Modelling the Transmission of Coxiella burnetii within a UK Dairy Herd: Investigating the Interconnected Relationship between the Parturition Cycle and Environment Contamination
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
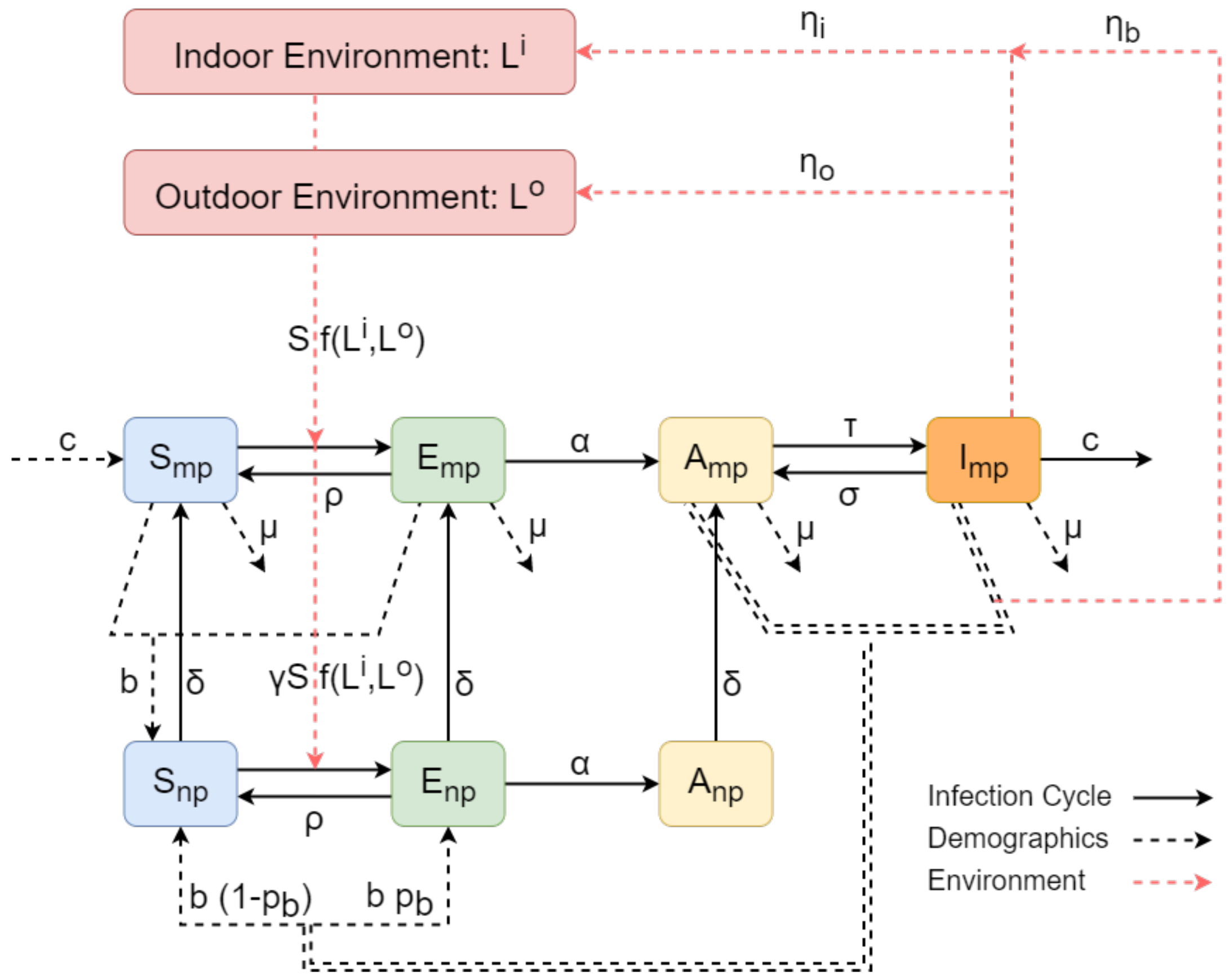
2.1. The C. burnetii within-Herd Transmission Model
2.1.1. The Infection Cycle
2.1.2. Farm Demographics
2.1.3. Contamination of the Environment
2.2. The Mathematical Formulation of the Model
2.2.1. Model Parameterization and Parameter Reduction
2.3. Initial Conditions
2.4. Sensitivity Analysis
3. Results
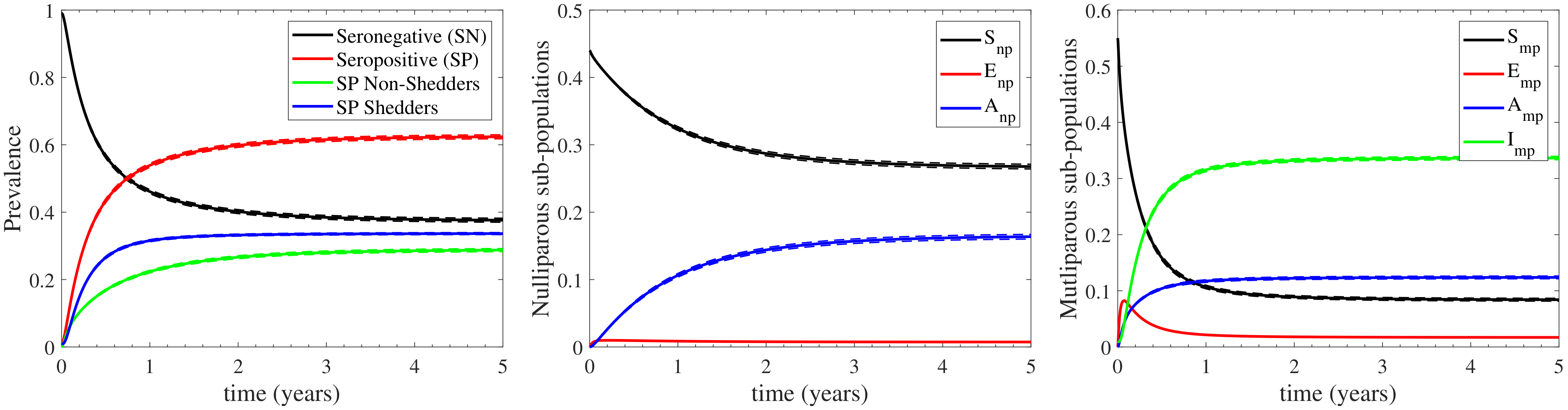
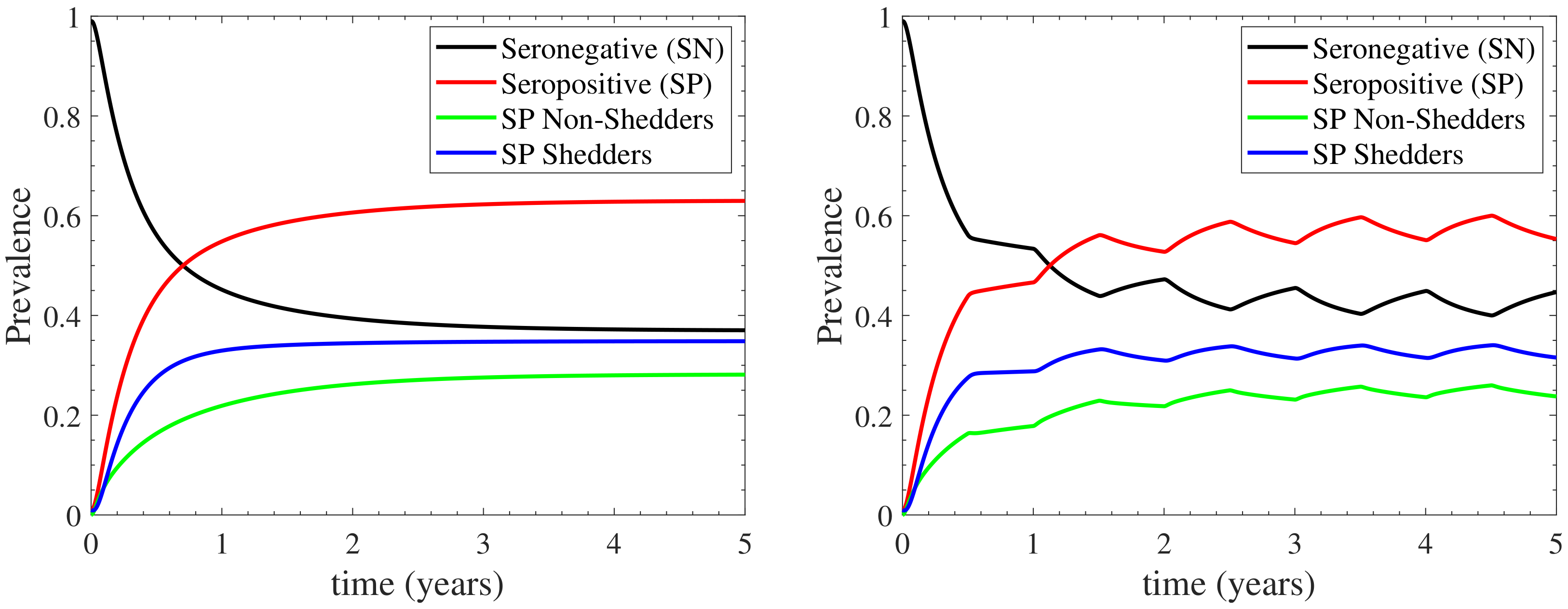
3.1. The Standard Case
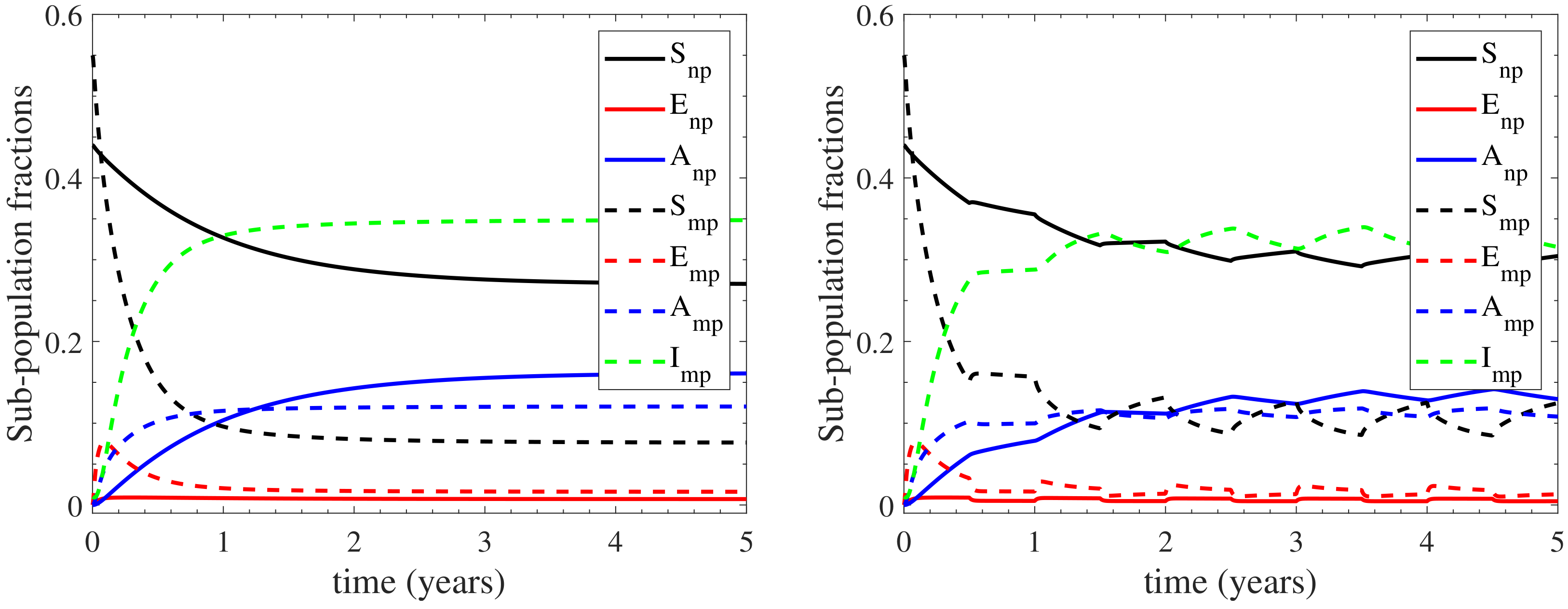
3.1.1. The Effect of Farm Demographics on the Infection Cycle
3.1.2. The Heterogeneity of the Environmental Contamination
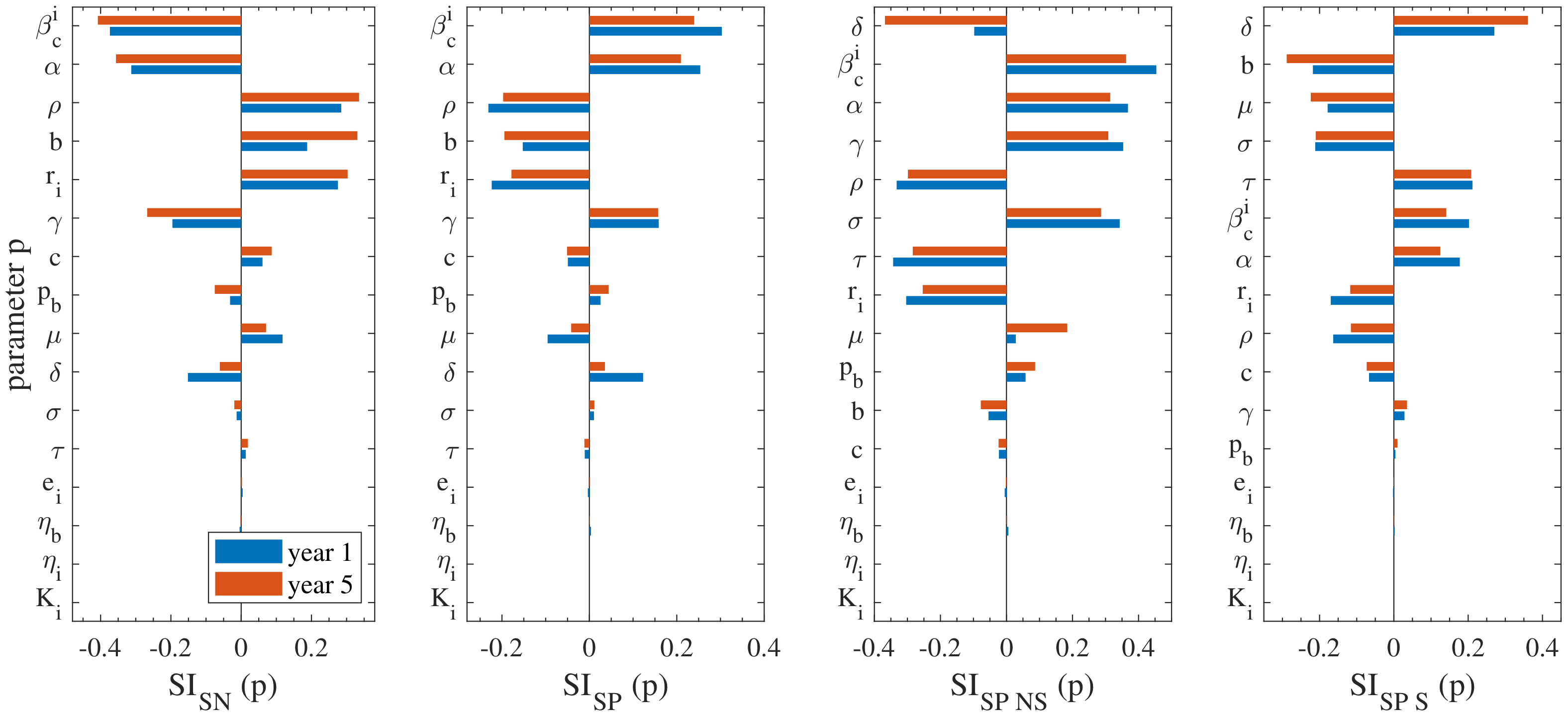
3.2. Sensitivity Analysis
- , that is, the reduction rate of transmission probability by the indoors environment, which essentially provides a measure of the uniformity of the transmission rate in the different environmental compartments: the higher the value of the more uniform the distribution of the transmission rates. There are currently no field or experimental data available in the literature for this parameter, therefore its uncertainty in the current study is high. Previous studies have not considered compartmentalisation of the environment, thus it is a new result highlighted by our model. Consequently, future experimental or field studies should focus on estimating this parameter. It is noted that the of is positive/negative for the seropositive/seronegative prevalence, suggesting that the more uniform the transmission rates become among the different environmental compartments, the more the seropositive sub-population.
- and , expressing the rates from to and vice versa, i.e., asymptomatic to infected. In the current study, data available from the literature were used to calibrate these parameters [7,12,23]; yet all the previous studies were performed in French cattle herds and did not distinguish between exposed and asymptomatic cattle. Therefore, there is some need for further research to enable a potentially more accurate description of these parameters.
- , that is the ratio of reduced transmission from nulliparous cattle. There is no data available in the literature for this parameter, hence, it is characterised by high uncertainty. As a result, further research is required to this regard. The importance of this parameter is also underlined by the fact that previous studies conclude that vaccination should be focused on nulliparous cattle [7,34,35];
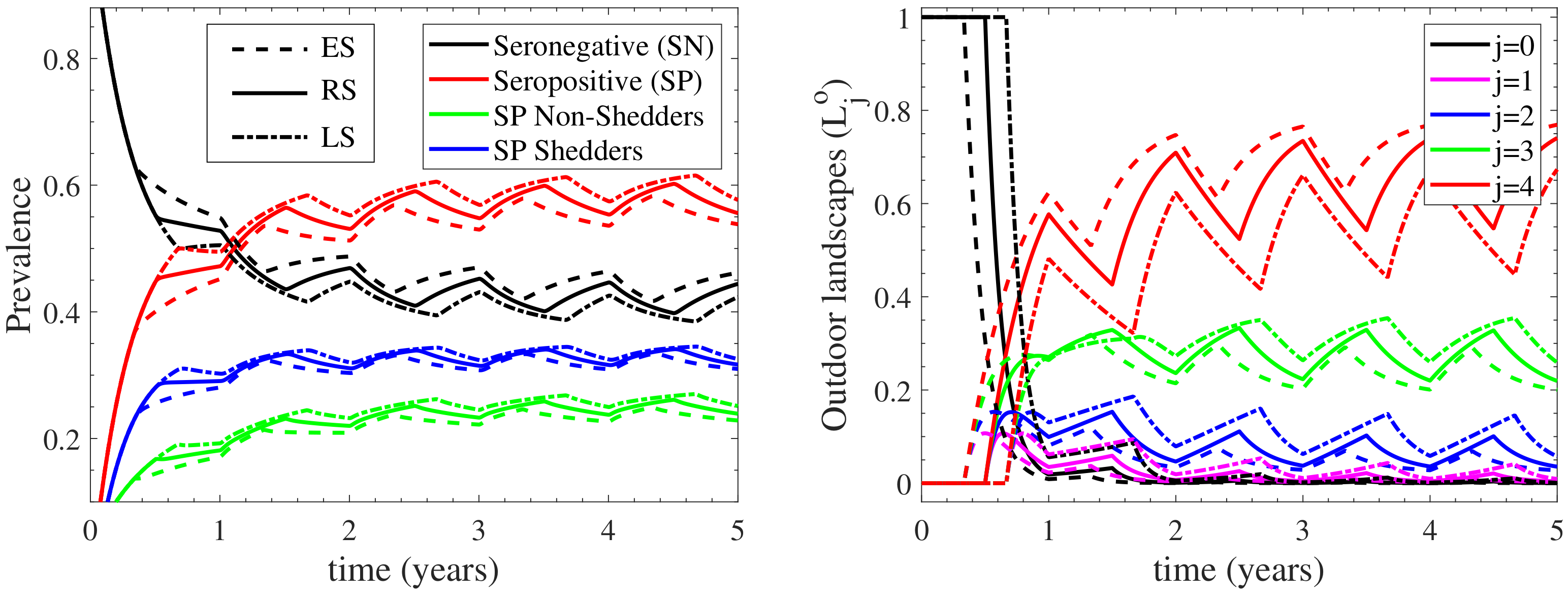
3.3. The Effect of the Outdoors Environment on the Infection Cycle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Parameterization of the Model
Appendix A.1. Transmission Related Parameters
Appendix A.2. Infection Cycle Related Parameters
Appendix A.3. Farm Demography Related Parameters
Appendix A.4. Environmental Contamination Related Parameters
Appendix B. The Equilibria of the Q Fever Model
Appendix C. Confidence Level of the Model’s Response
References
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.L.; Maurin, M.; Raoult, D. From Q fever to Coxiella burnetii infection: A paradigm change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [PubMed]
- Körner, S.; Makert, G.R.; Ulbert, S.; Pfeffer, M.; Mertens-Scholz, K. The prevalence of Coxiella Burnetii in hard ticks in Europe and their role in Q fever transmission revisited—A systematic review. Front. Vet. Sci. 2021, 8, 655715. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A. Q fever in dairy animals. Ann. N. Y. Acad. Sci. 2009, 1166, 90–93. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; López-Helguera, I.; Tutusaus, J.; Mur-Novales, R.; López-Gatius, F. Effects of long-term vaccination against Coxiella burnetii on the fertility of high-producing dairy cows. Acta Vet. Hung. 2015, 63, 223–233. [Google Scholar] [CrossRef]
- Serrano-Pérez, B.; Almería, S.; Tutusaus, J.; Jado, I.; Anda, P.; Monleón, E.; Badiola, J.; Garcia-Ispierto, I.; López-Gatius, F. Coxiella burnetii total immunoglobulin G, phase I and phase II immunoglobulin G antibodies, and bacterial shedding in young dams in persistently infected dairy herds. J. Vet. Diagn. Investig. 2015, 27, 167–176. [Google Scholar] [CrossRef]
- Kersh, G.J.; Fitzpatrick, K.A.; Self, J.S.; Priestley, R.A.; Kelly, A.J.; Lash, R.R.; Marsden-Haug, N.; Nett, R.J.; Bjork, A.; Massung, R.F.; et al. Presence and persistence of Coxiella burnetii in the environments of goat farms associated with a Q fever outbreak. Appl. Environ. Microbiol. 2013, 79, 1697–1703. [Google Scholar] [CrossRef]
- Courcoul, A.; Monod, H.; Nielen, M.; Klinkenberg, D.; Hogerwerf, L.; Beaudeau, F.; Vergu, E. Modelling the effect of heterogeneity of shedding on the within herd Coxiella burnetii spread and identification of key parameters by sensitivity analysis. J. Theor. Biol. 2011, 284, 130–141. [Google Scholar] [CrossRef]
- Bernard, K.W.; Parham, G.L.; Winkler, W.G.; Helmick, C.G. Q fever control measures: Recommendations for research facilities using sheep. Infect. Control Hosp. Epidemiol. 1982, 3, 461–465. [Google Scholar] [CrossRef]
- Nusinovici, S.; Hoch, T.; Brahim, M.L.; Joly, A.; Beaudeau, F. The Effect of Wind on C oxiella burnetii Transmission Between Cattle Herds: A Mechanistic Approach. Transbound. Emerg. Dis. 2017, 64, 585–592. [Google Scholar] [CrossRef]
- Tissot-Dupont, H.; Amadei, M.A.; Nezri, M.; Raoult, D. Wind in November, Q fever in December. Emerg. Infect. Dis. 2004, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Marcé, C.; Guatteo, R.; Bareille, N.; Fourichon, C. Dairy calf housing systems across Europe and risk for calf infectious diseases. Animal 2010, 4, 1588–1596. [Google Scholar] [CrossRef]
- Courcoul, A.; Vergu, E.; Denis, J.B.; Beaudeau, F. Spread of Q fever within dairy cattle herds: Key parameters inferred using a Bayesian approach. Proc. R. Soc. B Biol. Sci. 2010, 277, 2857–2865. [Google Scholar] [CrossRef]
- Pandit, P.; Hoch, T.; Ezanno, P.; Beaudeau, F.; Vergu, E. Spread of Coxiella burnetii between dairy cattle herds in an enzootic region: Modelling contributions of airborne transmission and trade. Vet. Res. 2016, 47, 48. [Google Scholar] [CrossRef]
- Hogerwerf, L.; Courcoul, A.; Klinkenberg, D.; Beaudeau, F.; Vergu, E.; Nielen, M. Dairy goat demography and Q fever infection dynamics. Vet. Res. 2013, 44, 28. [Google Scholar] [CrossRef] [PubMed]
- Courcoul, A.; Hogerwerf, L.; Klinkenberg, D.; Nielen, M.; Vergu, E.; Beaudeau, F. Modelling effectiveness of herd level vaccination against Q fever in dairy cattle. Vet. Res. 2011, 42, 68. [Google Scholar] [CrossRef] [PubMed]
- Bontje, D.; Backer, J.; Hogerwerf, L.; Roest, H.; Van Roermund, H. Analysis of Q fever in Dutch dairy goat herds and assessment of control measures by means of a transmission model. Prev. Vet. Med. 2016, 123, 71–89. [Google Scholar] [CrossRef]
- Asamoah, J.K.K.; Jin, Z.; Sun, G.Q.; Li, M.Y. A deterministic model for Q fever transmission dynamics within dairy cattle herds: Using sensitivity analysis and optimal controls. Comput. Math. Methods Med. 2020, 2020, 6820608. [Google Scholar] [CrossRef]
- Rodolakis, A.; Berri, M.; Hechard, C.; Caudron, C.; Souriau, A.; Bodier, C.; Blanchard, B.; Camuset, P.; Devillechaise, P.; Natorp, J.; et al. Comparison of Coxiella burnetii shedding in milk of dairy bovine, caprine, and ovine herds. J. Dairy Sci. 2007, 90, 5352–5360. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, R.; Seegers, H.; Joly, A.; Beaudeau, F. Prevention of Coxiella burnetii shedding in infected dairy herds using a phase I C. burnetii inactivated vaccine. Vaccine 2008, 26, 4320–4328. [Google Scholar] [CrossRef] [PubMed]
- Taurel, A.F.; Guatteo, R.; Joly, A.; Seegers, H.; Beaudeau, F. Seroprevalence of Q fever in naturally infected dairy cattle herds. Prev. Vet. Med. 2011, 101, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Roest, H.I.; Post, J.; van Gelderen, B.; van Zijderveld, F.G.; Rebel, J.M. Q fever in pregnant goats: Humoral and cellular immune responses. Vet. Res. 2013, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, R.; Beaudeau, F.; Joly, A.; Seegers, H. Performances of an ELISA applied to serum and milk for the detection of antibodies to Coxiella burnetii in dairy cattle. Rev. Med. Vet. 2007, 158, 250–252. [Google Scholar]
- Guatteo, R.; Beaudeau, F.; Joly, A.; Seegers, H. Coxiella burnetii shedding by dairy cows. Vet. Res. 2007, 38, 849–860. [Google Scholar] [CrossRef]
- Berri, M.; Souriau, A.; Crosby, M.; Crochet, D.; Lechopier, P.; Rodolakis, A. Relationships between the shedding of Coxiella burnetii, clinical signs and serological responses of 34 sheep. Vet. Rec. 2001, 148, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Bouvery, N.A.; Souriau, A.; Lechopier, P.; Rodolakis, A. Experimental Coxiella burnetii infection in pregnant goats: Excretion routes. Vet. Res. 2003, 34, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Huxley, J.; Hudson, C.; Kaler, J.; Gibbons, J.; Green, M. Field survey to evaluate space allowances for dairy cows in Great Britain. J. Dairy Sci. 2020, 103, 3745–3759. [Google Scholar] [CrossRef]
- Department for Environmental Food & Rural Affairs (DEFRA). Defra Statistics: Agricaltural Facts, England Regional Profiles. 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/972103/regionalstatistics_overview_23mar21.pdf (accessed on 13 December 2021).
- Department for Environmental Food & Rural Affairs (DEFRA). Farming Statistics—Livestock Populations at 1 December 2020, UK. 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/973322/structure-dec20-ukseries-25mar21i.pdf (accessed on 26 November 2021).
- Comparison in World Farming. The Life of: Dairy Cows. Available online: https://www.ciwf.org.uk/media/5235185/the-life-of-dairy-cows.pdf (accessed on 26 November 2021).
- Hanks, J.; Kossaibati, M. Key Performance Indicators for the UK National Dairy Herd: A Study of Herd Performance in 500 Holstein/Friesian Herds for the Year Ending 31st August 2021. 2021. Available online: https://www.nmr.co.uk/uploads/files/files/NMR500Herds-2021(Final).pdf (accessed on 5 July 2022).
- Gooderham, C. How Dairy Inseminations Will Impact Calf Numbers. 2021. Available online: https://ahdb.org.uk/news/how-dairy-inseminations-will-impact-calf-numbers (accessed on 5 July 2022).
- Freick, M.; Enbergs, H.; Walraph, J.; Diller, R.; Weber, J.; Konrath, A. Coxiella burnetii: Serological reactions and bacterial shedding in primiparous dairy cows in an endemically infected herd—Impact on milk yield and fertility. Reprod. Domest. Anim. 2017, 52, 160–169. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Q fever. Vet. Microbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef]
- Hogerwerf, L.; Van Den Brom, R.; Roest, H.I.; Bouma, A.; Vellema, P.; Pieterse, M.; Dercksen, D.; Nielen, M. Reduction of Coxiella burnetii prevalence by vaccination of goats and sheep, The Netherlands. Emerg. Infect. Dis. 2011, 17, 379. [Google Scholar] [CrossRef]
- De Cremoux, R.; Rousset, E.; Touratier, A.; Audusseau, G.; Nicollet, P.; Ribaud, D.; David, V.; Le Pape, M. Assessment of vaccination by a phase I Coxiella burnetii-inactivated vaccine in goat herds in clinical Q fever situation. FEMS Immunol. Med. Microbiol. 2012, 64, 104–106. [Google Scholar] [CrossRef]
- Heinzen, R.A.; Hackstadt, T.; Samuel, J.E. Developmental biology of Coxiella burnetii. Trends Microbiol. 1999, 7, 149–154. [Google Scholar] [CrossRef]


| Parameter | Unit | Description | Source | |
|---|---|---|---|---|
| , | - | Proportion of time per year for which cattle stay indoors and outdoors | Equation (6) | |
| , | day−1 | Transmission rates of becoming from each indoor and outdoor contaminated landscape | Equation (7) | |
| , | - | Reduction rate of transmission probability by indoor and outdoor environment | Assumed | |
| day−1 | Total indoor transmission rate | [12,17] | ||
| day−1 | Total outdoor transmission rate | CtM [12,26,27] | ||
| - | Ratio of reduced transmission rate for nulliparous cattle | Assumed | ||
| day−1 | Transition rate of eliminating the disease | [7,12] | ||
| day−1 | Transition rate of becoming | [21] | ||
| day−1 | Transition rate of becoming | CtM [7,12,23] | ||
| day−1 | Transition rate of becoming | [7,12] | ||
| c | day−1 | Removal rate of due to culling and isolation | [7] | |
| day−1 | Progression rate from nulliparous to multiparous cattle | CtM [28] | ||
| b | day−1 | Birth rate of multiparous cattle | [7,29,30,31] | |
| - | Probability of the offspring being exposed after birth from | [7,32] | ||
| b | day−1 | Natural death and removal rate | CtM [7,12,17] | |
| day−1 | Indoor shedding rate from | [7,12,17] | ||
| - | Indoor birth shedding rate from | CtM [16] | ||
| day−1 | Outdoor shedding rate from | Assumed | ||
| day−1 | Natural indoor environment decay rate | CtM [17] | ||
| day−1 | Natural outdoor environment decay rate | Assumed | ||
| day−1 | Active clearing rate of contaminated indoor environment | [17] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patsatzis, D.G.; Wheelhouse, N.; Tingas, E.-A. Modelling the Transmission of Coxiella burnetii within a UK Dairy Herd: Investigating the Interconnected Relationship between the Parturition Cycle and Environment Contamination. Vet. Sci. 2022, 9, 522. https://doi.org/10.3390/vetsci9100522
Patsatzis DG, Wheelhouse N, Tingas E-A. Modelling the Transmission of Coxiella burnetii within a UK Dairy Herd: Investigating the Interconnected Relationship between the Parturition Cycle and Environment Contamination. Veterinary Sciences. 2022; 9(10):522. https://doi.org/10.3390/vetsci9100522
Chicago/Turabian StylePatsatzis, Dimitrios G., Nick Wheelhouse, and Efstathios-Al. Tingas. 2022. "Modelling the Transmission of Coxiella burnetii within a UK Dairy Herd: Investigating the Interconnected Relationship between the Parturition Cycle and Environment Contamination" Veterinary Sciences 9, no. 10: 522. https://doi.org/10.3390/vetsci9100522
APA StylePatsatzis, D. G., Wheelhouse, N., & Tingas, E. -A. (2022). Modelling the Transmission of Coxiella burnetii within a UK Dairy Herd: Investigating the Interconnected Relationship between the Parturition Cycle and Environment Contamination. Veterinary Sciences, 9(10), 522. https://doi.org/10.3390/vetsci9100522






