Canine Stem Cell Conditioned Media Accelerates Epithelial Migration in the Canine Tympanic Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Canine Adipose Tissue-Derived Mesenchymal Stem Cells (cAD-MSCs)
2.2. Collection of Canine Keratinocyte-Conditioned Media (cKCM)
2.3. Quantification of Basic Fibroblast Growth Factor (bFGF), Interleukin-6 (IL-6), and Vascular Endothelial Growth Factor-A (VEGF-A) in SCCM
2.4. Laboratory Animals
2.5. Evaluation of the Ear Canals
2.6. Ink Spotting, Repeated Re-Evaluation, and cAD-MSCCM Application
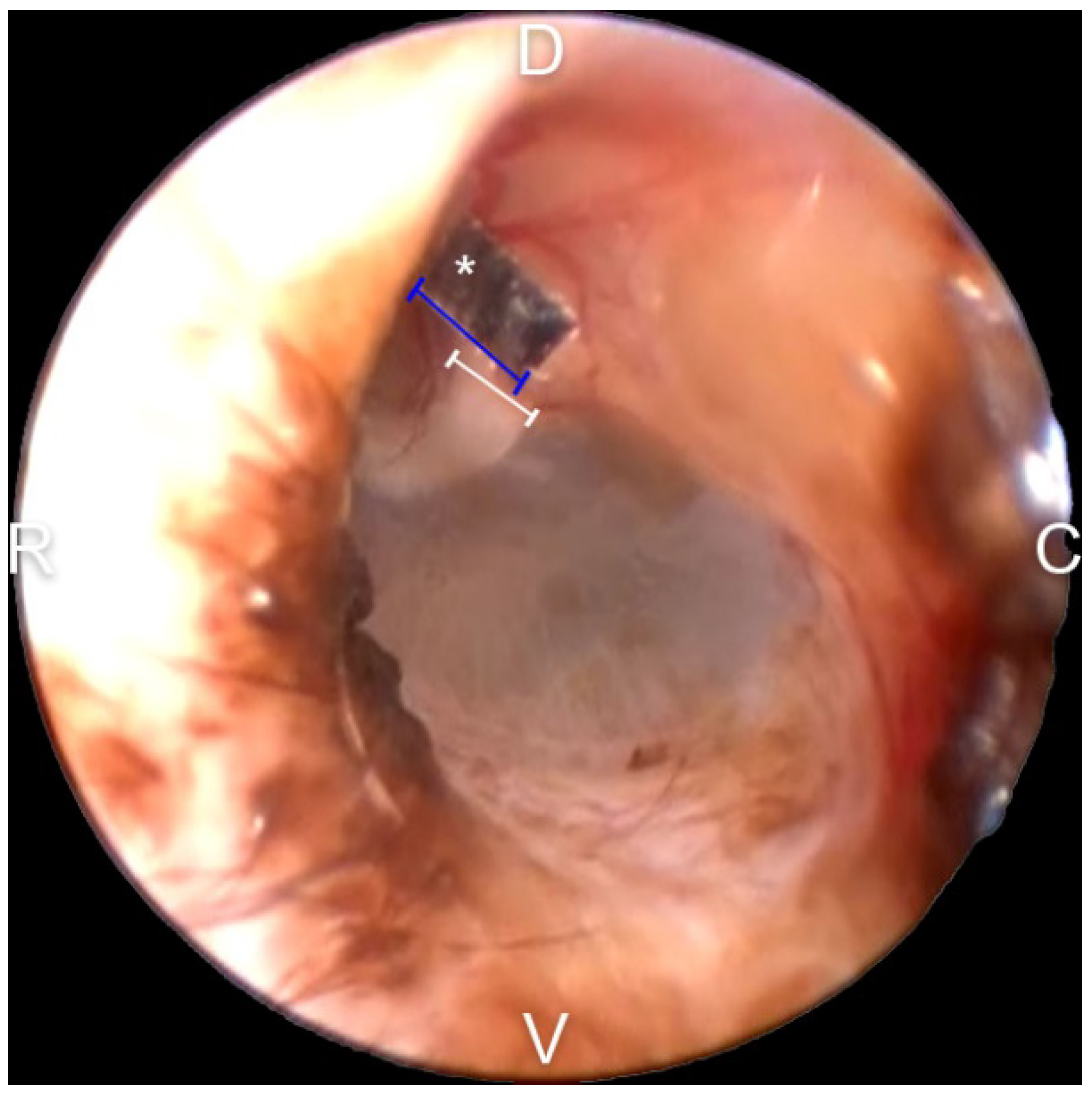
2.7. Malleus Width Measurement for Calibration
2.8. Evaluation of the TM EM Rate
2.9. Statistical Analysis
3. Results
3.1. Levels of bFGF, IL-6, and VEGF-A in cAD-MSCCM and cKCM
3.2. Malleus Width Measurement and Calibration
3.3. Evaluation of the TM EM Rate and Effect of cAD-MSCCM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cole, L.K. Anatomy and physiology of the canine ear. Vet. Dermatol. 2009, 20, 412–421. [Google Scholar] [CrossRef]
- Paterson, S.; Tobias, K. Atlas of Ear Diseases of the Dog and Cat; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 18–61. [Google Scholar]
- Alberti, P.W. The anatomy and physiology of the ear and hearing. In Occupational Exposure to Noise: Evaluation, Prevention, and Control; World Health Organization: Dortmund, Germany, 2001; pp. 53–62. [Google Scholar]
- Teh, B.M.; Marano, R.J. Tissue engineering of the tympanic membrane. Tissue Eng. Part B Rev. 2013, 19, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Hawke, M. The function of migratory epidermis in the healing of tympanic membrane perforations in guinea-pig: A photographic study. Acta Oto-Laryngol. 1987, 103, 81–86. [Google Scholar] [CrossRef]
- Tabacca, N.E.; Cole, L.K. Epithelial migration on the canine tympanic membrane. Vet. Dermatol. 2011, 22, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Z.X.; Shi, G.S. Age-related epithelial migration on the tympanic membrane of the Mongolian gerbil. Otolaryngol. Head Neck Surg. 1988, 98, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Tinling, S.P.; Chole, R.A. Gerbilline cholesteatoma development part I: Epithelial migration pattern and rate on the gerbil tympanic membrane: Comparisons with human and guinea pig. Otolaryngol. Head Neck Surg. 2006, 134, 788–793. [Google Scholar] [CrossRef]
- Makino, K.; Amatsu, M. Epithelial migration on the tympanic membrane and external canal. Arch. Otorhinolaryngol. 1986, 243, 39–42. [Google Scholar] [CrossRef]
- Alberti, P. Epithelial migration on the tympanic membrane. J. Laryngol. Otol. 1964, 78, 808–830. [Google Scholar] [CrossRef]
- Miller, W.H.; Karen, L.C. Muller and Kirk’s Small Animal Dermatology, 7th ed.; Saunders: St. Louis, MO, USA, 2012; p. 741. [Google Scholar]
- Rasheda, A.; Magada, A.M. Role of Stem Cells in Healing of Tympanic Membrane Perforations. Med. J. Cairo Univ. 2019, 87, 1951–1955. [Google Scholar] [CrossRef]
- Rahman, A.; Olivius, P. Stem cells and enhanced healing of chronic tympanic membrane perforation. Acta Otolarygol. 2008, 128, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jin, S.Y. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann. Dermatol. 2012, 24, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Unge, M.; Dirckx, J.J. Embryonic stem cells enhance the healing of tympanic membrane perforations. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, 215–219. [Google Scholar] [CrossRef]
- Joseph, A.; Baiju, I. Mesenchymal stem cell-conditioned media: A novel alternative of stem cell therapy for quality wound healing. J. Cell. Physiol. 2020, 235, 5555–5569. [Google Scholar] [CrossRef]
- Ong, H.T.; Redmond, S.L. Paracrine activity from adipose-derived stem cells on in vitro wound healing in human tympanic membrane keratinocytes. Stem Cells Dev. 2017, 26, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Yunseok, J.; Tae-Ho, O. Anti-inflammatory Effects of the Conditioned Media from Canine Adipose Tissue-derived Mesenchymal Stem Cells on Canine Keratinocytes. J. Vet. Clin. 2020, 37, 41. [Google Scholar]
- Lim, D.; Bae, S.; Oh, T. Anti-inflammatory effect of shea butter extracts in canine keratinocytes. J. Vet. Clin. 2021, 38, 27–31. [Google Scholar] [CrossRef]
- Nuttall, T.; Bensignor, E. A pilot study to develop an objective clinical score for canine otitis externa. Vet. Dermatol. 2014, 25, 530.e92. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.; Nuttall, T. Clinical Techniques: When and how to do a myringotomy—A practical guide. Vet. Dermatol. 2021, 32, 302.e82. [Google Scholar] [CrossRef]
- Shell, L.G. Otitis media and otitis interna: Etiology, diagnosis, and medical management. Vet. Clin. N. Am. Small Anim. Pract. 1988, 18, 885–899. [Google Scholar] [CrossRef]
- Cho, K.S.; Lee, D.G. The importance of vascular endothelial growth factor in the healing of acute tympanic membrane perforation. Am. J. Pathol. 2010, 31, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A.; Matthies, A.M. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am. J. Pathol. 2005, 167, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Grossman, R.M.; Krueger, J. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc. Natl. Acad. Sci. USA 1989, 86, 6367–6371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sogabe, Y.; Abe, M. Basic fibroblast growth factor stimulates human keratinocyte motility by Rac activation. Wound Repair Regen. 2006, 14, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.C.; Wang, Y.B. Healing outcomes of large (>50%) traumatic membrane perforations with inverted edges following no intervention, edge approximation and fibroblast growth factor application; a sequential allocation, three-armed trial. Clin. Otolaryngolol. 2013, 38, 289–296. [Google Scholar] [CrossRef]
- Ishimoto, S.I.; Ishibashi, T. Direct application of keratinocyte growth factor, basic fibroblast growth factor and Transforming Growth Factor-α during healing of tympanic membrane perforation in glucocorticoid-treated rats. Acta Otolaryngol. 2002, 122, 468–473. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z. Spontaneous healing of various tympanic membrane perforations in the rat. Acta Otolaryngolol. 2004, 124, 1141–1144. [Google Scholar] [CrossRef]
- Anderson, R.G.; Wright, C.G. Inflammatory effects of otic drops on the middle ear. Int. J. Pediatr. Otorhinolaryngolol. 1984, 7, 91–95. [Google Scholar] [CrossRef]




| Width of the Malleus (μm) | ||
|---|---|---|
| Left Ear (LE) | Right Ear (RE) | |
| Beagle 1 | 1129.4 | 957.3 |
| Beagle 2 | 1146.5 | 958.0 |
| Beagle 3 | 1217.9 | 1011.3 |
| Beagle 4 | 763.5 | 993.5 |
| Total | 1064.0 ± 204.2 | 980.0 ± 26.8 |
| 1022 ± 142.2 | ||
| TM EM Rate per Day (μm/d) | ||
|---|---|---|
| Side of Ear | Treatment Group | Control Group |
| LE | 208.7 ± 62.1 | 128.8 ± 26.0 |
| RE | 216.3 ± 79.9 | 136.3 ± 37.8 |
| Total | 212.5 ± 66.4 | 133.1 ± 30.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, H.; Kim, S.; Oh, T.; Bae, S. Canine Stem Cell Conditioned Media Accelerates Epithelial Migration in the Canine Tympanic Membrane. Vet. Sci. 2022, 9, 69. https://doi.org/10.3390/vetsci9020069
Suh H, Kim S, Oh T, Bae S. Canine Stem Cell Conditioned Media Accelerates Epithelial Migration in the Canine Tympanic Membrane. Veterinary Sciences. 2022; 9(2):69. https://doi.org/10.3390/vetsci9020069
Chicago/Turabian StyleSuh, Hyerin, Suhyun Kim, Taeho Oh, and Seulgi Bae. 2022. "Canine Stem Cell Conditioned Media Accelerates Epithelial Migration in the Canine Tympanic Membrane" Veterinary Sciences 9, no. 2: 69. https://doi.org/10.3390/vetsci9020069
APA StyleSuh, H., Kim, S., Oh, T., & Bae, S. (2022). Canine Stem Cell Conditioned Media Accelerates Epithelial Migration in the Canine Tympanic Membrane. Veterinary Sciences, 9(2), 69. https://doi.org/10.3390/vetsci9020069






