Black Pepper or Radish Seed Oils in a New Combination of Essential Oils Modulated Broiler Chickens’ Performance and Expression of Digestive Enzymes, Lipogenesis, Immunity, and Autophagy-Related Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Birds’ Management, Diets, and Experimental Design
2.3. Growth Performance
2.4. Digestibility Trail
2.5. Sampling
2.6. Cecal Bacterial Count
2.7. Serum Biochemical Analysis
2.8. Serum Immune Parameters and Assay of Phagocytosis
2.9. Gene Expression Analysis
2.10. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Nutrient Digestibility
3.3. Serum Biochemical Parameters
3.4. Serum Immune Parameters
3.5. Cecal Bacterial Count
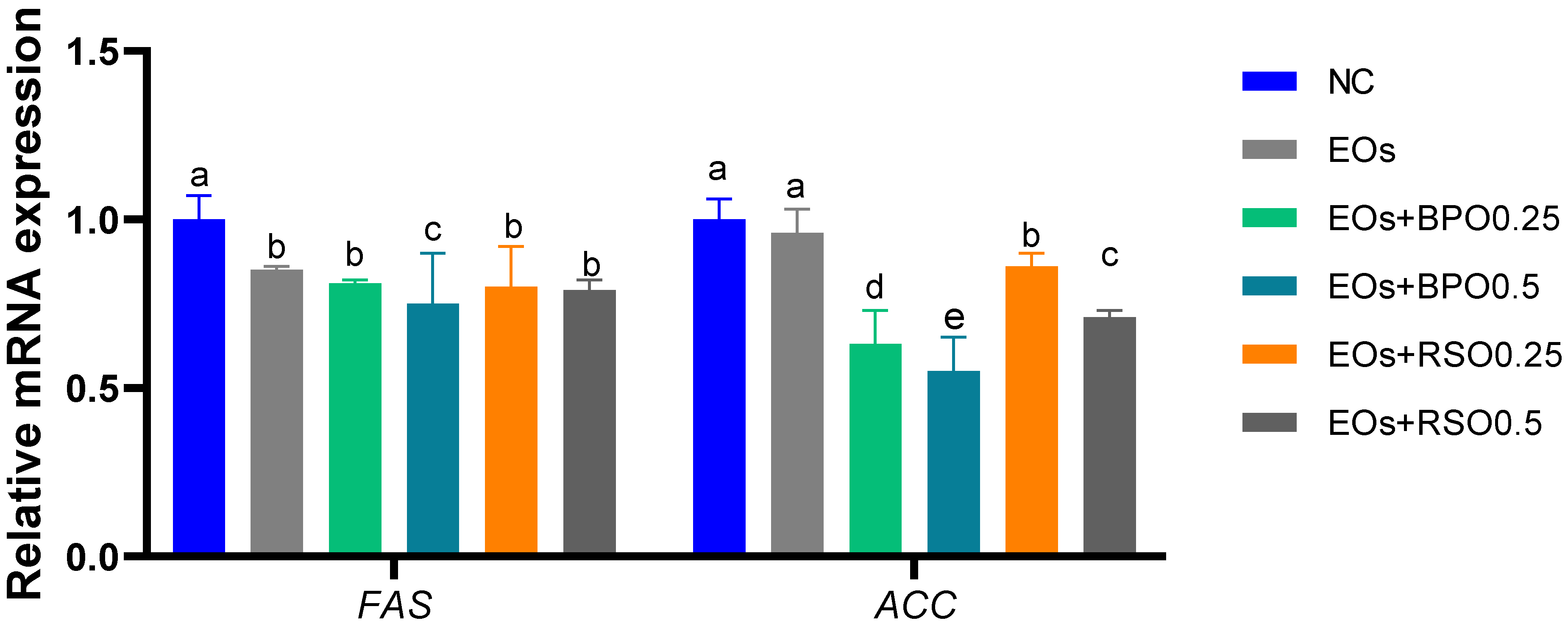
3.6. Expression of Digestive-Enzyme- and Lipogenesis-Related Genes
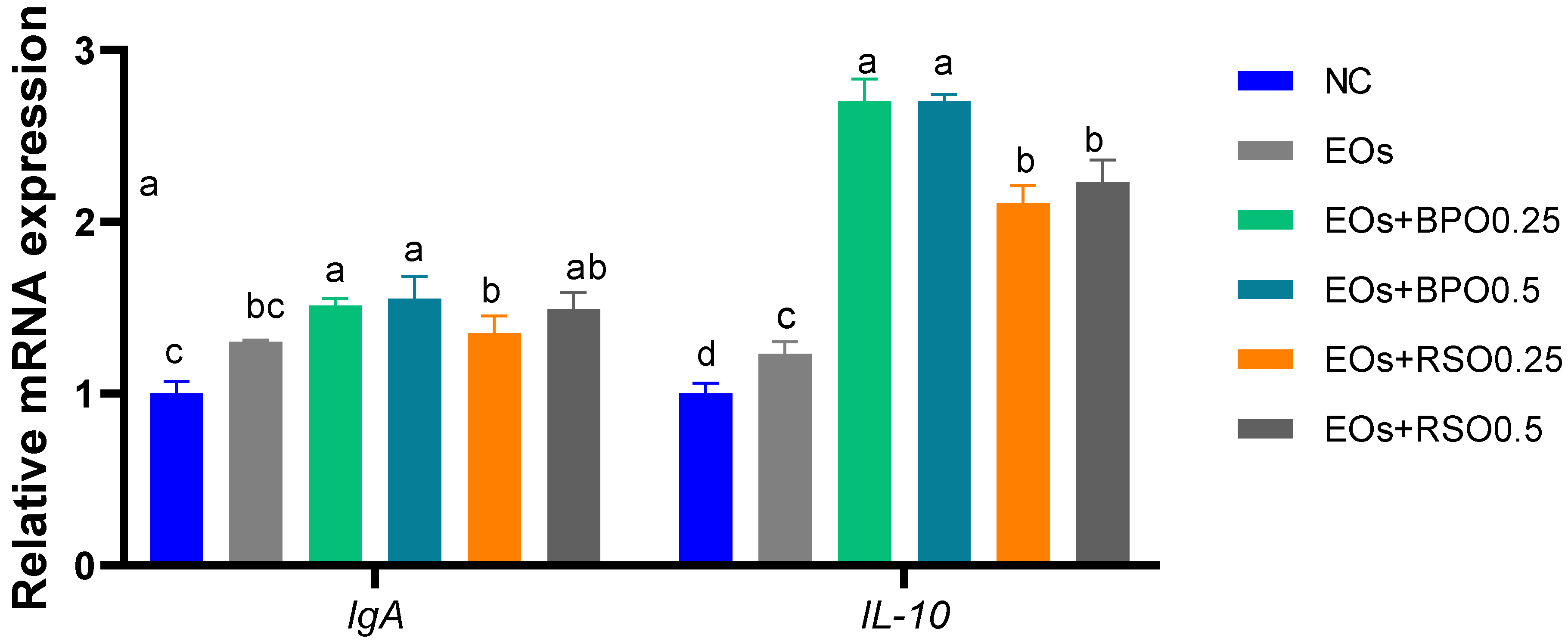
3.7. Expression of Immune- and Autophagy-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chowdhury, S.; Mandal, G.P.; Patra, A.K. Different essential oils in diets of chickens: 1. Growth performance, nutrient utilisation, nitrogen excretion, carcass traits and chemical composition of meat. Anim. Feed. Sci. Technol. 2018, 236, 86–97. [Google Scholar] [CrossRef]
- Du, E.; Wang, W.; Gan, L.; Liping, G.; Guo, S.; Guo, Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, D.; McDevitt, R.M.; Hillman, K.; Acamovic, T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007, 48, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Platel, K.; Srinivasan, K. Digestive stimulant action of spices: A myth or reality? Indian J. Med. Res. 2004, 119, 167. [Google Scholar] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Vichi, S.; Zitterl-Eglseer, K.; Jugl, M.; Franz, C. Determination of the presence of antioxidants deriving from sage and oregano extracts added to animal fat by means of assessment of the radical scavenging capacity by photochemiluminescence analysis. Food Nahrung 2001, 45, 101–104. [Google Scholar] [CrossRef]
- Bero, J.; Kpoviessi, S.; Quetin-Leclercq, J. Anti-Parasitic Activity of Essential Oils and their Active Constituents against Plasmodium, Trypanosoma and Leishmania. Nov. Plant Bioresour. Appl. Food Med. Cosmet. 2014, 33, 455–469. [Google Scholar] [CrossRef]
- Juglal, S.; Govinden, R.; Odhav, B. Spice Oils for the Control of Co-Occurring Mycotoxin-Producing Fungi. J. Food Prot. 2002, 65, 683–687. [Google Scholar] [CrossRef]
- Emadi, M.; Kermanshah, H.; Maroufyan, E. Effect of Varying Levels of Turmeric Rhizome Powder on Some Blood Parameters of Broiler Chickens Fed Corn-Soybean Meal Based Diets. Int. J. Poult. Sci. 2007, 6, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Trichet, V.V. Nutrition and immunity: An update. Aquac. Res. 2010, 41, 356–372. [Google Scholar] [CrossRef]
- Moorthy, M.; Ravi, S.; Ravikuma, M.; Viswanathan, K.; Edwin, S. Ginger, Pepper and Curry Leaf Powder as Feed Additives in Broiler Diet. Int. J. Poult. Sci. 2009, 8, 779–782. [Google Scholar] [CrossRef] [Green Version]
- El Tazi, S.M.; Mukhtar, M.A.; Mohamed, K.; Tabidi, M.H. Effect of using black pepper as natural feed additive on performance and carcass quality of broiler chicks. Int. J. Pharm. Res. Anal. 2014, 4, 108–113. [Google Scholar]
- Mahady, G.B.; Pendland, S.L.; Yun, G.S.; Lu, Z.-Z.; Stoia, A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of Cag A+ strains of Helicobacter pylori. Anticancer. Res. 2003, 23, 3699–3702. [Google Scholar] [PubMed]
- Sarica, S.; Çiftci, A.; Demir, E.; Kilinc, K.; Yildirim, Y. Use of an antibiotic growth promoter and two herbal natural feed additives with and without exogenous enzymes in wheat based broiler diets. South Afr. J. Anim. Sci. 2007, 35, 61–72. [Google Scholar] [CrossRef]
- Khalaf, N.A.; Shakya, A.K.; Al-Othman, A.; El-Agbar, Z.; Farah, H. Antioxidant activity of some common plants. Turk. J. Biol. 2008, 32, 51–55. [Google Scholar]
- Puvača, N.; Kostadinović, L.; Ljubojević, D.; Lukač, D.; Lević, J.; Popović, S.; Novakov, N.; Vidović, B.; Đuragić, O. Effect of garlic, black pepper and hot red pepper on productive performances and blood lipid profile of broiler chickens. Eur. Poult. Sci. 2015, 79, 1–13. [Google Scholar]
- Mansoub, N. Comparison of using different level of black pepper with probiotic on performance and serum composition of broiler chickens. J. Basic Appl. Sci. Res. 2011, 1, 2425–2428. [Google Scholar]
- El-Sayed, S.T. Purification and characterization of raphanin, A neutral protease, from Raphanus sativus leaves. Pak. J. Biol. Sci. 2001, 4, 564–568. [Google Scholar]
- Zaman, R. Study of cardioprotective activity of Raphanus sativus L. in the rabbits. Pak. J. Biol. Sci. 2004, 7, 843–847. [Google Scholar]
- Matsufuji, H.; Otsuki, T.; Takeda, T.; Chino, M.; Takeda, M. Identification of Reaction Products of Acylated Anthocyanins from Red Radish with Peroxyl Radicals. J. Agric. Food Chem. 2003, 51, 3157–3161. [Google Scholar] [CrossRef]
- Suh, S.-J.; Moon, S.-K.; Kim, C.-H. Raphanus sativus and its isothiocyanates inhibit vascular smooth muscle cells proliferation and induce G1 cell cycle arrest. Int. Immunopharmacol. 2006, 6, 854–861. [Google Scholar] [CrossRef]
- Vasudevan, M.; Gunnam, K.K.; Parle, M. Antinociceptive and Anti-Inflammatory Properties of Daucus carota Seeds Extract. J. Health Sci. 2006, 52, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.L.; Zhou, K.K.; Parry, J. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem. 2005, 91, 723–729. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Yoshinari, T.; Shams-Ghahfarokhi, P.M.; Rezaee, M.-B.; Nagasawa, H.; Sakuda, S. Dillapiol and Apiol as Specific Inhibitors of the Biosynthesis of Aflatoxin G1inAspergillus parasiticus. Biosci. Biotechnol. Biochem. 2007, 71, 2329–2332. [Google Scholar] [CrossRef] [Green Version]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef]
- Whitehouse, M.; Roberts, M.; Brooks, P. Over the counter (OTC) oral remedies for arthritis and rheumatism: How effective are they? Inflammopharmacology 1999, 7, 89–105. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, F.; Wang, X.; Yao, H.-Y. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res. Int. 2006, 39, 833–839. [Google Scholar] [CrossRef]
- Abbas, R.J. Effect of Using Fenugreek, Parsley and Sweet Basil Seeds as Feed Additives on the Performance of Broiler Chickens. Int. J. Poult. Sci. 2010, 9, 278–282. [Google Scholar] [CrossRef] [Green Version]
- Dew, M.J.; Evans, B.K.; Rhodes, J. Peppermint oil for the irritable bowel syndrome: A multicentre trial. Br. J. Clin. Pr. 1984, 38. [Google Scholar]
- Sharifi, S.D.; Khorsandi, S.H.; Khadem, A.A.; Salehi, A.; Moslehi, H. The effect of four medicinal plants on the performance, blood biochemical traits and ileal microflora of broiler chicks. Vet. Arh. 2013, 83, 69–80. [Google Scholar]
- Bupesh, G.; Amutha, C.; Nandagopal, S.; Ganeshkumar, A.; Sureshkumar, P.; Murali, K. Antibacterial activity of Mentha piperita L.(peppermint) from leaf extracts-a medicinal plant. Acta Agric. Slov. 2007, 89, 73. [Google Scholar] [CrossRef]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef]
- Aviagen, W. Ross 308: Broiler’s Management and Nutrition Specification; ROSS: Dublin, CA, USA, 2018. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 2012; Volume 13. [Google Scholar]
- Ibrahim, D.; Ismail, T.A.; Khalifa, E.; El-Kader, A.; Shaimaa, A.; Mohamed, D.I.; Mohamed, D.T.; Shahin, S.E.; El-Hamid, A.; Marwa, I. Supplementing Garlic Nanohydrogel Optimized Growth, Gastrointestinal Integrity and Economics and Ameliorated Necrotic Enteritis in Broiler Chickens Using a Clostridium perfringens Challenge Model. Animals 2021, 11, 2027. [Google Scholar] [CrossRef]
- Ibrahim, D.; Moustafa, A.; Metwally, A.; Nassan, M.; Abdallah, K.; Eldemery, F.; Tufarelli, V.; Laudadio, V.; Kishawy, A. Potential Application of Cornelian Cherry Extract on Broiler Chickens: Growth, Expression of Antioxidant Biomarker and Glucose Transport Genes, and Oxidative Stability of Frozen Meat. Animals 2021, 11, 1038. [Google Scholar] [CrossRef]
- Short, F.; Wiseman, J.; Boorman, K. Application of a method to determine ileal digestibility in broilers of amino acids in wheat. Anim. Feed. Sci. Technol. 1999, 79, 195–209. [Google Scholar] [CrossRef]
- Farahat, M.; Ibrahim, D.; Kishawy, A.; Abdallah, H.; Hernandez-Santana, A.; Attia, G. Effect of cereal type and plant extract addition on the growth performance, intestinal morphology, caecal microflora, and gut barriers gene expression of broiler chickens. Animal 2020, 15, 100056. [Google Scholar] [CrossRef]
- Langhout, D.; Schutte, J.; Van Leeuwen, P.; Wiebenga, J.; Tamminga, S. Effect of dietary high-and low-methylated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chicks. Br. Poult. Sci. 1999, 40, 340–347. [Google Scholar] [CrossRef]
- Bos, H.; de Souza, W. Phagocytosis of yeast: A method for concurrent quantification of binding and internalization using differential interference contrast microscopy. J. Immunol. Methods 2000, 238, 29–43. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kryukov, V.; Glebova, I. Antibacterial effect of essential oils of medicinal plants. Probl. Biol. Produktivn. Zhivotn 2017, 3, 5–25. [Google Scholar]
- Yang, C.; Chowdhury, M.K.; Huo, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [Green Version]
- Bonacucina, G.; Cespi, M.; Misici-Falzi, M.; Palmieri, G.F. Colloidal soft matter as drug delivery system. J. Pharm. Sci. 2009, 98, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, F.; Singh, U.; Chauhan, L. Review on microemulsion as futuristic drug delivery. Int. J. Pharm. Pharm. Sci 2013, 5, 39–53. [Google Scholar]
- Fernandes, C.; Mascarenhas, M.P.; Zibetti, F.M.; Lima, B.G.; Oliveira, R.P.; Rocha, L.; Falcão, D.Q. HLB value, an important parameter for the development of essential oil phytopharmaceuticals. Rev. Bras. Farm. 2013, 23, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Abou-Elkhair, R.; Ahmed, H.; Selim, S. Effects of Black Pepper (Piper Nigrum), Turmeric Powder (Curcuma Longa) and Coriander Seeds (Coriandrum Sativum) and Their Combinations as Feed Additives on Growth Performance, Carcass Traits, Some Blood Parameters and Humoral Immune. Asian-Australas. J. Anim. Sci. 2014, 27, 847–854. [Google Scholar] [CrossRef]
- Habibi, R.; Sadeghi, G.; Karimi, A. Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br. Poult. Sci. 2014, 55, 228–237. [Google Scholar] [CrossRef]
- Lee, K.-W.; Everts, H.; Kappert, H.; Frehner, M.; Losa, R.; Beynen, A. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sci. 2003, 44, 450–457. [Google Scholar] [CrossRef]
- Hassan, F.A.M.; Roushdy, E.M.; Kishawy, A.T.Y.; Zaglool, A.W.; Tukur, H.A.; Saadeldin, I.M. Growth Performance, Antioxidant Capacity, Lipid-Related Transcript Expression and the Economics of Broiler Chickens Fed Different Levels of Rutin. Animals 2018, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Kishawy, A.T.; A Amer, S.; El-Hack, M.E.A.; Saadeldin, I.M.; A Swelum, A. The impact of dietary linseed oil and pomegranate peel extract on broiler growth, carcass traits, serum lipid profile, and meat fatty acid, phenol, and flavonoid contents. Asian-Australas. J. Anim. Sci. 2019, 32, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Kishawy, A.T.; Omar, A.E.; Gomaa, A.M. Growth performance and immunity of broilers fed rancid oil diets that supplemented with pomegranate peel extract and sage oil. Jpn. J. Vet. Res. 2016, 64, S31–S38. [Google Scholar]
- Jang, I.S.; Ko, Y.H.; Yang, H.Y.; Ha, J.S.; Kim, J.Y.; Kang, S.Y.; Yoo, D.H.; Nam, D.S.; Kim, D.H.; Lee, C.Y. Influence of Essential Oil Components on Growth Performance and the Functional Activity of the Pancreas and Small Intestine in Broiler Chickens. Asian-Australas. J. Anim. Sci. 2004, 17, 394–400. [Google Scholar] [CrossRef]
- Bento, M.; Ouwehand, A.; Tiihonen, K.; Lahtinen, S.; Nurminen, P.; Saarinen, M.; Schulze, H.; Mygind, T.; Fischer, J. Essential oils and their use in animal feeds for monogastric animals--Effects on feed quality, gut microbiota, growth performance and food safety: A review. Vet. Med. 2013, 58, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Franz, C.; Baser, K.; Windisch, W. Essential oils and aromatic plants in animal feeding–a European perspective. A review. Flavour Fragr. J. 2010, 25, 327–340. [Google Scholar] [CrossRef]
- Kurekci, C.; Al Jassim, R.; Hassan, E.; Bishop-Hurley, S.L.; Padmanabha, J.; McSweeney, C. Effects of feeding plant-derived agents on the colonization of Campylobacter jejuni in broiler chickens. Poult. Sci. 2014, 93, 2337–2346. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Pendleton, S.J.; Crandall, P.G.; Ricke, S.C. Potential of plant essential oils and their components in animal agriculture–in vitro studies on antibacterial mode of action. Front. Vet. Sci. 2015, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Emami, N.K.; Samie, A.; Rahmani, H.; Ruiz-Feria, C. The effect of peppermint essential oil and fructooligosaccharides, as alternatives to virginiamycin, on growth performance, digestibility, gut morphology and immune response of male broilers. Anim. Feed Sci. Technol. 2012, 175, 57–64. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, L.-H.; Lin, L.; Zhang, R.; Du, Y.-C.; Chen, H.; Huang, M.; Guo, K.-W.; Yang, X.-Z. Essential Oil from Carpesium abrotanoides L. Induces Apoptosis via Activating Mitochondrial Pathway in Hepatocellular Carcinoma Cells. Curr. Med. Sci. 2018, 38, 1045–1053. [Google Scholar] [CrossRef]
- Akbari, M.; Torki, M.; Kaviani, K. Single and combined effects of peppermint and thyme essential oils on productive performance, egg quality traits, and blood parameters of laying hens reared under cold stress condition (6.8 ± 3 °C). Int. J. Biometeorol. 2015, 60, 447–454. [Google Scholar] [CrossRef]
- Grigoleit, H.-G.; Grigoleit, P. Pharmacology and preclinical pharmacokinetics of peppermint oil. Phytomedicine 2005, 12, 612–616. [Google Scholar] [CrossRef]
- Ürüşan, H.; Erhan, M.; Bölükbaşı, S. Effect of cold-press carrot seed oil on the performance, carcass characteristics, and shelf life of broiler chickens. JAPS J. Anim. Plant Sci. 2018, 28, 1662–1668. [Google Scholar]
- Alagawany, M.; El-Hack, M.; Farag, M.R.; Tiwari, R.; Dhama, K. Biological effects and modes of action of carvacrol in animal and poultry pro-duction and health-a review. Adv. Anim. Vet. Sci. 2015, 3, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Ndelekwute, E.; Afolabi, K.; Uzegbu, H.; Unah, U.; Amaefule, K. Effect of dietary Black pepper (Piper nigrum) on the performance of broiler. Bangladesh J. Anim. Sci. 2015, 44, 120–127. [Google Scholar] [CrossRef]
- Jamroz, D.; Wiliczkiewicz, A.; Wertelecki, T.; Orda, J.; Skorupińska, J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 2005, 46, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Best, P. Health boosters from botany. Feed Int. 2000, 6, 15–16. [Google Scholar]
- Tschirch, H. Use of natural plant extracts as productive enhancers in modern animal rearing practices. Zesz. Nauk. Akad. Rol. We Wroclawiu. Konf. 2000, 25–39. Available online: https://agris.fao.org/agris-search/search.do?recordID=PL2001000549 (accessed on 28 November 2021).
- Abaza, I. Effect of using fenugreek, chamomile and radish as feed additives on productive performance and digestibility coefficients of laying hens. Poult. Sci. 2007, 27, 199–218. [Google Scholar]
- Barbarestani, S.Y.; Jazi, V.; Mohebodini, H.; Ashayerizadeh, A.; Shabani, A.; Toghyani, M. Effects of dietary lavender essential oil on growth performance, intestinal function, and antioxidant status of broiler chickens. Livest. Sci. 2020, 233, 103958. [Google Scholar] [CrossRef]
- Brenes, A.; Roura, E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010, 158, 1–14. [Google Scholar] [CrossRef]
- Moharreri, M.; Vakili, R.; Oskoueian, E.; Rajabzadeh, G. Phytobiotic role of essential oil-loaded microcapsules in improving the health parameters in Clostridium perfringens-infected broiler chickens. Ital. J. Anim. Sci. 2021, 20, 2075–2085. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mandal, G.P.; Patra, A.K.; Kumar, P.; Samanta, I.; Pradhan, S.; Samanta, A.K. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018, 236, 39–47. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Skoufos, I.; Tzora, A.; Stylianaki, I.; Lazari, D.; Tsinas, A.; Christaki, E.; Florou-Paneri, P. Effect of herbal feed additives on performance parameters, intestinal microbiota, intestinal morphology and meat lipid oxidation of broiler chickens. Br. Poult. Sci. 2018, 59, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Martín-Orúe, S.M.; Roca, M.; Manzanilla, E.G.; Badiola, I.; Pérez, J.F.; Gasa, J. The response of gastrointestinal microbiota to avilamycin, butyrate, and plant extracts in early-weaned pigs1,2. J. Anim. Sci. 2006, 84, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Nofrarías, M.; Manzanilla, E.G.; Pujols, J.; Gibert, X.; Majo, N.; Segalés, J.; Gasa, J. Effects of spray-dried porcine plasma and plant extracts on intestinal morphology and on leukocyte cell subsets of weaned pigs1. J. Anim. Sci. 2006, 84, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Mekonnen, A.; Yitayew, B.; Tesema, A.; Taddese, S. In vitro antimicrobial activity of essential oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int. J. Microbiol. 2016, 2016, 9545693. [Google Scholar] [CrossRef] [Green Version]
- Murugesan, G.R.; Syed, B.; Haldar, S.; Pender, C. Phytogenic feed additives as an alternative to antibiotic growth promoters in broiler chickens. Front. Vet. Sci. 2015, 2, 21. [Google Scholar]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86 (Suppl. S14), E140–E148. [Google Scholar] [CrossRef]
- Jin, L.-Z.; Dersjant-Li, Y.; Giannenas, I. Application of aromatic plants and their extracts in diets of broiler chickens. Feed. Addit. 2020, 10, 159–185. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.; Montfort, G.R.-C.; Zavala, J.F.A.; González-Aguilar, G. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett. Appl. Microbiol. 2017, 66, 25–31. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.; von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Satoh, T.; Goto, M.; Igarashi, K. Effects of Protein Isolates from Radish and Spinach Leaves on Serum Lipids Levels in Rats. J. Nutr. Sci. Vitaminol. 1993, 39, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakumar, R.S.; Surya, D.; Senthilkumar, R.; Nalini, N. Hypolipidemic Effect of Black Pepper (Piper nigrum Linn.) in Rats Fed High Fat Diet. J. Clin. Biochem. Nutr. 2002, 32, 31–42. [Google Scholar] [CrossRef]
- Taniguchi, H.; Muroi, R.; Kobayashi-Hattori, K.; Uda, Y.; Oishi, Y.; Takita, T. Differing effects of water-soluble and fat-soluble extracts from Japanese radish (Raphanus sativus) sprouts on carbohydrate and lipid metabolism in normal and streptozotocin-induced diabetic rats. J. Nutr. Sci. Vitaminol. 2007, 53, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faramarzi, S.; Bozorgmehrifard, M.; Khaki, A.; Moomivand, H.; Ezati, M.; Rasoulinezhad, S.; Bahnamiri, A.; Dizaji, B.R. Study on the effect of Thymus vulgaris essential oil on humoral immunity and performance of broiler chickens after La Sota vaccination. Ann. Biol. Res. 2013, 4, 290–294. [Google Scholar]
- Krishan, G.; Narang, A. Use of essential oils in poultry nutrition: A new approach. J. Adv. Veter-Anim. Res. 2014, 1, 156. [Google Scholar] [CrossRef]
- Awaad, M.; Abdel-Alim, G.; Sayed, K.; Ahmed, A.; Nada, A.; Metwalli, A.; Alkhalaf, A. Immunostimulant effects of essential oils of peppermint and eucalyptus in chickens. Pak. Vet. J. 2010, 30, 61–66. [Google Scholar]
- Li, S.Y.; Ru, Y.J.; Liu, M.; Xu, B.; Péron, A.; Shi, X. The effect of essential oils on performance, immunity and gut microbial population in weaner pigs. Livest. Sci. 2012, 145, 119–123. [Google Scholar] [CrossRef]
- Puvača, N.; Ljubojević Pelić, D.; Čabarkapa, I.; Popović, S.; Tomičić, Z.; Nikolova, N.; Lević, J. Quality of broiler chickens carcass fed dietary addition of garlic, black pepper and hot red pepper. J. Agron. Technol. Eng. Manag. 2019, 2, 218–227. [Google Scholar]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Dridi, S.; Buyse, J.; Decuypere, E.; Taouis, M. Potential role of leptin in increase of fatty acid synthase gene expression in chicken liver. Domest. Anim. Endocrinol. 2005, 29, 646–660. [Google Scholar] [CrossRef]
- Kaushik, S.; Arias, E.; Kwon, H.J.; Lopez, N.M.; Athonvarangkul, D.; Sahu, S.; Schwartz, G.J.; E Pessin, J.; Singh, R. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Rep. 2012, 13, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archanco, M.; Muruzábal, F.J.; Llopiz, D.; Garayoa, M.; Gómez–Ambrosi, J.; Frühbeck, G.; Burrell, M.A. Leptin expression in the rat ovary depends on estrous cycle. J. Histochem. Cytochem. 2003, 51, 1269–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, C.; Martinez-Lopez, N.; Otten, E.G.; Carroll, B.; Maetzel, D.; Singh, R.; Sarkar, S.; Korolchuk, V.I. Autophagy, lipophagy and lysosomal lipid storage disorders. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2016, 1861, 269–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chen, M.; Wang, X.; Wang, Y.; Duan, C.; Gao, G.; Lu, L.; Wu, X.; Wang, X.; Yang, H. Piperine induces autophagy by enhancing protein phosphotase 2A activity in a rotenone-induced Parkinson’s disease model. Oncotarget 2016, 7, 60823–60843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Latif, A.S.A.; Saleh, N.; Allam, T.S.; Ghazy, E.W. The Effects of Rosemary (Rosemarinus afficinalis) and Garlic (Allium sativum) Essential Oils on Performance, Hematological, Biochemical and Immunological parameters of Broiler Chickens. Br. J. Poult. Sci. 2013, 2, 16–24. [Google Scholar]


| Experimental Diets | |||
|---|---|---|---|
| Starter | Grower | Finisher | |
| Yellow corn | 57.40 | 60.10 | 62.00 |
| Soybean meal, 47.5% | 34.66 | 29.00 | 25.00 |
| Corn gluten, 59.3% | 3.00 | 4.00 | 4.00 |
| Wheat bran | - | - | 1.90 |
| Soybean oil | 1.10 | 3.00 | 3.66 |
| Calcium carbonate | 1.00 | 1.00 | 0.90 |
| Dicalcium phosphate | 1.80 | 1.90 | 1.60 |
| Common salt | 0.30 | 0.30 | 0.30 |
| Premix 1 | 0.30 | 0.30 | 0.30 |
| DL-Methionine, 98% | 0.18 | 0.14 | 0.11 |
| Lysine, HCL, 78% | 0.16 | 0.16 | 0.13 |
| Antimycotoxin | 0.10 | 0.10 | 0.10 |
| Calculated composition | |||
| ME, Kcal/Kg | 3004.02 | 3157.17 | 3202.02 |
| CP, % | 23.01 | 21.10 | 19.57 |
| EE, % | 3.63 | 5.55 | 6.24 |
| CF, % | 2.66 | 2.53 | 2.64 |
| calcium, % | 0.97 | 0.98 | 0.86 |
| Available P% | 0.47 | 0.47 | 0.41 |
| Lysine, % | 1.37 | 1.22 | 1.10 |
| Methionine, % | 0.56 | 0.51 | 0.46 |
| Gene | Primer Sequence (5′-3′) | Accession No. |
|---|---|---|
| Digestive-enzyme-related genes | ||
| AMY2A | F: CGGAGTG↓GATGTTAACGACTGG R: ATGTTCGCAGACCCAGTCATTG | NM_001001473.2 |
| PNLIP | F: GCATCTGGGAAG↓GAACTAGGG R. TGAACCACAAGCATAGCCCA | NM_001277382.1 |
| CELA1 | F: AGCGTAAGGAAATGGGGTGG R. GTGGAGACCCCATGCAAGTC | XM_017007509.2 |
| CCK | F: AGGTTCCACTGGGAGGTTCT R: CGCCTGCTGTTCTTTAGGAG | XM_015281332.1 |
| Lipogenesis genes | ||
| FAS | F: GCAGCTTCGGTGCCTGTGGTT R: GCTGCTTGGCCCACACCTCC | NM205155 |
| ACC | F: TGCCTCCGAGAACCCTAA R: TCCAGGCTTGATACCACA | JQ080306 |
| Immune-related genes | ||
| IL-10 | F: GCTGAGGGTGAAGTTTGAGG R: AGACTGGCAGCCAAAGGTC | XM_025143715.1 |
| IgA | F: ACCACGGCTCTGACTGTACC R: CGATGGTCTCCTTCACATCA | S40610.1 |
| Autophagy-related genes | ||
| mTOR | F: CATGTCAGGCACTGTGTCTATTCTC R: CTTTCGCCCTTGTTTCTTCACT | XM_417614.5 |
| Atg5 | F: TCACCCCTGAAGATGGAGAGA R: TTTCCAGCATTGGCTCAATTC | NM_001006409 |
| Atg 7 | F: ACTGGCAATGCGTGTTTCAG R: CGATGAACCCAAAAGGTCAGA | NM_001030592 |
| Atg12 | F: GCACCCGCACCATCCA R: GAGGCCATCAGCTTCAGGAA | XM_003643073 |
| Housekeeping | ||
| GAPDH | F: CAACCCCCAATGTCTCTGTT R: TCAGCAGCAGCCTTCACTAC | NM205518 |
| Parameters | Experimental Groups | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| NC | EOs | EOs + BPO0.25 | EOs + BPO0.5 | EOs + RSO0.25 | EOs + RSO0.5 | |||
| Initial BW (g/bird) | 44.40 | 44.5 | 44.94 | 44.96 | 44.54 | 44.76 | 0.16 | 0. 904 |
| Final BW (g/bird) | 2259 d | 2375 c | 2378 c | 2422 ab | 2411 b | 2466 a | 21.11 | <0.001 |
| BWG (g/bird) | 2215 d | 2331 c | 2333 c | 2377 ab | 2366 b | 2421 a | 21.14 | <0.001 |
| FI (g/bird) | 3899 | 3893 | 3830 | 3825 | 3843 | 3879 | 9.71 | 0.075 |
| FCR | 1.76 a | 1.67 b | 1.64 bc | 1.61 c | 1.62 c | 1.60 c | 0.02 | 0.014 |
| RGR (%) | 192.29 | 192.63 | 192.58 | 192.69 | 192.74 | 192.85 | 0.07 | 0.08 |
| PER | 2.80 c | 2.94 b | 3.00 ab | 3.07 a | 3.04 a | 3.08 a | 0.03 | <0.001 |
| Experimental Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Digestion Coefficient, % | NC | EOs | EOs + BPO0.25 | EOs + BPO0.5 | EOs + RSO0.25 | EOs + RSO0.5 | SEM | p-Value |
| DM | 77.64 b | 79.00 b | 82.07 a | 82.99 a | 81.04 a | 81.68 a | 0.49 | <0.001 |
| CP | 70.75 d | 71.43 c | 72.780 b | 74.19 a | 72.37 b | 73.51 ab | 0.36 | 0.029 |
| EE | 80.36 e | 81.87 d | 83.20 b | 85.74 a | 81.61 cd | 82.74 c | 0.45 | <0.001 |
| Experimental Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | NC | EOs | EOs + BPO0.25 | EOs + BPO0.5 | EOs + RSO0.25 | EOs + RSO0.5 | SEM | p-Value |
| Total protein (g/ dL) | 3.32 d | 3.38 c | 3.50 b | 3.77 a | 3.49 b | 3.80 a | 0.03 | <0.001 |
| Albumin (g/dL) | 1.75 e | 1.80 d | 1.87 c | 1.93 b | 1.88 c | 1.97 a | 0.01 | <0.001 |
| Globulin (g/ dL) | 1.58 c | 1.58 bc | 1.63 b | 1.83 a | 1.61 bc | 1.83 a | 0.02 | <0.001 |
| Albumin/globulin ratio | 1.11 bc | 1.14 ab | 1.15 a | 1.06 d | 1.16 a | 1.07 cd | 0.01 | <0.001 |
| TAG (mg/ dL) | 60.68 a | 57.08 b | 54.54 d | 54.09 d | 55.33 c | 54.87 cd | 0.42 | <0.001 |
| Total cholesterol (mg/ dL) | 131.00 a | 124.45 b | 119.74 d | 119.39 d | 122.12 c | 121.73 c | 0.74 | <0.001 |
| HDL (mg/ dL) | 90.17 e | 96.03 d | 102.92 b | 105.59 a | 98.80 c | 99.88 c | 0.93 | <0.001 |
| VLDL (mg/ dL) | 12.14 a | 11.42 b | 10.91 d | 10.82 d | 11.07 c | 10.97 cd | 0.08 | <0.001 |
| LDL (mg/ dL) | 28.69 a | 17.00 b | 2.99 f | 5.92 e | 12.26 c | 10.87 d | 1.56 | <0.001 |
| ALT (U/L) | 18.00 | 17.92 | 18.22 | 19.12 | 18.06 | 18.78 | 0.19 | 0.40 |
| AST (U/L) | 17.76 | 18.42 | 17.92 | 18.94 | 18.48 | 18.82 | 0.19 | 0.41 |
| Experimental Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | NC | EOs | EOs + BPO0.25 | EOs + BPO0.5 | EOs + RSO0.25 | EOs + RSO0.5 | SEM | p-Value |
| Lysozymes (μ/mL) | 0.79 d | 0.81 d | 0.86 b | 1.00 a | 0.83 c | 0.87 b | 0.01 | <0.001 |
| IgM (mg/dL) | 13.26 d | 14.16 c | 16.11 b | 18.10 a | 14.44 c | 16.27 b | 0.31 | <0.001 |
| IgG (mg/dL) | 1.68 d | 1.84 c | 2.04 b | 2.40 a | 1.84 c | 2.06 b | 0.05 | <0.001 |
| Phagocytic, % | 61.28 d | 63.86 c | 68.56 b | 83.46 a | 64.68 c | 68.88 b | 1.37 | <0.001 |
| Parameters | Experimental Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| NC | EOs | EOs + BPO0.25 | EOs + BPO0.5 | EOs + RSO0.25 | EOs + RSO0.5 | SEM | p-Value | |
| Total bacterial count | 15.39 a | 13.51 b | 13.50 b | 11.41 c | 13.65 b | 12.00 c | 0.32 | <0.001 |
| Coliforms count | 7.39 a | 5.94 b | 5.50 c | 4.35 e | 5.66 bc | 5.00 d | 0.23 | <0.001 |
| Lactobacilli count | 5.36 e | 7.28 d | 7.91 cd | 9.69 a | 8.05 c | 8.79 b | 0.33 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishawy, A.T.Y.; Al-Khalaifah, H.S.; Nada, H.S.; Roushdy, E.M.; Zaglool, A.W.; Ahmed Ismail, T.; Ibrahim, S.M.; Ibrahim, D. Black Pepper or Radish Seed Oils in a New Combination of Essential Oils Modulated Broiler Chickens’ Performance and Expression of Digestive Enzymes, Lipogenesis, Immunity, and Autophagy-Related Genes. Vet. Sci. 2022, 9, 43. https://doi.org/10.3390/vetsci9020043
Kishawy ATY, Al-Khalaifah HS, Nada HS, Roushdy EM, Zaglool AW, Ahmed Ismail T, Ibrahim SM, Ibrahim D. Black Pepper or Radish Seed Oils in a New Combination of Essential Oils Modulated Broiler Chickens’ Performance and Expression of Digestive Enzymes, Lipogenesis, Immunity, and Autophagy-Related Genes. Veterinary Sciences. 2022; 9(2):43. https://doi.org/10.3390/vetsci9020043
Chicago/Turabian StyleKishawy, Asmaa T. Y., Hanan S. Al-Khalaifah, Hend S. Nada, Elshimaa M. Roushdy, Asmaa W. Zaglool, Tamer Ahmed Ismail, Seham M. Ibrahim, and Doaa Ibrahim. 2022. "Black Pepper or Radish Seed Oils in a New Combination of Essential Oils Modulated Broiler Chickens’ Performance and Expression of Digestive Enzymes, Lipogenesis, Immunity, and Autophagy-Related Genes" Veterinary Sciences 9, no. 2: 43. https://doi.org/10.3390/vetsci9020043
APA StyleKishawy, A. T. Y., Al-Khalaifah, H. S., Nada, H. S., Roushdy, E. M., Zaglool, A. W., Ahmed Ismail, T., Ibrahim, S. M., & Ibrahim, D. (2022). Black Pepper or Radish Seed Oils in a New Combination of Essential Oils Modulated Broiler Chickens’ Performance and Expression of Digestive Enzymes, Lipogenesis, Immunity, and Autophagy-Related Genes. Veterinary Sciences, 9(2), 43. https://doi.org/10.3390/vetsci9020043







