The Pivotal Role of Stem Cells in Veterinary Regenerative Medicine and Tissue Engineering
Abstract
:Simple Summary
Abstract
1. Introduction
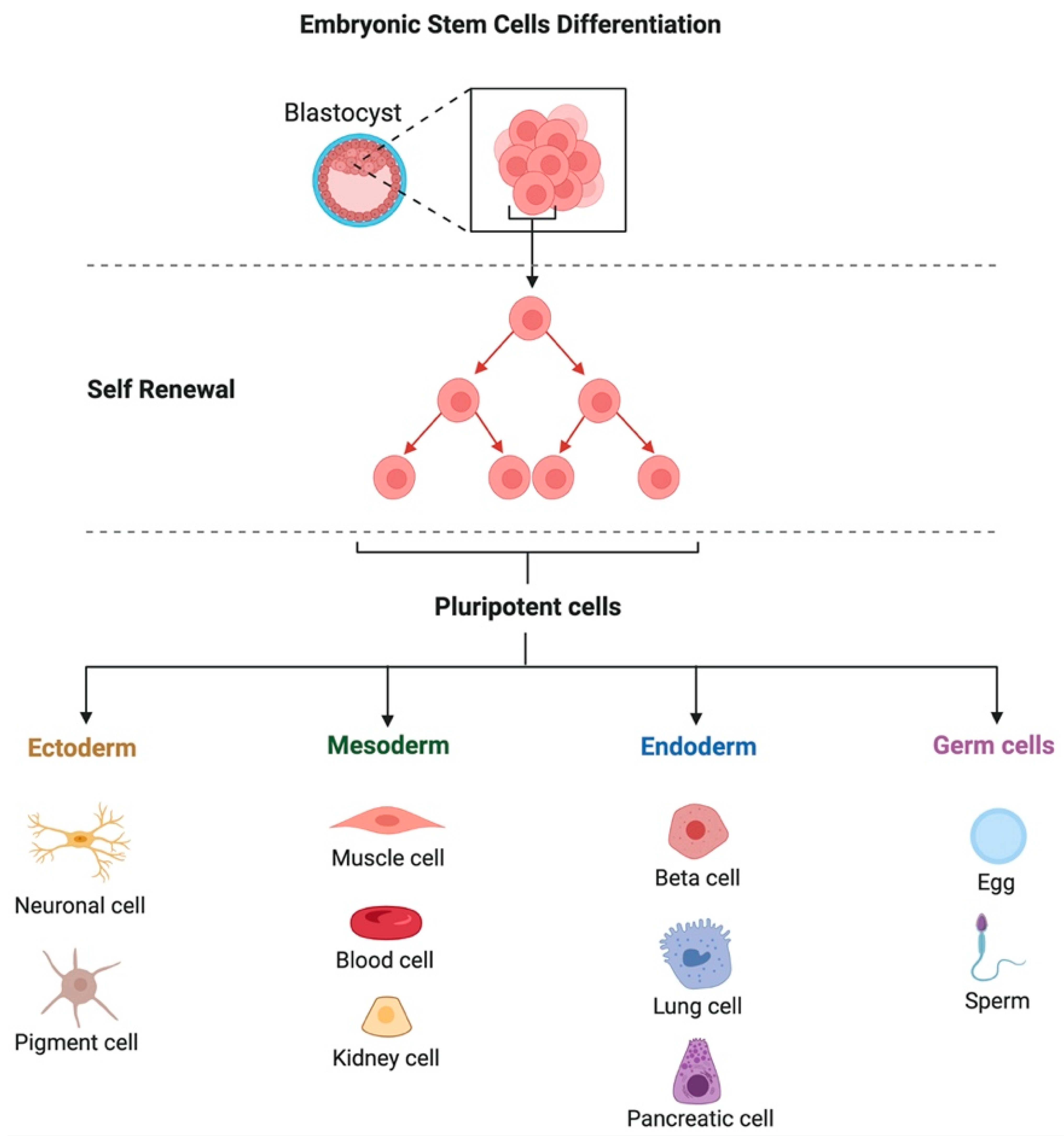
2. Stem Cells Classification
2.1. Differentiation Potential
2.2. Origin of Stem Cells
2.3. Relationship to the Recipient
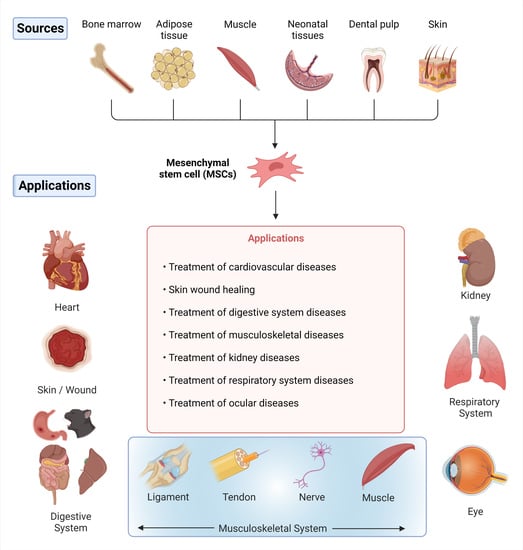
3. Sources of MSCs in Tissue
4. Autogenous Allogeneous, and Xenogenic MSCs
5. Immunomodulatory Potential of MSCs
6. Clinical Applications of Stem Cells in Regeneration and Bioengineering of Different Body Organs and Tissues in Veterinary Practice
6.1. Heart
| Body Systems and Tissues | The Type of Cells Used | Animal Model | Route of Administration | Outcomes | Refs. | |
|---|---|---|---|---|---|---|
| Heart | Adipose-derived mesenchymal stem cells (AD-MSCs) | Dobermann dogs with dilated cardiomyopathy | Retrograde coronary venous delivery | The stem cell therapy was safe. However, it did not prevent the progression of the disease | [82] | |
| Allogenic Cardiosphere-derived cells (CDCs) | Doberman pinscher dogs with spontaneous DCM | Intracoronary infusion | Safety was confirmed with an effective improvement of the heart functions | [83] | ||
| Allogenic puppy deciduous teeth stem cells (PDSCs) | Dogs with chronic valvular heart disease | Intravenous injection | Amelioration of the cardiac functions and improvement of the quality of the life scores | [84] | ||
| Skin | Caprine amniotic fluid and bone marrow-derived mesenchymal stem cells | New Zealand white rabbits | Subcutaneous injection | Amniotic fluid-derived cells were superior to bone marrow-derived cells for enhancement of skin wound healing | [85] | |
| Peripheral blood-derived MSCs | Sheep | Subcutaneous injection in the margins of the skin wound | Improvement of both superficial and deep wound healing | [86] | ||
| Umbilical cord-blood-derived equine MSCs | Horses | Subcutaneous injection in the margins of the skin wound | Stem cell therapy is a promising choice for the treatment of distal extremity wounds in horses | [87] | ||
| Digestive System | Mouth and teeth | Adipose-derived multi-lineage progenitor cells (ADMPC) | Micro-mini pig | Topical transplantation of autologous or allogeneic ADMPC-fibrin gel complex | ADMPC presented an immune-modulation action and enhanced periodontal tissue regeneration | [88] |
| Dental pulp stem cells (DPSCs) | Mongrel dogs | Direct pulp capping method | DPSCs have exhibited a promising capacity for regeneration of the damaged dentin. | [89] | ||
| Mobilized dental pulp stem cells (MDPSCs) isolated from the abdominal subcutaneous adipose tissue | Dogs | Topical transplantation in the pulpectomized dogs | Safety was confirmed with the enhancement of total pulp regeneration | [90] | ||
| Gastrointestinal tract | Adipose tissue-derived MSCs (AD-MSCs) | Dogs with inflammatory bowel disease (IBD) | Intravascular (IV) infusion | IV infusion of AD-MSCs was safe and effective in dogs with IBD | [91] | |
| Adipose-derived feline mesenchymal stem cells (fMSC) | Cats with chronic enteropathy | Intravenous injection | fMSCs were safe and effective for the treatment of cats with chronic enteropathy | [92] | ||
| Liver | Canine adipose-derived mesenchymal stem cells (cADSCs) | Dogs with induced acute liver injury | Intraperitoneal injection | cADSCs played an important role in the regeneration of canine liver | [93] | |
| Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Dogs with induced liver fibrosis | Intravenous infusion | Improvement of liver functions with no adverse effects | [94] | ||
| Musculoskeletal system | Tendons and ligament | Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Horses with tendon or ligament injuries | Topical intralesional injection | BM-MSCs were safe and successfully regenerated equine tendons and ligaments | [95] |
| Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Polo with an injured superficial digital flexor tendon | Topical injection at the lesion site | Improvement of the regeneration capacity of the injured tendon | [96] | ||
| Joints | Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Horses with a stifle injury | Intra-articular administration | Improvement of the stifle injury with enhanced ability of the animals to return to work | [97] | |
| Adipose-derived MSCs (AD-MSCs) | Horses with osteoarthritis | Intra-articular administration | AD-MSCs were efficient and safe | [98] | ||
| Muscles and nerves | Neurogenically-induced bone marrow-derived mesenchymal stem cells (NIBM-MSCs) | Dogs with paraplegia | Percutaneous transplantation | NIBM-MSC therapy was a promising option for the treatment of spinal cord injuries | [99] | |
| Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Dogs with paraplegia | Intralesional injection | Enhanced regeneration of the injured spinal cord | [100] | ||
| Kidneys | Bone marrow-derived or adipose tissue-derived MSCs (BM-MSCs or AD-MSCs) | Cats with chronic kidney disease (CKD) | Ultrasound-guided intrarenal injection | Improved kidney regeneration and function | [101] | |
| Amniotic membrane-derived MSCs (AMSCs) | Cats with chronic kidney disease (CKD) | Ultrasound-guided intrarenal injection and intravenous infusion | AMSCs exhibited a renoprotective effect and enhanced kidney function | [102] | ||
| Respiratory system | Bone marrow-derived mononuclear cells (BMMCs) | Horses with recurrent airway obstruction (RAO) | Intratracheal instillation | BMMCs could reduce inflammatory reactions | [103] | |
| Human umbilical cord-derived mesenchymal stem cells (MSCs) | Dogs with radiation-induced lung injury | Intratracheal transplantation | Reduced lung injury and declined inflammation | [104] | ||
| Eye | Bone marrow-derived mesenchymal stem cells (BM-MSCs) | Horses with unilateral immune-mediated keratitis (IMMK) | Subconjunctival injection | Improved corneal clarity with improved regeneration of the corneal tissue | [105] | |
| Feline adipose-derived mesenchymal stromal cells (fAd-MSCs) | Cats with feline eosinophilic keratitis (FEK) | Subconjunctival injection | fAd-MSCs were safe and effective for the treatment of FEK | [106] | ||
6.2. Skin
6.3. Digestive System
6.3.1. Mouth and Teeth
6.3.2. Gastrointestinal Tract
6.3.3. Liver
6.4. Musculoskeletal System
6.4.1. Tendons and Ligaments
6.4.2. Joints
6.4.3. Muscles and Nerves
6.5. Kidneys
6.6. Respiratory System
6.7. Eye
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- El-Husseiny, H.M. Evaluation of Some Prosthetic Implants for Surgical Management of Different Varieties of Hernias in Domestic Animals. Master’s Thesis, Faculty of Veterinary Medicine, Benha University, Benha, Egypt, 2017; pp. 42–43. [Google Scholar]
- El-Husseiny, H.M.; El-Maghraby, H.M.; Alakraa, A.M.; Kandiel, M.M.M. Platelet Rich Fibrin Augmented Versus Non-Augmented Glycerolized Bovine Pericardium and Polypropylene Mesh for Repairing of Large Abdominal Wall Defects. Eur. J. Med. Nat. Sci. 2019, 3, 33–48. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Doghish, A.S.; Tanaka, R. Stimuli-Responsive Hydrogels: Smart State of-the-Art Platforms for Cardiac Tissue Engineering. 2022. Available online: https://www.researchsquare.com/article/rs-2011475/v1 (accessed on 10 September 2022). [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Zewail, M.B.; Noshy, M.; Abdelfatah, A.M.; Doghish, A.S. Smart/stimuli-responsive hydrogels: State-of-the-art platforms for bone tissue engineering. Appl. Mater. Today 2022, 101560. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Abd Elkodous, M.; El-Husseiny, H.M.; El-Sayyad, G.S.; Hashem, A.H.; Doghish, A.S.; Elfadil, D.; Radwan, Y.; El-Zeiny, H.M.; Bedair, H.; Ikhdair, O.A.; et al. Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth. Nanotechnol. Rev. 2021, 10, 1662–1739. [Google Scholar] [CrossRef]
- Dziubińska, P.; Jaskólska, M.; Przyborowska, P.; Adamiak, Z. Stem cells in dentistry—Review of literature. Pol. J. Vet. Sci. 2013, 16, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Hendawy, H.; Uemura, A.; Ma, D.; Namiki, R.; Samir, H.; Ahmed, M.F.; Elfadadny, A.; El-Husseiny, H.M.; Chieh-Jen, C.; Tanaka, R. Tissue Harvesting Site Effect on the Canine Adipose Stromal Vascular Fraction Quantity and Quality. Animals 2021, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Zuba-Surma, E.; Ratajczak, J. Stem Cell Therapeutics—Hope and Concerns. Acta Haematol. Pol. 2009, 40, 289–303. [Google Scholar]
- Yamanaka, S. Induced Pluripotent Stem Cells: Past, Present, and Future. Cell Stem Cell 2012, 10, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Jayasuriya, C.T.; Hu, N.; Li, J.; Lemme, N.; Terek, R.; Ehrlich, M.G.; Chen, Q. Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Sci. Rep. 2018, 8, 7044. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Smith, R.K.W. Clinical update on the use of mesenchymal stem cells in equine orthopaedics. Equine Vet. J. 2010, 42, 86–89. [Google Scholar] [CrossRef]
- Chopra, H.; Kumar Hans, M.; Shetty, S. Stem cells-the hidden treasure: A strategic review. Dent. Res. J. 2013, 10, 421–427. [Google Scholar]
- Krampera, M.; Franchini, M.; Pizzolo, G.; Aprili, G. Mesenchymal stem cells: From biology to clinical use. Blood Transfus. 2007, 5, 120–129. [Google Scholar] [CrossRef]
- Emura, M. Stem cells of the respiratory epithelium and their in vitro cultivation. Vitr. Cell. Dev. Biol.-Anim. 1997, 33, 3–14. [Google Scholar] [CrossRef]
- Kolios, G.; Moodley, Y. Introduction to Stem Cells and Regenerative Medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, M.P.; Fuentes-Julián, S.; Alcaina, Y. Pluripotent Stem Cells: Origin, Maintenance and Induction. Stem Cell Rev. Rep. 2010, 6, 633–649. [Google Scholar] [CrossRef]
- Guercio, A.; Di Marco, P.; Casella, S.; Cannella, V.; Russotto, L.; Purpari, G.; Di Bella, S.; Piccione, G. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol. Int. 2012, 36, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Nantavisai, S.; Egusa, H.; Osathanon, T.; Sawangmake, C. Mesenchymal stem cell-based bone tissue engineering for veterinary practice. Heliyon 2019, 5, e02808. [Google Scholar] [CrossRef]
- Augello, A.; Kurth, T.B.; De Bari, C. Mesenchymal stem cells: A perspective from in vitro cultures to in vivo migration and niches. Eur. Cells Mater. 2010, 20, e33. [Google Scholar] [CrossRef] [PubMed]
- Majo, F.; Rochat, A.; Nicolas, M.; Jaoudé, G.A.; Barrandon, Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 2008, 456, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Burdzinska, A.; Idziak, M. Stem Cells in Veterinary Medicine—Facts and Myths. Mag. Weter 2013, 7, 695–704. [Google Scholar]
- Voga, M.; Adamic, N.; Vengust, M.; Majdic, G. Stem Cells in Veterinary Medicine—Current State and Treatment Options. Front. Vet. Sci. 2020, 7, 278. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-S.; Lin, G.; Lue, T.F. Allogeneic and Xenogeneic Transplantation of Adipose-Derived Stem Cells in Immunocompetent Recipients Without Immunosuppressants. Stem Cells Dev. 2012, 21, 2770–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arzi, B.; Peralta, S.; Fiani, N.; Vapniarsky, N.; Taechangam, N.; Delatorre, U.; Clark, K.C.; Walker, N.J.; Loscar, M.R.; Lommer, M.J.; et al. A multicenter experience using adipose-derived mesenchymal stem cell therapy for cats with chronic, non-responsive gingivostomatitis. Stem Cell Res. Ther. 2020, 11, 115. [Google Scholar] [CrossRef]
- Kalisiak, O.; Cegielski, M. Stem cells in treatment of tendon disorders in horses. Życie Weter. 2013, 88, 112–114. [Google Scholar]
- Cegielski, M.; Kalisiak, O. Stem Cells Therapy—A Hope For the Treatment of Tendons in Horses. Życie Weter. 2008, 83, 754–756. [Google Scholar]
- Cegielski, M.; Izykowska, I.; Chmielewska, M.; Wojciech, D.; Bochnia, M.; Calkonski, I.; Dziegiel, P. Characteristics of MIC-1 Antlerogenic Stem Cells and Their Effect on Hair Growth in Rabbits. Vivo 2013, 27, 97–106. [Google Scholar]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bearden, R.N.; Huggins, S.S.; Cummings, K.J.; Smith, R.; Gregory, C.A.; Saunders, W.B. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: A donor-matched comparative study. Stem Cell Res. Ther. 2017, 8, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godwin, E.E.; Young, N.J.; Dudhia, J.; Beamish, I.C.; Smith, R.K.W. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 2012, 44, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.E.; Watts, A.E. Biopsy Needle Advancement during Bone Marrow Aspiration Increases Mesenchymal Stem Cell Concentration. Front. Vet. Sci. 2016, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, R.M.; Kociba, G.J.; Ruoff, W.W. Monoclonal Gammopathy in a Horse with Defective Hemostasis. Vet. Pathol. 1983, 20, 643–647. [Google Scholar] [CrossRef]
- Durando, M.M.; Zarucco, L.; Schaer, T.P.; Ross, M.; Reef, V.B. Pneumopericardium in a horse secondary to sternal bone marrow aspiration. Equine Vet. Educ. 2006, 18, 75–79. [Google Scholar] [CrossRef]
- Désévaux, C.; Laverty, S.; Doizé, B. Sternal bone biopsy in standing horses. Vet. Surg. 2000, 29, 303–308. [Google Scholar]
- Kasashima, Y.; Ueno, T.; Tomita, A.; Goodship, A.E.; Smith, R.K.W. Optimisation of bone marrow aspiration from the equine sternum for the safe recovery of mesenchymal stem cells. Equine Vet. J. 2011, 43, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Rebelatto, C.K.; Aguiar, A.M.; Moretão, M.P.; Senegaglia, A.C.; Hansen, P.; Barchiki, F.; Oliveira, J.; Martins, J.; Kuligovski, C.; Mansur, F.; et al. Dissimilar Differentiation of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, and Adipose Tissue. Exp. Biol. Med. 2008, 233, 901–913. [Google Scholar] [CrossRef]
- Orbay, H.; Tobita, M.; Mizuno, H. Mesenchymal Stem Cells Isolated from Adipose and Other Tissues: Basic Biological Properties and Clinical Applications. Stem Cells Int. 2012, 2012, 461718. [Google Scholar] [CrossRef] [Green Version]
- Webb, T.L.; Quimby, J.M.; Dow, S.W. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J. Feline Med. Surg. 2012, 14, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, B.; Rubio, M.; Sopena, J.; Dominguez, J.M.; Vilar, J.; Morales, M.; Cugat, R.; Carrillo, J.M. Hip Osteoarthritis in Dogs: A Randomized Study Using Mesenchymal Stem Cells from Adipose Tissue and Plasma Rich in Growth Factors. Int. J. Mol. Sci. 2014, 15, 13437–13460. [Google Scholar] [CrossRef] [PubMed]
- Vilar, J.M.; Morales, M.; Santana, A.; Spinella, G.; Rubio, M.; Cuervo, B.; Cugat, R.; Carrillo, J.M. Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet. Res. 2013, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koerner, J.; Nesic, D.; Romero, J.D.; Brehm, W.; Mainil-Varlet, P.; Grogan, S.P. Equine Peripheral Blood-Derived Progenitors in Comparison to Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells 2006, 24, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Dhar, M.; Neilsen, N.; Beatty, K.; Eaker, S.; Adair, H.; Geiser, D. Equine peripheral blood-derived mesenchymal stem cells: Isolation, identification, trilineage differentiation and effect of hyperbaric oxygen treatment. Equine Vet. J. 2012, 44, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.H.; Schauwer, C.D.; Cornillie, P.; Meyer, E.; Soom, A.V.; Van de Walle, G.R. Culture and characterisation of equine peripheral blood mesenchymal stromal cells. Vet. J. 2013, 195, 107–113. [Google Scholar] [CrossRef]
- Chen, M.Y.; Lie, P.C.; Li, Z.L.; Wei, X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp. Hematol 2009, 37, 629–640. [Google Scholar] [CrossRef]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.J.S.; Claas, F.H.J.; Fibbe, W.E.; Kanhai, H.H.H. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Horse Embryonic Stem Cell Lines from the Proliferation of Inner Cell Mass Cells. Stem Cells Dev. 2006, 15, 523–531. [CrossRef]
- Passeri, S.; Nocchi, F.; Lamanna, R.; Lapi, S.; Miragliotta, V.; Giannessi, E.; Abramo, F.; Stornelli, M.R.; Matarazzo, M.; Plenteda, D.; et al. Isolation and expansion of equine umbilical cord-derived matrix cells (EUCMCs). Cell Biol. Int. 2009, 33, 100–105. [Google Scholar] [CrossRef]
- Prządka, P.; Kiełbowicz, Z.; Osiński, B.; Dzimira, S.; Madej, J.A.; Nowacki, W.; Kubiak, K.; Reichert, P.; Cegielski, M. Reconstruction of cranial cruciate ligament in rabbits using polyester implants saturated with PRP, antlerogenic stem cells MIC-1 and their homogenate. Connect. Tissue Res. 2017, 58, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Penha, E.M.; Meira, C.S.; Guimarães, E.T.; Mendonça, M.V.P.; Gravely, F.A.; Pinheiro, C.M.B.; Pinheiro, T.M.B.; Barrouin-Melo, S.M.; Ribeiro-dos-Santos, R.; Soares, M.B.P. Use of Autologous Mesenchymal Stem Cells Derived from Bone Marrow for the Treatment of Naturally Injured Spinal Cord in Dogs. Stem Cells Int. 2014, 2014, 437521. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Lu, A.; Gao, H.; Qian, C.; Zhang, J.; Lin, T.; Zhao, Y. Safety of Allogeneic Umbilical Cord Blood Stem Cells Therapy in Patients with Severe Cerebral Palsy: A Retrospective Study. Stem Cells Int. 2015, 2015, 325652. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Kim, W.; Lim, C.; Chung, S.G. Treatment of Lateral Epicondylosis by Using Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Pilot Study. Stem Cells 2015, 33, 2995–3005. [Google Scholar] [CrossRef]
- Sullivan, M.J. Banking on cord blood stem cells. Nat. Rev. Cancer 2008, 8, 555–563. [Google Scholar] [CrossRef]
- Bertoni, L.; Branly, T.; Jacquet, S.; Desancé, M.; Desquilbet, L.; Rivory, P.; Hartmann, D.-J.; Denoix, J.-M.; Audigié, F.; Galéra, P.; et al. Intra-Articular Injection of 2 Different Dosages of Autologous and Allogeneic Bone Marrow- and Umbilical Cord-Derived Mesenchymal Stem Cells Triggers a Variable Inflammatory Response of the Fetlock Joint on 12 Sound Experimental Horses. Stem Cells Int. 2019, 2019, 9431894. [Google Scholar] [CrossRef]
- Prządka, P.; Buczak, K.; Frejlich, E.; Gąsior, L.; Suliga, K.; Kiełbowicz, Z. The Role of Mesenchymal Stem Cells (MSCs) in Veterinary Medicine and Their Use in Musculoskeletal Disorders. Biomolecules 2021, 11, 1141. [Google Scholar] [CrossRef]
- Devine, S.M.; Bartholomew, A.M.; Mahmud, N.; Nelson, M.; Patil, S.; Hardy, W.; Sturgeon, C.; Hewett, T.; Chung, T.; Stock, W.; et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp. Hematol. 2001, 29, 244–255. [Google Scholar] [CrossRef]
- Saito, T.; Kuang, J.-Q.; Bittira, B.; Al-Khaldi, A.; Chiu, R.C.J. Xenotransplant cardiac chimera: Immune tolerance of adult stem cells. Ann. Thorac. Surg. 2002, 74, 19–24. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, J.-B.; Lee, J.K.; Park, E.-Y.; Park, E.-A.; Riew, K.D.; Rhee, S.-K. Transplanted xenogenic bone marrow stem cells survive and generate new bone formation in the posterolateral lumbar spine of non-immunosuppressed rabbits. Eur. Spine J. 2008, 17, 1515–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, R.; Gopinath, C.; Rao, N.M.; Banerjee, P.; Krishnamoorthy, V.; Venkataramana, N.K.; Totey, S. Functional recovery after transplantation of bone marrow-derived human mesenchymal stromal cells in a rat model of spinal cord injury. Cytotherapy 2010, 12, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Yao, W.; Liu, R.; Lam, K.S.; Nolta, J.; Jia, J.; Panganiban, B.; Meng, L.; Zhou, P.; Shahnazari, M.; et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat. Med. 2012, 18, 456–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Opiela, J.; Samiec, M. Characterization of mesenchymal stem cells and their application in experimental embryology. Pol. J. Vet. Sci. 2013, 16, 593–599. [Google Scholar] [CrossRef] [Green Version]
- de Vasconcellos Machado, C.; da Silva Telles, P.D.; Nascimento, I.L.O. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. E Hemoter. 2013, 35, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortier, L.A. Stem Cells: Classifications, Controversies, and Clinical Applications. Vet. Surg. 2005, 34, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through “pharmaceuticalization” for the best formulation. J. Control. Release 2019, 309, 11–24. [Google Scholar] [CrossRef]
- El-Husseiny, H.; Mady, E.; Shimada, K.; Hamabe, L.; Yoshida, T.; Ma, D.; Mandour, A.; Hendawy, H.; Sasaki, K.; Fukuzumi, S.; et al. Intraventricular pressure gradient: A promising tool to predict the post-infarction chronic congestive heart failure in rats. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, jeab289.390. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Ma, D.; Hamabe, L.; Takahashi, K.; Tanaka, R. Intraventricular pressure gradient: A novel tool to assess the post-infarction chronic congestive heart failure. Front. Cardiovasc. Med. 2022, 9, 944717. [Google Scholar] [CrossRef]
- Ma, D.; Mandour, A.S.; Elfadadny, A.; Hendawy, H.; Yoshida, T.; El-Husseiny, H.M.; Nishifuji, K.; Takahashi, K.; Zhao, Y.; Zhou, Z. Changes in cardiac function during the development of uremic cardiomyopathy and the effect of Salvianolic acid B administration in a rat model. Front. Vet. Sci. 2022, 734, 905759. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Mandour, A.S.; Hendawy, H.; Yoshida, T.; El-Husseiny, H.M.; Ozai, Y.; Takeuchi, A.; Takahashi, K.; Uemura, A.; Tanaka, R. Renovascular Hypertension-Induced Cardiac Changes in a Rat Model: Feasibility Of Conventional And Recent Echocardiography. J. Hypertens. 2021, 39, e403–e404. [Google Scholar] [CrossRef]
- Mandour, A.S.; Samir, H.; Yoshida, T.; Matsuura, K.; Abdelmageed, H.A.; Elbadawy, M.; Al-Rejaie, S.; El-Husseiny, H.M.; Elfadadny, A.; Ma, D.; et al. Assessment of the Cardiac Functions Using Full Conventional Echocardiography with Tissue Doppler Imaging before and after Xylazine Sedation in Male Shiba Goats. Animals 2020, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ma, D.; Mandour, A.S.; Ozai, Y.; Yoshida, T.; Matsuura, K.; Takeuchi, A.; Cheng, C.-J.; El-Husseiny, H.M.; Hendawy, H.; et al. Evaluation of Changes in the Cardiac Function before and after Transcatheter Edge-to-Edge Mitral Valve Repair in Healthy Dogs: Conventional and Novel Echocardiography. Animals 2022, 12, 56. [Google Scholar] [CrossRef]
- Yairo, A.; Mandour, A.S.; Matsuura, K.; Yoshida, T.; Ma, D.; Kitpipatkun, P.; Kato, K.; Cheng, C.-J.; El-Husseiny, H.M.; Tanaka, T.; et al. Effect of Loading Changes on the Intraventricular Pressure Measured by Color M-Mode Echocardiography in Rats. Diagnostics 2021, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Mandour, A.S.; Matsuura, K.; Shimada, K.; El-Husseiny, H.M.; Hamabe, L.; Yilmaz, Z.; Uemura, A.; Tanaka, R. Changes in the Pulmonary Artery Wave Reflection in Dogs with Experimentally-Induced Acute Pulmonary Embolism and the Effect of Vasodilator. Animals 2021, 11, 1977. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsuura, K.; Chieh-Jen, C.; Aboshi, Y.; Yamada, S.; Yotsuida, H.; Hasegawa, M.; Hendawy, H.A.; El-Husseiny, H.M.; Takahashi, Y.; et al. Surgical treatment for left atrial rupture due to myxomatous mitral valve disease in three dogs: A case report. Vet. Med. Sci. 2022, 1–7. [Google Scholar] [CrossRef]
- Hamabe, L.; Shimada, K.; Mandour, A.S.; Yoshida, T.; Hirose, M.; Hendawy, H.; El-Husseiny, H.M.; Tanaka, R. Evaluation of Left Ventricular Function in Healthy Retrievers Using Standard and 2D Speckle-Tracking Echocardiography. Vet. Sci. 2022, 9, 529. [Google Scholar] [CrossRef]
- Pogue, B.; Estrada, A.H.; Sosa-Samper, I.; Maisenbacher, H.W.; Lamb, K.E.; Mincey, B.D.; Erger, K.E.; Conlon, T.J. Stem-cell therapy for dilated cardiomyopathy: A pilot study evaluating retrograde coronary venous delivery. J. Small Anim. Pract. 2013, 54, 361–366. [Google Scholar] [CrossRef]
- Hensley, M.T.; Tang, J.; Woodruff, K.; Defrancesco, T.; Tou, S.; Williams, C.M.; Breen, M.; Meurs, K.; Keene, B.; Cheng, K. Intracoronary allogeneic cardiosphere-derived stem cells are safe for use in dogs with dilated cardiomyopathy. J. Cell. Mol. Med. 2017, 21, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Petchdee, S.; Sompeewong, S. Intravenous administration of puppy deciduous teeth stem cells in degenerative valve disease. Vet. World 2016, 9, 1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratheesh, M.D.; Dubey, P.K.; Gade, N.E.; Nath, A.; Sivanarayanan, T.B.; Madhu, D.N.; Somal, A.; Baiju, I.; Sreekumar, T.R.; Gleeja, V.L.; et al. Comparative study on characterization and wound healing potential of goat (Capra hircus) mesenchymal stem cells derived from fetal origin amniotic fluid and adult bone marrow. Res. Vet. Sci. 2017, 112, 81–88. [Google Scholar] [CrossRef]
- Martinello, T.; Gomiero, C.; Perazzi, A.; Iacopetti, I.; Gemignani, F.; DeBenedictis, G.M.; Ferro, S.; Zuin, M.; Martines, E.; Brun, P.; et al. Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Vet. Res. 2018, 14, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Textor, J.A.; Clark, K.C.; Walker, N.J.; Aristizobal, F.A.; Kol, A.; LeJeune, S.S.; Bledsoe, A.; Davidyan, A.; Gray, S.N.; Bohannon-Worsley, L.K.; et al. Allogeneic Stem Cells Alter Gene Expression and Improve Healing of Distal Limb Wounds in Horses. Stem Cells Transl. Med. 2017, 7, 98–108. [Google Scholar] [CrossRef]
- Venkataiah, V.S.; Handa, K.; Njuguna, M.M.; Hasegawa, T.; Maruyama, K.; Nemoto, E.; Yamada, S.; Sugawara, S.; Lu, L.; Takedachi, M.; et al. Periodontal Regeneration by Allogeneic Transplantation of Adipose Tissue Derived Multi-Lineage Progenitor Stem Cells in vivo. Sci. Rep. 2019, 9, 921. [Google Scholar] [CrossRef]
- Abdelaz, P.; ElZoghbi, A.; Shokry, M.; Ahmed, A.-Z.; Rasha, H. Reparative dentin formation using stem cell therapy versus calcium hydroxide in direct pulp capping: An animal study. Braz. Dent. J. 2019, 30, 542–549. [Google Scholar] [CrossRef]
- Iohara, K.; Utsunomiya, S.; Kohara, S.; Nakashima, M. Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Res. Ther. 2018, 9, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Duque-Carrasco, J.; Zaragoza-Bayle, C.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Vilafranca-Compte, M.; Barrera-Chacón, R.; Gualtieri, M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: Endoscopic and histological outcomes. Vet. J. 2015, 206, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.L.; Webb, C.B. Stem cell therapy in cats with chronic enteropathy: A proof-of-concept study. J. Feline Med. Surg. 2015, 17, 901–908. [Google Scholar] [CrossRef]
- Yan, Y.; Fang, J.; Wen, X.; Teng, X.; Li, B.; Zhou, Z.; Peng, S.; Arisha, A.H.; Liu, W.; Hua, J. Therapeutic applications of adipose-derived mesenchymal stem cells on acute liver injury in canines. Res. Vet. Sci. 2019, 126, 233–239. [Google Scholar] [CrossRef]
- Matsuda, T.; Takami, T.; Sasaki, R.; Nishimura, T.; Aibe, Y.; Paredes, B.D.; Quintanilha, L.F.; Matsumoto, T.; Ishikawa, T.; Yamamoto, N.; et al. A canine liver fibrosis model to develop a therapy for liver cirrhosis using cultured bone marrow–derived cells. Hepatol. Commun. 2017, 1, 691–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renzi, S.; Riccò, S.; Dotti, S.; Sesso, L.; Grolli, S.; Cornali, M.; Carlin, S.; Patruno, M.; Cinotti, S.; Ferrari, M. Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: A clinical report. Res. Vet. Sci. 2013, 95, 272–277. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Korda, M.; Blunn, G.W.; Goodship, A.E. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet. J. 2003, 35, 99–102. [Google Scholar] [CrossRef]
- Ferris, D.J.; Frisbie, D.D.; Kisiday, J.D.; McIlwraith, C.W.; Hague, B.A.; Major, M.D.; Schneider, R.K.; Zubrod, C.J.; Kawcak, C.E.; Goodrich, L.R. Clinical Outcome After Intra-Articular Administration of Bone Marrow Derived Mesenchymal Stem Cells in 33 Horses With Stifle Injury. Vet. Surg. 2014, 43, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariñas-Pardo, L.; Rodríguez-Hurtado, I.; Rodríguez-García, M.I.; Núñez-Naveira, L.; Hermida-Prieto, M. Allogeneic Adipose-Derived Mesenchymal Stem Cells (Horse Allo 20) for the Treatment of Osteoarthritis-Associated Lameness in Horses: Characterization, Safety, and Efficacy of Intra-Articular Treatment. Stem Cells Dev. 2018, 27, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Besalti, O.; Aktas, Z.; Can, P.; Akpinar, E.; Elcin, A.E.; Elcin, Y.M. The use of autologous neurogenically-induced bone marrow-derived mesenchymal stem cells for the treatment of paraplegic dogs without nociception due to spinal trauma. J. Vet. Med. Sci. 2016, 78, 1465–1473. [Google Scholar] [CrossRef] [Green Version]
- Bhat, I.A.; Sivanarayanan, T.B.; Somal, A.; Pandey, S.; Bharti, M.K.; Panda, B.S.; Verma, M.; Sonwane, A.; Kumar, G.S.; Chandra, V.; et al. An allogenic therapeutic strategy for canine spinal cord injury using mesenchymal stem cells. J. Cell. Physiol. 2019, 234, 2705–2718. [Google Scholar] [CrossRef]
- Quimby, J.M.; Webb, T.L.; Gibbons, D.S.; Dow, S.W. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: A pilot study. J. Feline Med. Surg. 2011, 13, 418–426. [Google Scholar] [CrossRef]
- Vidane, A.S.; Pinheiro, A.O.; Casals, J.B.; Passarelli, D.; Hage, M.; Bueno, R.S.; Martins, D.S.; Ambrósio, C.E. Transplantation of amniotic membrane-derived multipotent cells ameliorates and delays the progression of chronic kidney disease in cats. Reprod. Domest. Anim. 2017, 52, 316–326. [Google Scholar] [CrossRef] [Green Version]
- Barussi, F.C.M.; Bastos, F.Z.; Leite, L.M.B.; Fragoso, F.Y.I.; Senegaglia, A.C.; Brofman, P.R.S.; Nishiyama, A.; Pimpão, C.T.; Michelotto, P.V. Intratracheal therapy with autologous bone marrow-derived mononuclear cells reduces airway inflammation in horses with recurrent airway obstruction. Respir. Physiol. Neurobiol. 2016, 232, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ran, Y.; Lu, B.; Li, J.; Zhang, J.; Feng, C.; Fang, J.; Ma, R.; Qiao, Z.; Dai, X.; et al. Therapeutic Effects of Human Umbilical Cord–Derived Mesenchymal Stem Cells on Canine Radiation-Induced Lung Injury. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.B.; Schnabel, L.V.; Gilger, B.C. Subconjunctival bone marrow-derived mesenchymal stem cell therapy as a novel treatment alternative for equine immune-mediated keratitis: A case series. Vet. Ophthalmol. 2019, 22, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Claros, S.; Fernández, V.; Alcoholado, C.; Fariñas, F.; Moreno, A.; Becerra, J.; Andrades, J.A. Safety and efficacy of the mesenchymal stem cell in feline eosinophilic keratitis treatment. BMC Vet. Res. 2018, 14, 116. [Google Scholar] [CrossRef] [Green Version]
- Wernly, B.; Mirna, M.; Rezar, R.; Prodinger, C.; Jung, C.; Podesser, B.K.; Kiss, A.; Hoppe, U.C.; Lichtenauer, M. Regenerative Cardiovascular Therapies: Stem Cells and Beyond. Int. J. Mol. Sci. 2019, 20, 1420. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.M.; Dow, S.W. Concise Review: Stem Cell Trials Using Companion Animal Disease Models. Stem Cells 2016, 34, 1709–1729. [Google Scholar] [CrossRef]
- Fox, P.R. Pathology of myxomatous mitral valve disease in the dog. J. Vet. Cardiol. 2012, 14, 103–126. [Google Scholar] [CrossRef]
- Ojeh, N.; Pastar, I.; Tomic-Canic, M.; Stojadinovic, O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. [Google Scholar] [CrossRef] [Green Version]
- Johnson, V.; Webb, T.; Norman, A.; Coy, J.; Kurihara, J.; Regan, D.; Dow, S. Activated Mesenchymal Stem Cells Interact with Antibiotics and Host Innate Immune Responses to Control Chronic Bacterial Infections. Sci. Rep. 2017, 7, 9575. [Google Scholar] [CrossRef] [Green Version]
- El-Tookhy, O.S.; Shamaa, A.A.; Shehab, G.G.; Abdallah, A.N.; Azzam, O.M. Histological Evaluation of Experimentally Induced Critical Size Defect Skin Wounds Using Exosomal Solution of Mesenchymal Stem Cells Derived Microvesicles. Int. J. Stem Cells 2017, 10, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Spaas, J.H.; Broeckx, S.; Van de Walle, G.R.; Polettini, M. The effects of equine peripheral blood stem cells on cutaneous wound healing: A clinical evaluation in four horses. Clin. Exp. Dermatol. 2013, 38, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Lanci, A.; Merlo, B.; Mariella, J.; Castagnetti, C.; Iacono, E. Heterologous Wharton’s Jelly Derived Mesenchymal Stem Cells Application on a Large Chronic Skin Wound in a 6-Month-Old Filly. Front. Vet. Sci. 2019, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.; Rosenkrantz, W.S.; Hong, J.; Griffin, C.E.; Mendelsohn, C. Evaluation of the potential use of adipose-derived mesenchymal stromal cells in the treatment of canine atopic dermatitis: A pilot study. Vet. Ther. 2010, 11, E1–E14. [Google Scholar] [PubMed]
- Villatoro, A.J.; Hermida-Prieto, M.; Fernández, V.; Fariñas, F.; Alcoholado, C.; Rodríguez-García, M.I.; Mariñas-Pardo, L.; Becerra, J. Allogeneic adipose-derived mesenchymal stem cell therapy in dogs with refractory atopic dermatitis: Clinical efficacy and safety. Vet. Rec. 2018, 183, 654. [Google Scholar] [CrossRef] [PubMed]
- Babu, N.C.; Gomes, A.J. Systemic manifestations of oral diseases. J. Oral Maxillofac. Pathol. 2011, 15, 144. [Google Scholar] [CrossRef]
- Javdani, M.; Nikousefat, Z. Prevalence of dental problems in pet dogs in Shiraz, Iran Res. Opin. Anim. Vet. Sci 2011, 1, 666–668. [Google Scholar]
- Niemiec, B.; Gawor, J.; Nemec, A.; Clarke, D.; McLeod, K.; Tutt, C.; Gioso, M.; Steagall, P.V.; Chandler, M.; Morgenegg, G.; et al. World Small Animal Veterinary Association Global Dental Guidelines. J. Small Anim. Pract. 2020, 61, E36–E161. [Google Scholar] [CrossRef]
- El-Zekrid, M.H.; Mahmoud, S.H.; Ali, F.A.; Helal, M.E.; Grawish, M.E. Healing Capacity of Autologous Bone Marrow–derived Mesenchymal Stem Cells on Partially Pulpotomized Dogs’ Teeth. J. Endod. 2019, 45, 287–294. [Google Scholar] [CrossRef]
- Sánchez-Garcés, M.À.; Alvira-González, J.; Sánchez, C.M.; Cairó, J.R.B.; Del Pozo, M.R.; Gay-Escoda, C. Bone Regeneration Using Adipose-Derived Stem Cells with Fibronectin in Dehiscence-Type Defects Associated with Dental Implants: An Experimental Study in a Dog Model. Int. J. Oral Maxillofac. Implant. 2017, 32, e97–e106. [Google Scholar] [CrossRef] [Green Version]
- Bellei, E.; Dalla, F.; Masetti, L.; Pisoni, L.; Joechler, M. Surgical therapy in chronic feline gingivostomatitis (FCGS). Vet. Res. Commun. 2008, 32, 231–234. [Google Scholar] [CrossRef]
- Hennet, P.R.; Camy, G.A.L.; McGahie, D.M.; Albouy, M.V. Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus-positive cats with caudal stomatitis: A randomised, multi-centre, controlled, double-blind study in 39 cats. J. Feline Med. Surg. 2011, 13, 577–587. [Google Scholar] [CrossRef]
- Lommer, M.J. Efficacy of Cyclosporine for Chronic, Refractory Stomatitis in Cats: A Randomized, Placebo-Controlled, Double-Blinded Clinical Study. J. Vet. Dent. 2013, 30, 8–17. [Google Scholar] [CrossRef]
- Druet, I.; Hennet, P. Relationship between Feline calicivirus Load, Oral Lesions, and Outcome in Feline Chronic Gingivostomatitis (Caudal Stomatitis): Retrospective Study in 104 Cats. Front. Vet. Sci. 2017, 4, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winer, J.N.; Arzi, B.; Verstraete, F.J.M. Therapeutic Management of Feline Chronic Gingivostomatitis: A Systematic Review of the Literature. Front. Vet. Sci. 2016, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Arzi, B.; Mills-Ko, E.; Verstraete, F.J.M.; Kol, A.; Walker, N.J.; Badgley, M.R.; Fazel, N.; Murphy, W.J.; Vapniarsky, N.; Borjesson, D.L. Therapeutic Efficacy of Fresh, Autologous Mesenchymal Stem Cells for Severe Refractory Gingivostomatitis in Cats. Stem Cells Transl. Med. 2015, 5, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arzi, B.; Clark, K.C.; Sundaram, A.; Spriet, M.; Verstraete, F.J.M.; Walker, N.J.; Loscar, M.R.; Fazel, N.; Murphy, W.J.; Vapniarsky, N.; et al. Therapeutic Efficacy of Fresh, Allogeneic Mesenchymal Stem Cells for Severe Refractory Feline Chronic Gingivostomatitis. Stem Cells Transl. Med. 2017, 6, 1710–1722. [Google Scholar] [CrossRef]
- Craven, M.; Simpson, J.W.; Ridyard, A.E.; Chandler, M.L. Canine inflammatory bowel disease: Retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J. Small Anim. Pract. 2004, 45, 336–342. [Google Scholar] [CrossRef]
- Hall, E.J. Inflammatory Bowel Disease in Dogs and Cats; Hill’s Pet Nutrition, Inc.: Topeka, KS, USA, 2009; Available online: https://protrain.hs.llnwd.net/e1/sitefiles/642/Documents/GI%20technical%20booklet.pdf (accessed on 10 September 2022).
- Nishimura, T.; Takami, T.; Sasaki, R.; Aibe, Y.; Matsuda, T.; Fujisawa, K.; Matsumoto, T.; Yamamoto, N.; Tani, K.; Taura, Y.; et al. Liver regeneration therapy through the hepatic artery-infusion of cultured bone marrow cells in a canine liver fibrosis model. PLoS ONE 2019, 14, e0210588. [Google Scholar] [CrossRef]
- Gardin, C.; Ferroni, L.; Bellin, G.; Rubini, G.; Barosio, S.; Zavan, B. Therapeutic Potential of Autologous Adipose-Derived Stem Cells for the Treatment of Liver Disease. Int. J. Mol. Sci. 2018, 19, 4064. [Google Scholar] [CrossRef] [Green Version]
- Nam, A.; Han, S.M.; Go, D.M.; Kim, D.Y.; Seo, K.W.; Youn, H.Y. Long-Term Management with Adipose Tissue-Derived Mesenchymal Stem Cells and Conventional Treatment in a Dog with Hepatocutaneous Syndrome. J. Vet. Intern. Med. 2017, 31, 1514–1519. [Google Scholar] [CrossRef]
- de Witte, S.F.H.; Luk, F.; Sierra Parraga, J.M.; Gargesha, M.; Merino, A.; Korevaar, S.S.; Shankar, A.S.; O’Flynn, L.; Elliman, S.J.; Roy, D.; et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 2018, 36, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Dakin, S.G.; Jespers, K.; Warner, S.; O’hara, L.K.; Dudhia, J.; Goodship, A.E.; Wilson, A.M.; Smith, R.K.W. The relationship between in vivo limb and in vitro tendon mechanics after injury: A potential novel clinical tool for monitoring tendon repair. Equine Vet. J. 2011, 43, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Petrov, R.; MacDonald, M.H.; Tesch, A.M.; Hoogmoed, L.M.V. Influence of topically applied cold treatment on core temperature and cell viability in equine superficial digital flexor tendons. Am. J. Vet. Res. 2003, 64, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.-M.; Fu, S.-C. Anti-inflammatory management for tendon injuries-friends or foes? BMC Sport. Sci. Med. Rehabil. 2009, 1, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliashar, E.; Schramme, M.C.; Schumacher, J.; Smith, R.K.W.; Ikada, Y. Use of a bioabsorbable implant for the repair of severed digital flexor tendons in four horses. Vet. Rec. 2001, 148, 506–509. [Google Scholar] [CrossRef]
- Filomeno, P.; Dayan, V.; Touriño, C. Stem cell research and clinical development in tendon repair. Muscles Ligaments Tendons J. 2012, 2, 204. [Google Scholar]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacini, S.; Spinabella, S.; Trombi, L.; Fazzi, R.; Galimberti, S.; Dini, F.; Carlucci, F.; Petrini, M. Suspension of Bone Marrow–Derived Undifferentiated Mesenchymal Stromal Cells for Repair of Superficial Digital Flexor Tendon in Race Horses. Tissue Eng. 2007, 13, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.J. Medical management of superficial digital flexor tendonitis: A comparative study in 219 horses (1992–2000). Equine Vet. J. 2004, 36, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.K.W.; Werling, N.J.; Dakin, S.G.; Alam, R.; Goodship, A.E.; Dudhia, J. Beneficial Effects of Autologous Bone Marrow-Derived Mesenchymal Stem Cells in Naturally Occurring Tendinopathy. PLoS ONE 2013, 8, e75697. [Google Scholar] [CrossRef]
- Van Loon, V.J.F.; Scheffer, C.J.W.; Genn, H.J.; Hoogendoorn, A.C.; Greve, J.W. Clinical follow-up of horses treated with allogeneic equine mesenchymal stem cells derived from umbilical cord blood for different tendon and ligament disorders. Vet. Q. 2014, 34, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.R.; Moreno, B.; de Blas, I.; Sanz, A.; Vázquez, F.J.; Vitoria, A.; Junquera, C.; et al. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Vet. J. 2017, 224, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Geburek, F.; Roggel, F.; van Schie, H.T.M.; Beineke, A.; Estrada, R.; Weber, K.; Hellige, M.; Rohn, K.; Jagodzinski, M.; Welke, B.; et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: A controlled experimental trial. Stem Cell Res. Ther. 2017, 8, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harasen, G. Diagnosing rupture of the cranial cruciate ligament. Can. Vet. J. 2002, 43, 475. [Google Scholar]
- Chuang, C.; Ramaker, M.A.; Kaur, S.; Csomos, R.A.; Kroner, K.T.; Bleedorn, J.A.; Schaefer, S.L.; Muir, P. Radiographic Risk Factors for Contralateral Rupture in Dogs with Unilateral Cranial Cruciate Ligament Rupture. PLoS ONE 2014, 9, e106389. [Google Scholar] [CrossRef]
- Mölsä, S.H.; Hyytiäinen, H.K.; Hielm-Björkman, A.K.; Laitinen-Vapaavuori, O.M. Long-term functional outcome after surgical repair of cranial cruciate ligament disease in dogs. BMC Vet. Res. 2014, 10, 266. [Google Scholar] [CrossRef] [Green Version]
- Taroni, M.; Cabon, Q.; Fèbre, M.; Cachon, T.; Saulnier, N.; Carozzo, C.; Maddens, S.; Labadie, F.; Robert, C.; Viguier, E. Evaluation of the Effect of a Single Intra-articular Injection of Allogeneic Neonatal Mesenchymal Stromal Cells Compared to Oral Non-Steroidal Anti-inflammatory Treatment on the Postoperative Musculoskeletal Status and Gait of Dogs over a 6-Month Period after Tibial Plateau Leveling Osteotomy: A Pilot Study. Front. Vet. Sci. 2017, 4, 83. [Google Scholar] [CrossRef] [Green Version]
- Linon, E.; Spreng, D.; Rytz, U.; Forterre, S. Engraftment of autologous bone marrow cells into the injured cranial cruciate ligament in dogs. Vet. J. 2014, 202, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Muir, P.; Hans, E.C.; Racette, M.; Volstad, N.; Sample, S.J.; Heaton, C.; Holzman, G.; Schaefer, S.L.; Bloom, D.D.; Bleedorn, J.A.; et al. Autologous Bone Marrow-Derived Mesenchymal Stem Cells Modulate Molecular Markers of Inflammation in Dogs with Cruciate Ligament Rupture. PLoS ONE 2016, 11, e0159095. [Google Scholar] [CrossRef]
- Canapp, S.O.; Leasure, C.S.; Cox, C.; Ibrahim, V.; Carr, B.J. Partial Cranial Cruciate Ligament Tears Treated with Stem Cell and Platelet-Rich Plasma Combination Therapy in 36 Dogs: A Retrospective Study. Front. Vet. Sci. 2016, 3, 112. [Google Scholar] [CrossRef] [Green Version]
- Frisbie, D.D.; Stewart, M.C. Cell-based Therapies for Equine Joint Disease. Vet. Clin. N. Am. Equine Pract. 2011, 27, 335–349. [Google Scholar] [CrossRef]
- Rossdale, P.; Hopes, R.; Digby, N. Epidemiological study of wastage among racehorses 1982 and 1983. Vet. Rec. 1985, 116, 66–69. [Google Scholar] [CrossRef]
- Walmsley, J.P.; Phillips, T.J.; Townsend, H.G.G. Meniscal tears in horses: An evaluation of clinical signs and arthroscopic treatment of 80 cases. Equine Vet. J. 2003, 35, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Nicpoń, J.; Marycz, K.; Grzesiak, J. Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol. J. Vet. Sci. 2013, 16, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Joswig, A.-J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R.; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohoric, L.; Zorko, B.; Ceh, K.; Majdic, G. Blinded placebo study of bilateral osteoarthritis treatment using adipose derived mesenchymal stem cells. Slov. Vet. Res. 2016, 53, 167–174. [Google Scholar]
- Black, L.L.; Gaynor, J.; Gahring, D.; Adams, C.; Aron, D.; Harman, S.; Gingerich, D.A.; Harman, R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: A randomized, double-blinded, multicenter controlled trial. Vet. Ther. 2007, 8, 272. [Google Scholar]
- Srzentić Dražilov, S.; Mrkovački, J.; Spasovski, V.; Fazlagić, A.; Pavlović, S.; Nikčević, G. The use of canine mesenchymal stem cells for the autologous treatment of osteoarthritis. Acta Vet. Hung. 2018, 66, 376–389. [Google Scholar] [CrossRef] [Green Version]
- Harman, R.; Carlson, K.; Gaynor, J.; Gustafson, S.; Dhupa, S.; Clement, K.; Hoelzler, M.; McCarthy, T.; Schwartz, P.; Adams, C. A Prospective, Randomized, Masked, and Placebo-Controlled Efficacy Study of Intraarticular Allogeneic Adipose Stem Cells for the Treatment of Osteoarthritis in Dogs. Front. Vet. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Shah, K.; Drury, T.; Roic, I.; Hansen, P.; Malin, M.; Boyd, R.; Sumer, H.; Ferguson, R. Outcome of Allogeneic Adult Stem Cell Therapy in Dogs Suffering from Osteoarthritis and Other Joint Defects. Stem Cells Int. 2018, 2018, 7309201. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.-y.; Wang, B.-y.; Li, S.-c.; Luo, D.-z.; Zhan, X.; Chen, S.-f.; Chen, Z.-s.; Liu, C.-y.; Ji, H.-q.; Bai, Y.-s.; et al. Evaluation of the Curative Effect of Umbilical Cord Mesenchymal Stem Cell Therapy for Knee Arthritis in Dogs Using Imaging Technology. Stem Cells Int. 2018, 2018, 1983025. [Google Scholar] [CrossRef]
- Cabon, Q.; Febre, M.; Gomez, N.; Cachon, T.; Pillard, P.; Carozzo, C.; Saulnier, N.; Robert, C.; Livet, V.; Rakic, R.; et al. Long-Term Safety and Efficacy of Single or Repeated Intra-Articular Injection of Allogeneic Neonatal Mesenchymal Stromal Cells for Managing Pain and Lameness in Moderate to Severe Canine Osteoarthritis Without Anti-inflammatory Pharmacological Support: Pilot Clinical Study. Front. Vet. Sci. 2019, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Duan, X.; Fan, Z.; Chen, L.; Xing, F.; Xu, Z.; Chen, Q.; Xiang, Z. Mesenchymal Stem Cells in Combination with Hyaluronic Acid for Articular Cartilage Defects. Sci. Rep. 2018, 8, 9900. [Google Scholar] [CrossRef] [Green Version]
- Kornicka-Garbowska, K.; Pędziwiatr, R.; Woźniak, P.; Kucharczyk, K.; Marycz, K. Microvesicles isolated from 5-azacytidine-and-resveratrol-treated mesenchymal stem cells for the treatment of suspensory ligament injury in horse—A case report. Stem Cell Res. Ther. 2019, 10, 394. [Google Scholar] [CrossRef] [Green Version]
- Bach, F.S.; Rebelatto, C.L.K.; Fracaro, L.; Senegaglia, A.C.; Fragoso, F.Y.I.; Daga, D.R.; Brofman, P.R.S.; Pimpão, C.T.; Engracia Filho, J.R.; Montiani-Ferreira, F.; et al. Comparison of the Efficacy of Surgical Decompression Alone and Combined With Canine Adipose Tissue-Derived Stem Cell Transplantation in Dogs With Acute Thoracolumbar Disk Disease and Spinal Cord Injury. Front. Vet. Sci. 2019, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Bone Marrow-Derived Mesenchymal Stem Cells as Autologous Therapy in Dogs with Naturally Occurring Intervertebral Disc Disease: Feasibility, Safety, and Preliminary Results. Tissue Eng. Part C Methods 2017, 23, 643–651. [CrossRef] [PubMed]
- Wu, G.-H.; Shi, H.-J.; Che, M.-T.; Huang, M.-Y.; Wei, Q.-S.; Feng, B.; Ma, Y.-H.; Wang, L.-J.; Jiang, B.; Wang, Y.-Q.; et al. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials 2018, 181, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Adin, C.A.; Gregory, C.R.; Kyles, A.E.; Cowgill, L. Diagnostic Predictors of Complications and Survival after Renal Transplantation in Cats. Vet. Surg. 2001, 30, 515–521. [Google Scholar] [CrossRef]
- Quimby, J.M.; Webb, T.L.; Habenicht, L.M.; Dow, S.W. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: Results of three sequential pilot studies. Stem Cell Res. Ther. 2013, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Quimby, J.M.; Webb, T.L.; Randall, E.; Marolf, A.; Valdes-Martinez, A.; Dow, S.W. Assessment of intravenous adipose-derived allogeneic mesenchymal stem cells for the treatment of feline chronic kidney disease: A randomized, placebo-controlled clinical trial in eight cats. J. Feline Med. Surg. 2016, 18, 165–171. [Google Scholar] [CrossRef]
- Trzil, J.E.; Masseau, I.; Webb, T.L.; Chang, C.-H.; Dodam, J.R.; Liu, H.; Quimby, J.M.; Dow, S.W.; Reinero, C.R. Intravenous adipose-derived mesenchymal stem cell therapy for the treatment of feline asthma: A pilot study. J. Feline Med. Surg. 2016, 18, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Marfe, G.; Massaro-Giordano, M.; Ranalli, M.; Cozzoli, E.; Di Stefano, C.; Malafoglia, V.; Polettini, M.; Gambacurta, A. Blood derived stem cells: An ameliorative therapy in veterinary ophthalmology. J. Cell. Physiol. 2012, 227, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Saldinger, L.K.; Nelson, S.G.; Bellone, R.R.; Lassaline, M.; Mack, M.; Walker, N.J.; Borjesson, D.L. Horses with equine recurrent uveitis have an activated CD4+ T-cell phenotype that can be modulated by mesenchymal stem cells in vitro. Vet. Ophthalmol. 2020, 23, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villatoro, A.J.; Fernández, V.; Claros, S.; Rico-Llanos, G.A.; Becerra, J.; Andrades, J.A. Use of Adipose-Derived Mesenchymal Stem Cells in Keratoconjunctivitis Sicca in a Canine Model. BioMed Res. Int. 2015, 2015, 527926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgrignoli, M.R.; Silva, D.A.; Nascimento, F.F.; Sgrignoli, D.A.M.; Nai, G.A.; da Silva, M.G.; de Barros, M.A.; Bittencourt, M.K.W.; de Morais, B.P.; Dinallo, H.R.; et al. Reduction in the inflammatory markers CD4, IL-1, IL-6 and TNFα in dogs with keratoconjunctivitis sicca treated topically with mesenchymal stem cells. Stem Cell Res. 2019, 39, 101525. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Husseiny, H.M.; Mady, E.A.; Helal, M.A.Y.; Tanaka, R. The Pivotal Role of Stem Cells in Veterinary Regenerative Medicine and Tissue Engineering. Vet. Sci. 2022, 9, 648. https://doi.org/10.3390/vetsci9110648
El-Husseiny HM, Mady EA, Helal MAY, Tanaka R. The Pivotal Role of Stem Cells in Veterinary Regenerative Medicine and Tissue Engineering. Veterinary Sciences. 2022; 9(11):648. https://doi.org/10.3390/vetsci9110648
Chicago/Turabian StyleEl-Husseiny, Hussein M., Eman A. Mady, Mahmoud A. Y. Helal, and Ryou Tanaka. 2022. "The Pivotal Role of Stem Cells in Veterinary Regenerative Medicine and Tissue Engineering" Veterinary Sciences 9, no. 11: 648. https://doi.org/10.3390/vetsci9110648
APA StyleEl-Husseiny, H. M., Mady, E. A., Helal, M. A. Y., & Tanaka, R. (2022). The Pivotal Role of Stem Cells in Veterinary Regenerative Medicine and Tissue Engineering. Veterinary Sciences, 9(11), 648. https://doi.org/10.3390/vetsci9110648