| 2 | DSH | M | 15 | Head (upper lip) | 4 | Focal | 6 | X | X | X | None | ||||
| 3 | DSH | M | 14 | Neck, dorsum, perineum, limbs | Diffuse | 24 | X | X | X | X | Papules, crusts | Otitis | |||
| 4 | DSH | F | 14 | Flank | 4 | Focal | 2 weeks | X | X | X | X | None | |||
| 5 | DSH | M | 9 | Head (upper lip, periocular) | 1 | Focal | 18 | X | X | X | X | None | |||
| 6 | DSH | M | 11 | Head (eyelid) | Focal | 12 | X | ||||||||
| 7 | DSH | M | 16 | Thorax-lumbar | 10 | Focal | 24 | X | X | X | X | None | |||
| 8 | DSH | F | 10 | Neck, axillae, dorsum | Multifocal | 7 | X | X | X | X | Anorexia, hyperthermia, melena, weight loss | ||||
| 9 | DSH | F | 14 | Head (periocular), hindlimb (metatarsus), tail | 5 | Multifocal | 6 | X | X | X | X | None | |||
| 10 | DSH | F | 10 | Head (upper lip), flank (bilateral) | 5 | Multifocal | 3 | X | X | X | Hyperpigmentation | None | |||
| 11 | DSH | F | 10 | Flank | Focal | 9 | X | ||||||||
| 12 | DSH | F | 13 | Forelimb (elbow) | 4 | Focal | 2 | X | X | X | None | ||||
| 13 | DSH | F | 10 | Thorax, axillae | 4 | Multifocal | 12 | X | X | X | None | ||||
| 14 | DSH | M | 7 | Head, abdomen (bilateral) | Diffuse | 3 | X | X | X | X | Weight loss, submandibular lymphadenomegaly, FIV-positive | ||||
| 15 | DSH | F | 9 | Abdomen | 1 | Focal | 2 | Nodule | None | ||||||
| 16 | DSH | M | 14 | Hindlimb (3rd digit) | 0.2 | Focal | 12 | X | X | Nodule | None | ||||
| 17 | DSH | F | 7 | Head (perilabial), neck, abdomen, trunk, hindlimb (thighs) | Diffuse | 24 | X | X | X | X | Bronchitis | ||||
| 18 | DSH | F | 13 | Neck, flank, hindlimb (thigh) | 20 | Multifocal | 60 | X | X | X | None | ||||
| 19 | DSH | F | 14 | Head, dorsum, abdomen | 30 | Multifocal | 6 | X | X | X | X | Dryness, crusts | None | ||
| Case No. | Signalment | Clinical Features of the Lesions | Other Clinical Findings | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breed | Sex | Age (Years) | Site | Longest Diameter (cm) | Distribution | Duration (Months) | U | Pr | A | Er | Ex | Pl | Additional Features | ||
| 1 | Beagle | F | 13 | Abdomen, hindlimb | 2 | Multifocal | 2 weeks | X | None | ||||||
| 2 | Beagle | M | 6 | Head (ear), perianal | 3 | Multifocal | 1 | X | X | X | None | ||||
| 3 | Shar Pei | F | 12 | Thorax | 2 | Multifocal | 1 | X | X | None | |||||
| 4 | Toy poodle | F | 13 | Dorsum, flank, abdomen | 2 | Multifocal | 1 | X | X | X | X | Lymphadenomegaly | |||
| 5 | Mixed breed | M | 8 | Thorax | 7 | Focal | 5 | X | None | ||||||
| 6 | Breton | M | 11 | Neck, flank, thorax | 20 | Multifocal | 2 | X | X | X | Crusts | None | |||
| 7 | Mixed breed | F | 15 | Perianal | 8 | Focal | 1 | X | X | X | Lymphocytic leukemia, mesenchymal gastric tumour | ||||
| 8 | Mixed breed | F | 12 | Trunk, flank | 2 | Multifocal | 10 | X | X | X | X | X | Ataxia, hypoglycemic crisis | ||
| 9 | Akita Inu | F | 9 | Axilla, forelimb (shoulder), hindlimb | Diffuse | 12 | X | X | X | Anemia, thrombocytopenia | |||||
| 10 | Dachshund | F | 8 | Abdomen | 5 | Multifocal | 2 weeks | X | X | X | None | ||||
| Case No. | PARR | Staging | Therapy | Response to Therapy | Overall Survival (Days) | Cause of Death |
|---|---|---|---|---|---|---|
| 1 | neg | neg (HB, U, Rx) | Corticosteroids | PR | 365 | Lost to follow-up |
| 2 | neg | neg (HB) | None | SD | 1080 | Unrelated (renal failure) |
| 3 | neg | n.a. | None | SD | 720 | Unrelated (renal failure) |
| 4 | neg | n.a. | None | SD | 240 | Unknown |
| 5 | neg | n.a. | Corticosteroids, chlorambucil | PR | 540 | Lost to follow-up |
| 6 | neg | n.a. | None | SD | 90 | Unrelated (renal failure) |
| 7 | neg | n.a. | Corticosteroids, chlorambucil | SD | 570 | Lost to follow-up |
| 8 | neg | n.a. | Corticosteroids | SD | 30 | Unknown |
| 9 | neg | neg (lnC) | Corticosteroids, cyclosporine | PR | 480 | Alive |
| 10 | neg | neg (HB, U) | Corticosteroids, cyclosporine | PR | 1080 | Alive |
| 11 | neg | n.a. | n.a. | n.a. | n.a. | n.a. |
| 12 | neg | n.a. | Corticosteroids | CR | 1080 | Alive |
| 13 | neg | n.a. | Chlorambucil | SD | 81 | Alive |
| 14 | neg | Submandibular lymphoadenomegaly (U, CT) | Corticosteroids | PR | 90 | Unknown |
| 15 | neg | n.a. | Surgery | CR | 365 | Unknown (not investigated visceral mass) |
| 16 | neg | n.a. | Surgery | CR | 570 | Alive |
| 17 | neg | neg (HB, U) | Corticosteroids | PR | 180 | Alive |
| 18 | neg | neg (HB, spC, livC) | Corticosteroids | PR | 1680 | Alive |
| 19 | neg | neg (HB, spC, livC) | Corticosteroids, chlorambucil | SD | 1080 | Alive |
| Case No. | PARR | Staging | Therapy | Response to Therapy | Overall Survival (Days) | Cause of Death |
|---|---|---|---|---|---|---|
| 1 | neg | n.a. | None | CR | 28 | Lost to follow-up |
| 2 | TCR/BCR clonal | neg (lnC) | Corticosteroids, cyclosporine | SD | 365 | Lost to follow-up |
| 3 | BCR clonal | neg (HB, US, spC, livC) | Surgery | CR | 630 | Alive |
| 4 | neg | neg (HB, US, spC, livC) | Lomustine, chlorambucil * | PD | 360 | Related (euthanasia for progressive disease) |
| 5 | neg | n.a. | Surgery | CR | 720 | Alive |
| 6 | BCR clonal | neg (HB, US, spC, livC) | Corticosteroids, chlorambucil | SD | 240 | Alive |
| 7 | BCR clonal | lymphocitic leukemia (HB, U) | Corticosteroids, lomustine | PR | 240 | Alive |
| 8 | neg | neg (HB, US, spC, livC) | Lomustine, chlorambucil * | PR | n.a. | Unknown |
| 9 | TCR clonal | mild anemia and thrombocytopenia (HB) | Corticosteroids, cyclosporine, chlorambucil, lomustine * | PR (after drug withdrawal) | 334 | Alive |
| 10 | BCR clonal | neg (U, lnC) | Surgery | CR | 150 | Alive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albanese, F.; Abramo, F.; Marino, M.; Massaro, M.; Marconato, L.; Minoli, L.; Martini, V.; Aresu, L. Feline and Canine Cutaneous Lymphocytosis: Reactive Process or Indolent Neoplastic Disease? Vet. Sci. 2022, 9, 26. https://doi.org/10.3390/vetsci9010026
Albanese F, Abramo F, Marino M, Massaro M, Marconato L, Minoli L, Martini V, Aresu L. Feline and Canine Cutaneous Lymphocytosis: Reactive Process or Indolent Neoplastic Disease? Veterinary Sciences. 2022; 9(1):26. https://doi.org/10.3390/vetsci9010026
Chicago/Turabian StyleAlbanese, Francesco, Francesca Abramo, Michele Marino, Maria Massaro, Laura Marconato, Lucia Minoli, Valeria Martini, and Luca Aresu. 2022. "Feline and Canine Cutaneous Lymphocytosis: Reactive Process or Indolent Neoplastic Disease?" Veterinary Sciences 9, no. 1: 26. https://doi.org/10.3390/vetsci9010026
APA StyleAlbanese, F., Abramo, F., Marino, M., Massaro, M., Marconato, L., Minoli, L., Martini, V., & Aresu, L. (2022). Feline and Canine Cutaneous Lymphocytosis: Reactive Process or Indolent Neoplastic Disease? Veterinary Sciences, 9(1), 26. https://doi.org/10.3390/vetsci9010026







