Antinociceptive Effect of a Sacro-Coccygeal Epidural of Morphine and Lidocaine in Cats Undergoing Ovariohysterectomy
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Anaesthetic Management
2.3. Blind Study
2.4. Anaesthetic Drugs and Technique Used to Perform the Sacrococcygeal Epidural Anaesthesia
2.5. Intraoperative Analgesia Assessments and Rescue Analgesia
2.6. Postoperative Pain Assessments and Rescue Analgesia
- UNESP-Botucatu multidimensional composite pain scale (UNESP-Botucatu-MCPS) with increasing pain levels reflected by a score from 0 to 27, based on specific behaviours, posture, attitude, and reactions to physical contact. Due to the difficulty in measuring blood pressure in non-anaesthetized and/or sedated cats [37], this parameter was not evaluated postoperatively (sub-scale 3-physiological variables).
- Wound sensitivity by measuring the minimal mechanical nociceptive threshold (MNT) using a pneumatic device (ELECALL ELK-50 Digital Dynamometer Force Measuring), with an 8 mm round, flat tip, applied near the surgical wound.
2.7. Statistical Analysis
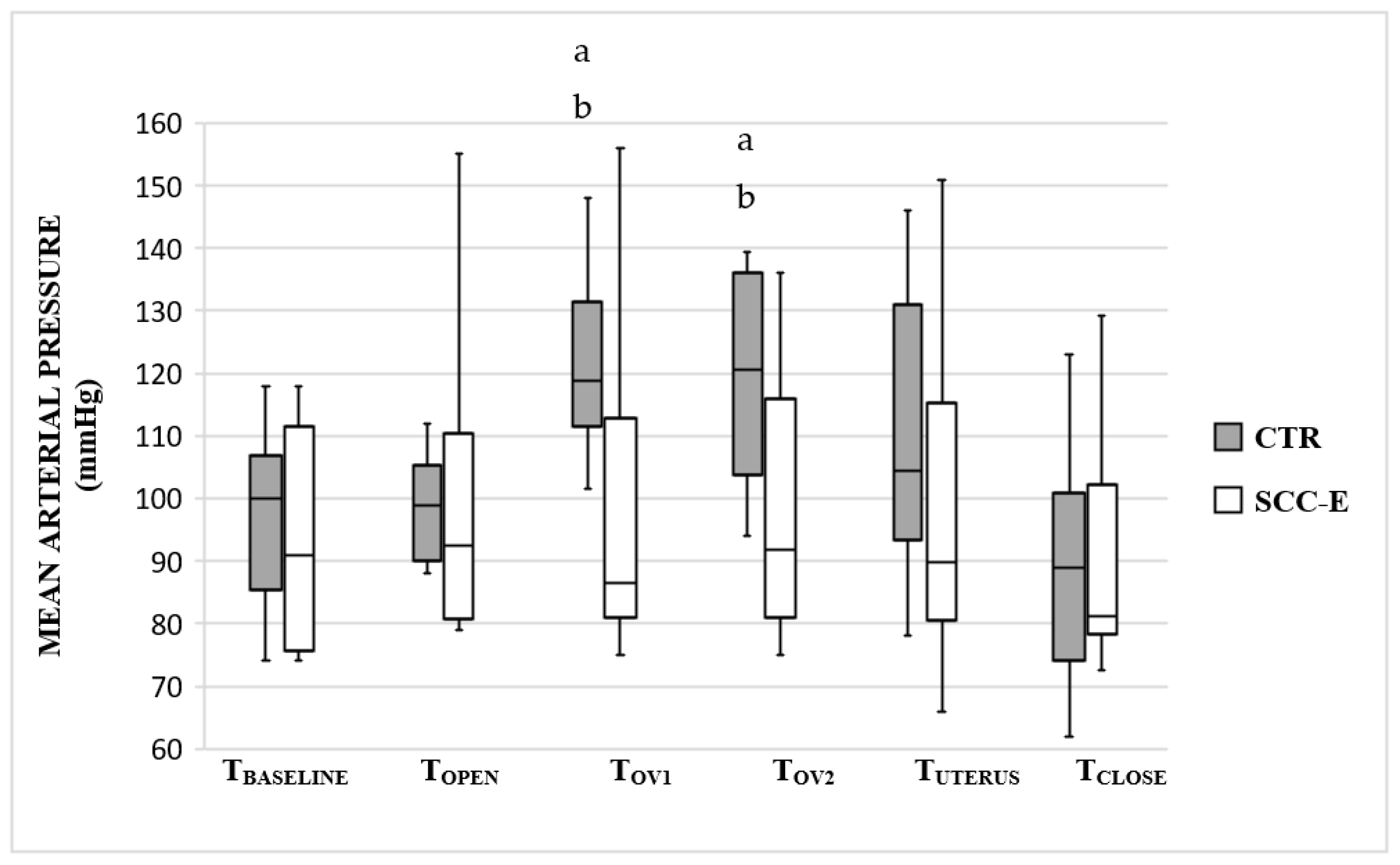
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trevejo, R.; Yang, M.; Lund, E.M. Epidemiology of Surgical Castration of Dogs and Cats in the United States. J. Am. Vet. Med. Assoc. 2011, 238, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Aida, S.; Baba, H.; Yamakura, T.; Taga, K.; Fukuda, S.; Shimoji, K. The Effectiveness of Preemptive Analgesia Varies According to the Type of Surgery: A Randomized, Double-Blind Study. Anesth. Analg. 1999, 89, 711. [Google Scholar] [CrossRef] [PubMed]
- Glerum, L.; Egger, C.; Allen, S.; Haag, M. Analgesic Effect of the Transdermal Fentanyl Patch during and after Feline Ovariohysterectomy. Vet. Surg. 2001, 30, 351–358. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, J.; O’Donnell, B.; Curley, G.; Heffernan, A.; Power, C.; Laffey, J. The Analgesic Efficacy of Transversus Abdominis Plane Block After Abdominal Surgery: A Prospective Randomized Controlled Trial. Anesth. Analg. 2007, 104, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Hawson, A.; Correll, L. Transversus Abdominis Plane Block and Treatment of Viscerosomatic Abdominal Pain. Reg. Anesth. Pain Med. 2015, 40, 731–732. [Google Scholar] [CrossRef]
- Bubalo, V.; Moens, Y.; Holzmann, A.; Coppens, P. Anaesthetic Sparing Effect of Local Anaesthesia of the Ovarian Pedicle during Ovariohysterectomy in Dogs. Vet. Anaesth. Analg. 2008, 35, 537–542. [Google Scholar] [CrossRef]
- Höglund, O.; Hagman, R.; Olsson, K.; Olsson, U.; Lagerstedt, A.-S. Intraoperative Changes in Blood Pressure, Heart Rate, Plasma Vasopressin, and Urinary Noradrenalin during Elective Ovariohysterectomy in Dogs: Repeatability at Removal of the 1st and 2nd Ovary. Vet. Surg. 2014, 43, 852–859. [Google Scholar] [CrossRef]
- Höglund, O.; Lövebrant, J.; Olsson, U.; Höglund, K. Blood Pressure and Heart Rate during Ovariohysterectomy in Pyometra and Control Dogs: A Preliminary Investigation. Acta Vet. Scand. 2016, 58, 80. [Google Scholar] [CrossRef]
- Slingsby, L.; Bortolami, E.; Murrell, J. Methadone in Combination with Medetomidine as Premedication Prior to Ovariohysterectomy and Castration in the Cat. J. Feline Med. Surg. 2015, 17, 864–872. [Google Scholar] [CrossRef]
- Polson, S.; Taylor, P.; Yates, D. Analgesia after Feline Ovariohysterectomy under Midazolam-Medetomidine-Ketamine Anaesthesia with Buprenorphine or Butorphanol, and Carprofen or Meloxicam: A Prospective, Randomised Clinical Trial. J. Feline Med. Surg. 2012, 14, 553–559. [Google Scholar] [CrossRef]
- Mahdmina, A.; Evans, A.; Yates, D.; White, K.L. Comparison of the Effects of Buprenorphine and Methadone in Combination with Medetomidine Followed by Intramuscular Alfaxalone for Anaesthesia of Cats Undergoing Ovariohysterectomy. J. Feline Med. Surg. 2020, 22, 77–83. [Google Scholar] [CrossRef]
- Shah, M.; Yates, D.; Hunt, J.; Murrell, J. Comparison between Methadone and Buprenorphine within the QUAD Protocol for Perioperative Analgesia in Cats Undergoing Ovariohysterectomy. J. Feline Med. Surg. 2018, 21, 723–731. [Google Scholar] [CrossRef]
- Schlereth, T.; Birklein, F. The Sympathetic Nervous System and Pain. Neuromol. Med. 2008, 10, 141–147. [Google Scholar] [CrossRef]
- Padilha, S.; Steagall, P.; Monteiro, B.; Kahvegian, M.A.P.; Ubukata, R.; Rodrigues, E.O.; Rosa, A.L.; Aguiar, A.J.A. A Clinical Comparison of Remifentanil or Alfentanil in Propofol-Anesthetized Cats Undergoing Ovariohysterectomy. J. Feline Med. Surg. 2011, 13, 738–743. [Google Scholar] [CrossRef]
- Srithunyarat, T.; Höglund, O.; Hagman, R.; Olsson, U.; Stridsberg, M.; Lagerstedt, A.-S.; Pettersson, A. Catestatin, Vasostatin, Cortisol, Temperature, Heart Rate, Respiratory Rate, Scores of the Short Form of the Glasgow Composite Measure Pain Scale and Visual Analog Scale for Stress and Pain Behavior in Dogs before and after Ovariohysterectomy. BMC Res. Notes 2016, 9, 381. [Google Scholar] [CrossRef]
- Sández, I.; Soto, M.; Torralbo, D.; Rioja, E. Effect of Different Analgesic Techniques on Hemodynamic Variables Recorded with an Esophageal Doppler Monitor during Ovariohysterectomy in Dogs. Can. Vet. J. 2018, 59, 419–424. [Google Scholar] [PubMed]
- Lamont, L.A. Multimodal Pain Management in Veterinary Medicine: The Physiologic Basis of Pharmacologic Therapies. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1173–1186. [Google Scholar] [CrossRef]
- Robertson, S.; Taylor, P. Pain Management in Cats—Past, Present and Future. Part 2. Treatment of Pain—Clinical Pharmacology. J. Feline Med. Surg. 2004, 6, 321–333. [Google Scholar] [CrossRef]
- Bortolami, E.; Love, E.J. Practical Use of Opioids in Cats: A State-of-the-Art, Evidence-Based Review. J. Feline Med. Surg. 2015, 17, 283–311. [Google Scholar] [CrossRef]
- Almeida, R.; Escobar, A.; Maguilnik, S. Comparison of Analgesia Provided by Lidocaine, Lidocaine-Morphine or Lidocaine-Tramadol Delivered Epidurally in Dogs Following Orchiectomy. Vet. Anaesth. Analg. 2010, 37, 542–549. [Google Scholar] [CrossRef]
- DeRossi, R.; Hermeto, L.; Jardim, P.; Bicudo, N.D.A.; De Assis, K.T. Postoperative Pain Control in Cats: Clinical Trials with Pre-EmptiveLidocaine Epidural Co-Administered with Morphine or Methadone. J. Feline Med. Surg. 2016, 18, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Sande, J.Q.; Anjos, N.P.; Filho, E.F.M.; Barbosa, V.F. Avaliação Da Anestesia Epidural Com Lidocaína Associada Ao Tramadol Ou à Dexmedetomidina, Em Gatas Submetidas à Ovariosalpingohisterectomia, Anestesiadas Com Propofol Avaliação Da Anestesia Epidural Com Lidocaína Associada Ao Tramadol Ou à Dexmedetomidina. Arq. Bras. Med. Vet. Zootec 2019, 71, 1901–1908. [Google Scholar] [CrossRef]
- Fernandez-Parra, R.; Zilberstein, L.; Fontaine, C.; Adami, C. Comparison of Intratesticular Lidocaine, Sacrococcygeal Epidural Lidocaine and Intravenous Methadone in Cats Undergoing Castration: A Prospective, Randomized, Investigator-Blind Clinical Trial. Vet. Anaesth. Analg. 2017, 44, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Tung, A.; Yaksh, T. The Antinociceptive Effects of Epidural Opiates in the Cat: Studies on the Pharmacology and the Effects of Lipophilicity in Spinal Analgesia. Pain 1982, 12, 343–356. [Google Scholar] [CrossRef]
- Lima, D.A.S.D.; Souza, A.P.; Santana, V.L.; Araújo, A.L.; Lima, W.C.; Mendes, R.S.; Neto, P.I.N. Anestesia Epidural Com Associação Medetomidina e Lidocaína, Em Gatos Pré-Medicados Com Acepromazina e Midazolam [Epidural Anesthesia with Lidocaine and Association Medetomidine in Cats Pre-Medicated with Acepromazine and Midazolam]. Arq. Bras. Med. Vet. Zootec. 2011, 63, 308–316. [Google Scholar] [CrossRef]
- Hermeto, L.C.; De Rossi, R.; Marques, B.C.; Jardim, P.H.A. Potentiation of Epidural Lidocaine by Co-Administering Tramadol by Either Intramuscular or Epidural Route in Cats. Can. J. Vet. Res. 2015, 79, 214–220. [Google Scholar] [PubMed]
- Souza, S.S.; Intelisano, T.R.; De Biaggi, C.P.; Moura, C.A.; Selmi, A.L.; Dias, R.A.; Cortopassi, S.R.G. Cardiopulmonary and Isoflurane-Sparing Effects of Epidural or Intravenous Infusion of Dexmedetomidine in Cats Undergoing Surgery with Epidural Lidocaine. Vet. Anaesth. Analg. 2010, 37, 106–115. [Google Scholar] [CrossRef]
- Otero, P.; Campoy, L. Epidural and Spinal Anesthesia. In Small Animals Regional Anesthesia and Analgesia; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- Diniz, M.S.; Kanashiro, G.P.; Bernardi, C.A.; Nicácio, G.M.; Cassu, R.N. Extradural Anesthesia with Lidocaine Combined with Fentanyl or Methadone to Ovariohisterectomy in Dogs. Acta Cir. Bras. 2013, 28, 531–536. [Google Scholar] [CrossRef]
- Fletcher, T.F.; Malkmus, S.A. Spinal anatomy of experimental animals. In Spinal Drug Delivery; Yaksh, T.L., Ed.; Elsevier: New York, NY, USA, 1999; pp. 71–96. [Google Scholar]
- Maierl, H.; Liebich, J. Investigations on the Postnatal Development of the Macroscopic Proportions and the Topographic Anatomy of the Feline Spinal Cord. Anat. Histol. Embryol. 1998, 27, 375–379. [Google Scholar] [CrossRef]
- O’Hearn, A.; Wright, B.D. Coccygeal Epidural with Local Anesthetic for Catheterization and Pain Management in the Treatment of Feline Urethral Obstruction. J. Vet. Emerg. Crit. Care 2011, 21, 50–52. [Google Scholar] [CrossRef]
- Credie, L.; Luna, S. The Use of Ultrasound to Evaluate Sacrococcygeal Epidural Injections in Cats. Can. Vet. J. 2018, 59, 143–146. [Google Scholar] [PubMed]
- Otero, P.; Verdier, N.; Zaccagnini, A.; Fuensalida, S.E.; Tarragona, L.; Portela, D.A. The Use of a Nerve Stimulation Test to Confirm Sacrococcygeal Epidural Needle Placement in Cats. Vet. Anaesth. Analg. 2014, 42, 115–118. [Google Scholar] [CrossRef]
- Otero, P.; Portela, D. Manual de Anestesia Regional En Animales de Compañía: Anatomía Para Bloqueos Guiados Por Ecografía y Neuroestimulación; Inter-Meédica: Buenos Aires, Argentina, 2017. [Google Scholar]
- Rondelli, V.; Otero, P.E.; Romano, F.; Verdier, N.; Bettschart-Wolfensberger, R.; Portela, D.A. Incidence of Dural Sac Puncture during Neuraxial Anesthesia in Cats: An Observational, Retrospective Study. J. Feline Med. Surg. 2021, 1098612X211021292. [Google Scholar] [CrossRef]
- Brondani, J.T.; Mama, K.R.; Luna, S.P.L.; Wright, B.D.; Niyom, S.; Ambrosio, J.; Vogel, P.R.; Padovani, C.R. Validation of the English Version of the UNESP-Botucatu Multidimensional Composite Pain Scale for Assessing Postoperative Pain in Cats. BMC Vet. Res. 2013, 9, 143. [Google Scholar] [CrossRef]
- Kanai, A.; Hoka, S. A Comparison of Epidural Blockade Produced by Plain 1% Lidocaine and 1% Lidocaine Prepared by Dilution of 2% Lidocaine Wilt the Same Volume of Saline. Anesth. Analg. 2006, 102, 1851–1855. [Google Scholar] [CrossRef]
- Höglund, O.; Olsson, K.; Hagman, R.; Öhlund, M.; Olsson, U.; Lagerstedt, A. Comparison of Haemodynamic Changes during Two Surgical Methods for Neutering Female Dogs. Res. Vet. Sci. 2011, 91, 159–163. [Google Scholar] [CrossRef]
- Rosengren, E.; Sjöuberg, N. The Adrenergic Nerve Supply to the Female Reproductive Tract of the Cat. Am. J. Anat. 1967, 121, 271–283. [Google Scholar] [CrossRef]
- Chien, C.; Li, S.; Shen, C. The Ovarian Innervation in the Dog: A Preliminary Study for the Base for Electro-Acupuncture. J. Auton. Nerv. Syst. 1991, 35, 185–192. [Google Scholar] [CrossRef]
- Sinowatz, F. Development of the Urogenital System. In Essentials of Domestic Animal Embryology; Saunders: Oxford, UK, 2010; pp. 252–285. [Google Scholar]
- McGeady, T.A.; Quinn, P.J.; Fitzpatrick, E.S.; Ryan, M.T.; Kilroy, D.; Lonergan, P. Male and Female Reproductive Systems. In Veterinary Embryology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 251–273. [Google Scholar]
- Okutomi, T.; Hoka, S. Epidural Saline Solution Prior to Local Anaesthetic Produces Differential Nerve Block. Can. J. Anesth. 1998, 45, 1091–1093. [Google Scholar] [CrossRef]
- Inagaki, Y.; Mashimo, T.; Kuzukawa, A.; Tsuda, Y.; Yoshiya, I. Epidural Lidocaine Delays Arousal from Isoflurane Anesthesia. Anesth. Analg. 1994, 79, 368–372. [Google Scholar] [CrossRef]
- Brondani, J.T.; Luna, S.; Minto, B.W.; Santos, B.P.R. Alidity and Responsiveness of a Multidimensional Composite Scale to Assess Postoperative Pain in Cats (English Abstract). Arq. Bras. Med. Veterinária Zootec. 2012, 64, 1529–1538. [Google Scholar] [CrossRef]
- Benito-de-la-Víbora, J.; Lascelles, B.; García-Fernandez, P.; Freire, M.; Segura, I.A.G.D. Efficacy of Tolfenamic Acid and Meloxicam in the Control of Postoperative Pain Following Ovariohysterectomy in the Cat. Vet. Anaesth. Analg. 2008, 35, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.; Monteiro, B.; Lavoie, A.; Beauchamp, G.; Lascelles, B.D.X.; Steagall, P.V. Analgesic Efficacy of Intraperitoneal Administration of Bupivacaine in Cats. J. Feline Med. Surg. 2015, 18, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Yamagishi, N.; Oboshi, K.; Yamada, H. Distribution of New Methylene Blue Injected into the Lumbosacral Epidural Space in Cats. Vet. Anaesth. Analg. 2004, 31, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Son, W.; Jang, M.; Jo, S.; Yoon, J.; Lee, I. The volume effect of lidocaine on thoracic epidural anesthesia in conscious Beagle dogs. Vet. Anaesth. Analg. 2014, 42, 414–424. [Google Scholar] [CrossRef]
- Vesovski, S.; Makara, M.; Martinez-Taboada, F. Computer Tomographic Comparison of Cranial Spread of Contrast in Lumbosacral and Sacrococcygeal Epidural Injections in Dog Cadavers. Vet. Anaesth. Analg. 2019, 46, 510–515. [Google Scholar] [CrossRef]
- Romano, M.; Portela, D.; Breghi, G.; Otero, P.E. Stress-Related Biomarkers in Dogs Administered Regional Anaesthesia or Fentanyl for Analgesia during Stifle Surgery. Vet. Anaesth. Analg. 2016, 43, 44–54. [Google Scholar] [CrossRef]
- Sibanda, S.; Hughes, J.; Pawson, P.; Kelly, G.; Bellenger, C.R. The Effects of Preoperative Extradural Bupivacaine and Morphine on the Stress Response in Dogs Undergoing Femoro-Tibial Joint Surgery. Vet. Anaesth. Analg. 2006, 33, 246–257. [Google Scholar] [CrossRef]
- Gent, T.; Iff, I.; Bettschart-Wolfensberger, R.; Mosing, M. Neuraxial Morphine Induced Pruritus in Two Cats and Treatment with Sub Anaesthetic Doses of Propofol. Vet. Anaesth. Analg. 2013, 40, 517–520. [Google Scholar] [CrossRef]
- Troncy, E.; Junot, S.; Keroack, S.; Sammut, V.; Pibarot, P.; Genevois, J.-P.; Cuvelliez, S. Results of Preemptive Epidural Administration of Morphine with or without Bupivacaine in Dogs and Cats Undergoing Surgery: 265 Cases (1997–1999). J. Am. Vet. Med. Assoc. 2002, 221, 666–672. [Google Scholar] [CrossRef]
- Castro, D.S.; Silva, M.F.A.; Shih, A.C.; Motta, P.P.A.; Pires, M.V.M.; Scherer, P.O. Comparison between the Analgesic Effects of Morphine and Tramadol Delivered Epidurally in Cats Receiving a Standardized Noxious Stimulation. J. Feline Med. Surg. 2009, 11, 948–953. [Google Scholar] [CrossRef]
- Pypendop, B.H.; Siao, K.T.; Pascoe, P.J.; Ilkiw, J.E. Effects of Epidurally Administered Morphine or Buprenorphine on the Thermal Threshold in Cats. Am. J. Vet. Res. 2008, 69, 983–987. [Google Scholar] [CrossRef]
- Chadwick, H. Toxicity and Resuscitation in Lidocaineor Bupivacaine-Infused Cats. Anesthesiology 1985, 63, 385–390. [Google Scholar] [CrossRef]
- Lemke, K. Pain Management II: Local and Regional Anaesthetic Techniques. In BSAVA Manual of Canine and Feline Anaesthesia and Analgesia; Seymour, C., Duke-Nova Kovski, T., Eds.; British Small Animal Veterinary Association: Aberystwyth, UK, 2010; pp. 104–114. [Google Scholar]
- Duke-NovaKovski, T. Pain Management II: Local and Regional Anaesthetic Techniques. In BSAVA Manual of Canine and Feline Anaesthesia and Analgesia; Duke-Novakovski, T., Vries, M., Seymour, C., Eds.; British Small Animal Veterinary Association: Aberystwyth, UK, 2016; pp. 143–158. [Google Scholar]




| VARIABLE | CTR | SCC-E | p-Value |
|---|---|---|---|
| Age (months, median (IQR)) | 11.5 (6.25) | 12.0 (20.0) | 0.393 |
| Weight (kg, mean ± SD) | 3.0 ± 0.5 | 3.1 ± 0.5 | 0.621 |
| Body condition (1–9, median (IQR)) | 6.0 (2.0) | 6.0 (1.0) | 0.353 |
| Duration of the anaesthesia (minutes, median (IQR)) | 40.5 (6.5) | 44.5 (4.8) | 0.063 |
| Duration of the surgery (minutes, median (IQR)) | 14.5 (3.8) | 16.0 (4.3) | 0.315 |
| Time to extubation (minutes, median (IQR)) | 7.5 (8.5) | 8.5 (24.0) | 0.393 |
| Time to sternal recumbency (minutes, median (IQR) | 2.0 (11.0) | 11.5 (34.8) | 0.143 |
| Number of Analgesic Rescues | Animals with Rescues | Total Amount of Methadone (μg): Mean ± SD | |||||
|---|---|---|---|---|---|---|---|
| TOPEN | TOV1 | TOV2 | TUTERUS | TCLOSE | |||
| CTR | 0 | 8 | 8 | 8 | 5 | 10/10 (100%) | 18.7 ± 3.7 a |
| SCC-E | 1 | 0 | 2 | 3 | 1 | 4/10 (40%) | 4.9 ± 2.5 |
| Variable | Group | TBASELINE | TOPEN | TOV1 | TOV2 | TUTERUS | TCLOSE |
|---|---|---|---|---|---|---|---|
| RR (breaths minute−1 ± SD) | SCC-E | 23 ± 6 | 22 ± 6 | 22 ± 6 | 21 ± 5 | 19 ± 7 | 20 ± 8 |
| CTR | 29 ± 6 | 28 ± 6 | 26 ± 6 | 23 ± 5 | 23 ± 7 | 23 ± 8 | |
| ETCO2 (mmHg ± SD) | SCC-E | 41 ± 5 | 40 ± 5 | 41 ± 5 | 39 ± 5 | 40 ± 7 | 40 ± 6 |
| CTR | 39 ± 4 | 36 ± 6 | 36 ± 5 | 36 ± 6 | 34 ± 9 | 36 ± 5 | |
| TEMP (C° ± SD) | SCC-E | 36.8 ± 0.6 | 36.9 ± 0.6 | 36.8 ± 0.6 | 36.7 ± 0.6 | 36.6 ± 0.6 | 36.5 ± 0.6 |
| CTR | 36.7 ± 0.3 | 36.8 ± 0.4 | 36.6 ± 0.5 | 36.4 ± 0.4 | 36.3 ± 0.3 | 35.9 ± 1.2 | |
| SEVO (% ± SD) | SCC-E | 2 ± 0 | 2 ± 0.5 | 2 ± 0 | 2 ± 0 | 2 ± 0.5 | 1.5 ± 0.5 |
| CTR | 2 ± 0 | 2 ± 0.5 | 2 ± 1 | 2 ± 0.5 | 2 ± 0 | 2 ± 0.5 |
| Score | Recovery Quality | CTR | % | SCC-E | % |
|---|---|---|---|---|---|
| 1 | Poor: Evident signs of excitement during recovery to sternal recumbency such as sudden movements around and lack of awareness of the surrounding environment; growl that does not respond to the animal’s touch. | 0/10 | 0 | 0/10 | 0 |
| 2 | Moderate: slight signs of excitement during recovery to sternal recumbency such as sudden movements around and lack of awareness of the surrounding environment; growling that does respond to the animal’s touch. | 1/10 | 10 | 1/10 | 10 |
| 3 | Good: mild signs of excitement that resolve quickly and the animal becomes calm during recovery to sternal recumbency. | 5/10 | 50 | 1/10 | 10 |
| 4 | Excellent: calm and relaxed animal during recovery to sternal recumbency. | 4/10 | 40 | 8/10 | 80 |
| T1 | T2 | T3 | T4 | T6 | T8 | Total Number of Rescue Animals | Total Amount of Methadone (mg) | |
|---|---|---|---|---|---|---|---|---|
| CTR | 5 | 3 | 3 | 1 | 2 | 0 | 7/10 (70%) | 7.4 |
| SCC-E | 2 | 0 | 0 | 1 | 0 | 0 | 3/10 (30%) | 1.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dourado, A.; Gomes, A.; Teixeira, P.; Lobo, L.; Azevedo, J.T.; Dias, I.R.; Pinelas, R. Antinociceptive Effect of a Sacro-Coccygeal Epidural of Morphine and Lidocaine in Cats Undergoing Ovariohysterectomy. Vet. Sci. 2022, 9, 623. https://doi.org/10.3390/vetsci9110623
Dourado A, Gomes A, Teixeira P, Lobo L, Azevedo JT, Dias IR, Pinelas R. Antinociceptive Effect of a Sacro-Coccygeal Epidural of Morphine and Lidocaine in Cats Undergoing Ovariohysterectomy. Veterinary Sciences. 2022; 9(11):623. https://doi.org/10.3390/vetsci9110623
Chicago/Turabian StyleDourado, Amândio, Anabela Gomes, Paulo Teixeira, Luís Lobo, Jorge T. Azevedo, Isabel R. Dias, and Rui Pinelas. 2022. "Antinociceptive Effect of a Sacro-Coccygeal Epidural of Morphine and Lidocaine in Cats Undergoing Ovariohysterectomy" Veterinary Sciences 9, no. 11: 623. https://doi.org/10.3390/vetsci9110623
APA StyleDourado, A., Gomes, A., Teixeira, P., Lobo, L., Azevedo, J. T., Dias, I. R., & Pinelas, R. (2022). Antinociceptive Effect of a Sacro-Coccygeal Epidural of Morphine and Lidocaine in Cats Undergoing Ovariohysterectomy. Veterinary Sciences, 9(11), 623. https://doi.org/10.3390/vetsci9110623






