Abstract
Medial compartment disease is a common occurrence in dogs affected by elbow dysplasia. Despite many treatments suggested in the literature, only few studies reported comparative outcomes in the short and long term. The aim of this study is to report and compare short- and long-term clinical and radiographic outcomes of dogs treated for medial compartment disease (MCD) by distal dynamic ulnar ostectomy (DUO), bi-oblique dynamic proximal ulnar osteotomy (BODPUO) and conservative management (CM). From 2016 to 2018, all medium to large dogs, aged between 5 and 12 months, affected by uni/bilateral MCD and treated by DUO, BODPUO or CM, were enrolled in this study and followed up for 24 months. Orthopedic and radiographic examinations were performed at T0, T2, T12 and T24 months after treatment. Lameness score, elbow arthralgia, elbow range of motion (ROM), osteoarthritis (OA) score and percentage of ulnar subtrochlear sclerosis (%STS) were evaluated at each time point. According to the treatment performed, dogs were divided into three groups: DUO, BODPUO and CM. Forty-five elbows from twenty-six dogs, treated with DUO (n = 17), BODPUO (n = 17) or CM (n = 11), were prospectively enrolled in the study. The patients enrolled in the CM group were older and showed more severe radiographic signs of OA, compared to those enrolled in the other two groups. Lameness and arthralgia scores (p < 10−4) were significantly decreased in patients that underwent surgical treatment and increased in patients managed conservatively (lameness p < 10−4, arthralgia p = 0.3068), at T12 and T24. OA score (p < 0.0040) and ROM (DUO, CM p < 10−4; BODPUO p = 0.0740) worsened in every study group, but %STS decreased in DUO (p = 0.0108), increased in the CM group (p = 0.0025) and remained unchanged in the BODPUO group (p = 0.2740). This study supports the clinical efficacy of DUO and BODPUO in reducing lameness, arthralgia and progression of %STS. Early diagnosis and surgical attention in patients affected by MCD can improve the short- and long-term outcome and reduce the progression of secondary changes.
1. Introduction
Elbow dysplasia (ED) is a polygenic and multifactorial disease most commonly affecting young large breed dogs. ED includes medial coronoid process disease (MCPD), osteochondrosis/osteochondritis dissecans (OC/OCD) of the humeral trochlea, ununited anconeal process (UAP) and joint incongruence (INC). These conditions can occur alone, or in association with each other [1,2,3,4,5,6,7,8,9,10,11].
MCPD is the most prevalent condition and encompasses fragmentation (FCP), fissuring, sclerosis and cartilage damage of the medial coronoid process [5,6,7,8,9,10,11]. MCPD can be associated with lesions of the joint cartilage of the humeral trochlea, resulting from humeroulnar conflict (kissing lesions) [12,13,14,15,16,17]. Cartilage degradation and ED primary lesions that affect the medial elbow compartment lead to osteoarthritis (OA) of the medial compartment joint. This advanced stage of ED, involving only the medial aspect of the canine elbow joint, is referred to as medial compartment disease (MCD). Medial compartment disease is characterized by an alternation of inflammation and degeneration of the elbow joint, that compromises dysplastic patients’ quality of life, causing advanced stage of OA, reduced range of motion, pain, lameness and reluctance to move [5,6,7,8,18].
Currently, there is no single treatment for all recognized pathological manifestations, but there are various therapeutic options [5,6,13,16,18,19,20,21,22,23,24,25,26]. The therapeutic choice must be based on the type and extension of the intra-articular lesion, on the severity of pre-existing OA and cartilage damage, on the patient’s age and on the severity of clinical signs [8,27].
In recent years, research has been focused on a decision-making algorithm suggesting the most appropriate treatment for the patient [8,27]. Early treatments to correct the suspected underlying cause are preferred in young dogs with absent or minimal articular degeneration. The objective of treatments is to positively impact the development of the disease, slow down the progression of OA and improve patients’ quality of life [8,27]. Early treatments include fragment removal (in case of FCP and OCD), subtotal coronoid ostectomy (SCO) [6], and removal and debridement or replacement of degenerated cartilage [20], in association with dynamic ulnar osteotomy/ectomy that aims to homogeneously distribute the intra-articular loads [8,16,27,28,29].
Distal dynamic ulnar ostectomy (DUO) and bi-oblique dynamic proximal ulnar osteotomy (BODPUO) are early surgical procedures [8,27,30,31] that rely on the forces acting on the proximal ulnar segment to allow it to displace into a more appropriate position, dictated by the action of soft tissues, articular interface interaction and loading forces [9,28,32,33]. The orientation of the proximal ulna relative to the radius and humerus changes when it is unconstrained after osteotomy, which may reduce humero-ulnar conflict [8,16,29,34].
There is a broad agreement about the necessity of an early diagnosis and treatment for a better prognosis [12]. Only few studies directly compared different treatments and a prospective analysis with objective measurements of clinical development and OA evolution in patients treated following the algorithm is lacking. The aim of this article is to report and compare short- and long-term outcomes obtained from clinical and radiographic evaluation after DUO, BODPUO and conservative management (CM) in dogs affected by MCD. We hypothesized that the clinical outcomes would improve after surgery, while the radiographic evaluation would indicate a constant progression of OA, regardless of the performed treatment.
2. Materials and Methods
2.1. Animals, Clinical and Radiographic Examinations
Medium to large breed dogs, of less than a year of age, with unilateral or bilateral MCD, were prospectively enrolled in the study. Dogs affected by any other orthopedic or neurologic condition were excluded from the study. Patients underwent complete orthopedic and neurologic examination. Standard International Elbow Working Group (IEWG) radiographic projections of the affected elbows (neutral mediolateral, flexed mediolateral and craniocaudal 15° pronated) were acquired to confirm MCD diagnosis. When the MCD diagnosis was confirmed, the recruited dysplastic joints were divided into study groups according to the therapy received: DUO, BODPUO or CM (DUO, BODPUO and CM groups, respectively).
Clinical and radiographic evaluations were performed by an expert orthopedic surgeon (A.P.P) the day of treatment, or when the CM was started, (T0), at 2 months (T2), at 12 months (T12) and at 24 months (T24) after treatment. Age, breed, body weight (BW), body condition score (BCS), lameness and arthralgia scores and ROM were recorded at each time point by the same operator.
The degree of lameness and arthralgia was assessed by an expert orthopedic surgeon (A.P.P) using a modified Numerical Rating Scale (NRS) proposed by Vasseur et al. (1995) [35,36] (Table 1). ROM was clinically measured with an orthopedic goniometer [37].

Table 1.
NRS used to assess the degree of lameness and arthralgia.
OA was assessed with the modified IEWG scoring system using the form by Lang et al. 1998: score 0 = normal (grade 0); score 1 = borderline (grade BL); score 2–4 = mild OA (grade 1); score 5–8 = moderate OA (grade 2); score > 8 severe OA (grade 3) [38]. The same IEWG modified scoring system was used to classify the INC, based on magnitude of radio-ulnar and humero-ulnar steps: mild INC, step < 2 mm; moderate INC, <4 mm; severe INC, >4 mm. The subtrochlear sclerosis of the ulna (STS) was measured using a percentage scale (% STS), as previously described [10,39,40,41] (Figure 1).
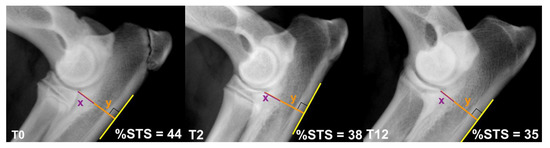
Figure 1.
The %STS was calculated as 100(x/y). The craniocaudal ulnar depth (y) was measured from the most proximocaudal aspect of the radial head to the most caudal margin of the ulnar proximal metaphyseal cortex; the depth of sclerosis (x) was measured from the most proximocaudal aspect of the radial head to the STS caudal border. The figure represents the %STS detected on the same elbow at 0, 2 and 12 months from DUO.
2.2. Treatment
The selection of the appropriate treatment for each patient was performed according to the treatment algorithms available in the literature [8,12,27,30].
Four- to six-month-old symptomatic puppies with mild radiographic changes (presence of STS without osteophytes and/or mild INC and MCPD) were treated with DUO surgery. Four- to eight-month-old symptomatic dogs with more severe radiographic changes (presence of STS and moderate INC, MCPD and/or OCD-kissing lesion) were treated with BODPUO surgery.
Four- to twelve-month-old symptomatic puppies with radiographic signs of severe MCD (severe INC, MCPD and/or OCD-kissing lesion and sign of OA) were treated with CM. CM was performed also in dogs where surgical options were declined by the owners. Conservative management consisted of weight control, a joint-type diet, modulation of on-lead exercise and 14 days of oral carprofen (4 mg/kg for 7 days followed by oral carprofen 2 mg/kg for 7 days). Administration of carprofen was repeated as needed.
Patients from DUO and BODPUO groups also underwent a diagnostic elbow arthroscopy. If present, fissure/fracture of the medial coronoid process was arthroscopically removed. A modified Robert Jones bandage was applied for 24 h postoperatively and carprofen (4 mg/kg orally once daily) was administered for 7 days in all dogs. Physical activity was restricted during postoperative phase with lead walks for 2 months. A weight control and joint-type diet were subsequently prescribed.
2.3. Statistical Analysis
Degree of lameness and arthralgia, BCS and OA scores were compared between groups using Kruskal–Wallis test followed by Dunn’s multiple comparison test, or using Mann–Whitney test, where appropriate, at each time point. Friedman analysis followed by Dunn’s test were used to perform a multiple comparison between different time points within each group.
Cardinal data were assessed for normality using D’Agostino–Pearson test. Range of motion and %STS were compared between groups using One-Way ANOVA analysis followed by Holm–Sidak post hoc test or using Student’s t-test, where appropriate. A comparison between different time points within each group was performed using Repeated Measures ANOVA followed by Holm–Sidak test.
Statistical analysis was performed in GraphPad Prism, version 8.2.1 (GraphPad Software Inc., San Diego, CA, USA) and p < 0.05 was considered statistically significant.
3. Results
3.1. Animals, Diagnosis and Treatment
Twenty-six dogs (45 elbows) were enrolled in the study. Thirteen breeds were represented: nine Labrador Retriever, three German Shepherd, two Boxer, two White Swiss Shepherd Dog, two Border Collie, one Saint Bernard, one Chow Chow, one Bernese Mountain Dog, one Golden Retriever, one English Bulldog, one Great Dane, one Tchorny Terrier and one American Staffordshire Terrier, for a total of nineteen males and seven females. Nineteen dogs were bilaterally affected and seven unilaterally. Forty-five elbows were enrolled in the study. Twenty-one right and twenty-four left joints were treated. Mean ± SD age at T0 was 6.7 ± 1.3 months. Mean ± SD BW and BCS were 25.2 ± 7.9 kg and 4.6 ± 0.6, at T0, 27.8 ± 8.5 kg and 4.6 ± 0.6 at T2, 33.2 ± 10.3 kg and 5.5 ± 0.9 at T12, and 36.8 ± 9.9 kg and 6.4 ± 1.3 at T24, respectively.
The DUO group included 17 elbows, the BODPUO group included 17 elbows and the CM group included 11 elbows (Table 2). In DUO, BODPUO and CM groups, mean ± SD age at the moment of treatment was 5.9 ± 0.6, 6.6 ± 0.6 and 8.0 ± 1.0 months, respectively. BW and BCS means ± SD were 24.3 ± 6.2 kg and 4.6 ± 0.5, 24.2 ± 6.3 kg and 4.6 ± 0.9, and 28.3 ± 10.9 kg and 4.5 ± 0.5 at T0, respectively. They were 26.9 ± 6.7 kg and 4.6 ± 0.5, 26.6 ± 7.1 kg and 4.6 ± 0.9, and 30.9 ± 11.7 kg and 4.6 ± 0.5 at T2. At T12 they were 32.6 ± 8.0 kg and 5.2 ± 0.8, 31.4 ± 9.9 kg and 5.6 ± 0.9, and 36.2 ± 12.3 kg and 5.7 ± 0.8, while they were 38.9 ± 7.8 kg and 5.6 ± 1.0, 35.9±12.1 kg and 7.0 ± 0.6, and 34.7 ± 6.5 kg and 6.5 ± 2.6 at T24.

Table 2.
Elbows enrolled in the study. Diagnosis and treatment.
All the 45 elbows were evaluated after therapy at T2 and T12, while only 22 joints (13 dogs) were evaluated at T24 follow-up. In particular, seven elbows in the DUO group, seven elbows in the BODPUO group and eight elbows in the CM group were evaluated ad T24.
Within the BODPUO group, the radiographic follow-ups showed an excessive proximal ulnar displacement in five elbows [28]. Therefore, the BODPUO group was further divided into the BODPUO-D group, which included five joints with proximal segment dislocated, and the BODPUO-ND group, which included 12 joints with proximal segment not dislocated.
3.2. Clinical Evaluation Findings
The lameness score decreased significantly in the DUO group (χ2r = 15.00; p = 0.0018) and in the BODPUO group (χ2r = 31.26; p < 0.10−4) at long-term evaluation compared with preoperative values (DUO: T12 p = 0.0065, T24 p = 0.0140; BODPUO: T12, T24 p = 0.0003). In the CM group the lameness score increased during follow-up (χ2r = 25.150; p < 10−4): at T0 and T2 it was lower in the CM group compared with BODPUO (T0 p = 0.0028, T2 p = 0.0031) and DUO (T0 p = 0.1047, T2 p = 0.0987) groups, but it was higher at T12 (DUO p = 0.0592, BODPUO p = 0.0122) and T24 (DUO p = 0.0192, BODPUO p = 0.0029) (Figure 2a). The results of the comparative statistical analysis performed on the DUO, BODPUO-ND and CM groups (Figure 2c) are very similar to that on the DUO, BODPUO and CM groups. There was no statistically significant difference between the lameness score of patients from the BODPUO and BODPUO-ND groups, when compared to DUO and CM groups (Figure 2c). Lameness score significantly decreased in BODPUO-ND group in long-term evaluations (χ2r = 32.44; p < 10−4) and it was lower than BODPUO-D group at T2 (T = 25.50; p = 0.6833), T12 and T24 (T = 15.00; p = 0.0924) (Figure 2b) (Table 3).
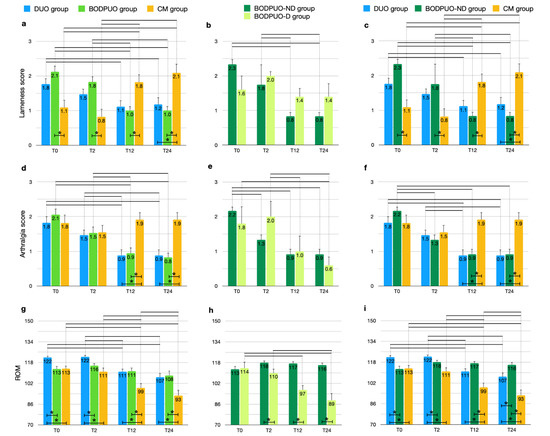
Figure 2.
Comparison of lameness score, arthralgia score and ROM (means and SEM) between DUO, BODPUO and CM groups (a,d,g), between BODPUO-ND and BODPUO-D groups (b,e,h), and between DUO, BODPUO-ND and CM groups (c,f,i) at the beginning of treatment (T0) and at 2 (T2), 12 (T12) and 24 (T24) months after treatment. Asterisk (*) indicates a significant difference (p < 0.05) between groups and the black line (—) indicates a significant difference between time points within the same group.

Table 3.
Comparison of lameness and arthralgia (mean ± SD) between study groups and within each group (on the gray rows) at T0, T2, T12 and T24.
Arthralgia score decreased in DUO (χ2r = 25.47; p < 10−4) and BODPUO groups (χ2r = 31.20; p < 10−4) at long-term evaluation compared with preoperative values (DUO: T12, T24 p = 0.0009; BODPUO: T12, T24 p < 10−4), while in the CM group there was no significant difference between time points (χ2r = 3.610; p = 0.3068). At T12 (H = 12.27; p = 0.0022) and T24 (H = 14.60; p = 0.0007) a higher arthralgia score was detected in the CM group in comparison to DUO (T12 p = 0.0037, T24 p = 0.0028) and BODPUO (T12 p = 0.0072, T24 p = 0.0013) groups (Figure 2d). In BODPUO-ND the arthralgia scores decreased significantly at T2 compared with T0 values (p = 0.0114). There was no significant difference in arthralgia scores between BODPUO-ND and BODPUO-D groups at each time point (p > 0.05) (Figure 2e,f) (Table 3).
ROM significantly decreased in DUO (F = 20.00; p < 10−4) and in CM (F = 61.88; p < 10−4) groups at T24, while there was no significant difference in BODPUO groups (F = 2.461; p = 0.0740). There was significant difference in ROM between groups at each time point. At T0 and T2 ROM was higher in elbows treated by DUO compared with those treated with BODPUO (T0 p = 0.0027, T2 p = 0.0147) and CM (T0 p = 0.0062, T2 p = 0.0004) groups. However, at T12 and T24 DUO (T12 p = 0.0083, T24 p = 0.0210) and BODPUO (T12 p = 0.0083, T24 p = 0.0167), values were significantly higher compared with the CM group (Figure 2g). ROM significantly decreased in the BODPUO-D group (F = 8.589; p = 0.0337), while there was no difference in the BODPUO-ND group (F = 25.48; p = 0.1157). ROM in the BODPUO-ND group was higher than in the BODPUO-D group at T2 (t = 2.624; p = 0.0192), T12 (t = 6.472; p < 0.10−4) and T24 (t = 6.295; p < 0.10−4) (Figure 2h), and significantly higher than in the DUO group at T24 (p = 0.0378) (Figure 2i) (Table 4).

Table 4.
Comparison of ROM and BCS (mean ± SD) between study groups and within each group (on the gray rows) at T0, T2, T12 and T24.
There was no significant difference of BCS between all study groups (p > 0.05), except for the comparison between BODPUO subgroups (p ≤ 0.0123). An improving trend in the BCS was instead appreciated within each group (p ≤ 0.0004) (Table 4).
3.3. Radiographic Examination Findings
The radiographic OA scores significantly increased in all study groups at long-term evaluations (p < 0.05) (Figure 3a). Radiographic signs of OA were more noticeable in the BODPUO-D group than in the BODPUO-ND group at T12 (T = 5.000; p = 0.0068) and T24 (T = 4.500; p = 0.0040), because in the BODPUO-D group the score was significantly increased (χ2r = 14.47; p < 10−4) at T12 (p = 0.0373) and T24 (p = 0.0022) (Figure 3b). From the comparison of DUO, BODPUO-ND and CM groups there was a difference between BODPUO-ND and CM group at T12 (p = 0.0577) and T24 (p = 0.0156). At T12 there was no significant difference between BODPUO and CM groups (p = 0.3289) (Figure 3c) (Table 5).
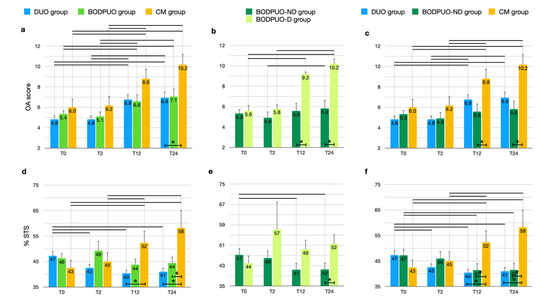
Figure 3.
Comparison of OA score and %STS (means and SEM) between DUO, BODPUO and CM groups (a,d), between BODPUO-ND and BODPUO-D groups (b,e), and between DUO, BODPUO-ND and CM groups (c,f) at the time of treatment (T0) and at 2 (T2), 12 (T12) and 24 (T24) months after treatment. Asterisk (*) indicates a significant difference (p < 0.05) between groups and the black line (—) indicates a significant difference between time points within the same group.

Table 5.
Comparison of OA and %STS (mean ± SD) between study groups and within each group (on the gray rows) at T0, T2, T12 and T24.
In the BODPUO group the %STS remained almost unchanged during follow-up (F = 1.347; p = 0.2740), while in the DUO group it decreased (F = 6.348; p = 0.0108) at T2 (p = 0.0373), T12 (p = 0.0018) and T24 (p = 0.0039). In the CM group it increased (F = 13.20; p = 0.0025) compared with pretreatment values. The %STS was lower in the DUO group compared to CM group at T12 (p = 0.0110) and T24 (p = 0.0050) and it was lower in the BODPUO group compared with the CM group at T24 (p = 0.0189) (Figure 3d). Comparing the means of %STS in DUO, BODPUO-ND and CM groups, a significant difference was also detected between BODPUO-ND and CM groups at T12 (p = 0.0235) (Figure 3f). The %STS decreased in the BODPUO-ND group (F = 13.33; p = 0.0040) and increased in the BODPUO-D group (F = 0.7874; p = 0.4487). At T24, the BODPUO-ND group showed significantly lower %STS than the BODPUO-D group (p = 0.0481) (Figure 3e).
In the BODPUO-D group, immediate postoperative radiographs were used to measure the osteotomy geometry. The means ± SD of the caudo-cranial osteotomy angle and the latero-medial osteotomy angles were 53.2 ± 4.9° and 49.7 ± 1.0°, respectively, while the most caudo-proximal point of osteotomy was situated at 32.7 ± 10.0% of the total ulnar length (Figure 4).
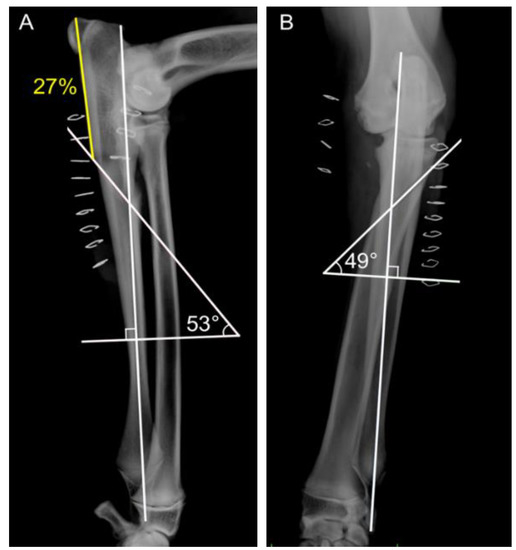
Figure 4.
Postoperative medio-lateral (A) and cranio-caudal (B) radiographic views, following BODPUO, of a patient. The and most caudo-proximal point of osteotomy was situated at 27% of the total ulnar length. (B) Latero-medial osteotomy angle was 49°.
4. Discussion
This prospective study reports and compares short- and long-term clinical and radiographic outcomes in dogs that underwent surgical and conservative treatment to manage MCD.
Division into study groups was challenging because of the broad variety of clinical presentations and surgical techniques associated with MCD [5,6,16,18,19,20,21,22,23,24]. This difficulty explains the current paucity of studies that directly compares all techniques proposed by the decision-making algorithm [8,10,19,27,42]. In the present study, the patients were divided according to whether osteotomies were performed and if DUO or BODPUO were executed.
Ulnar subtrochlear sclerosis is an early sign of elbow dysplasia [30] and has been reported to increase with the progression of the underlying condition [41]. Our results confirmed that the %STS can be useful to assess the progression of the disease, in the short and long term. In addition to that, our results showed that %STS decreased significantly two months after surgery in the DUO group, while it decreased significantly twelve months after surgery in the BODPUO-ND group. This finding supports the hypothesis that DUO and BODPUO may be effective in slowing down the progression of MCD [43]. Our results are consistent with recent clinical studies which showed that the progression of %STS could reduce if the INC is addressed [33,43]. At T0, the lameness score in the CM group was lower compared to DUO and BODPUO groups, despite more severe radiographic evidence of OA. However, increased radiographic evidence of OA is not always clinically directly related to the lameness score. It is possible that the increased peri-articular fibrosis associated with the progression of the disease may, to some extent at least in the short term, have increased joint stability and possibly affected the lameness score. In the short term (T2), lameness score in the CM group was significantly lower when compared to the DUO and BODPUO groups. This finding can be explained by the expected postoperative recovery time, following surgical treatment, in the DUO and BODPUO groups. However, the clinical long-term outcome obtained in the DUO and BODPUO groups was significantly superior to the CM group. Lameness and arthralgia scores decreased, in the long term (T12–T24), in patients treated by DUO and BODPUO, according to previous clinical studies [30]. The decreased lameness and arthralgia scores observed in our study might be explained by a homogenous re-distribution of the intra-articular loads, following DUO/BODPUO [29].
The ROM decreased and OA score increased in all our study groups. Due to osteophytes and fibrosis interfering with the motion of the joint, moderate inverse correlation between ROM and OA has been previously reported [10,44]. Progression of OA and decreased ROM, in the DUO and BODPUO groups, in spite of an improvement of the clinical outcome, is consistent with previous reviews [10,19,29,45].
A recent study demonstrated that BODPUO does not completely restore the INC and increases the humeroulnar rotational instability [29]. The instability could be responsible for the continuous progression of OA and the absence of a clear improvement in ROM. However, at T12 and T24, the progression of OA and the reduced ROM were significantly lower in the DUO and BODPUO groups compared to the CM.
The ROM of elbows treated by DUO was significantly higher than the mean ROM of BODPUO and CM groups before surgery and at T2. This finding was expected, considering that DUO is a surgical procedure recommended in young patients with low cartilage degeneration and with mild clinical symptoms [12,30].
Analyzing elbows treated by BODPUO, we suspected that the excessive proximal ulnar displacement in five elbows had a negative impact on the outcome of the BODPUO group. In fact, at long-term evaluation, the results obtained in the BODPUO-D group were significantly worse than the BODPUO-ND group (excluding lameness and arthralgia score, in which there was no statistical difference).
The mean osteotomy angles and osteotomy position in our study was comparable with what was previously reported by Caron and Fitzpatrick in six elbows with the same complication [28]. In their study, there was no significant difference between the osteotomy angle and position in patients that did and did not develop excessive proximal ulnar displacement. However, the authors supposed that a more acute osteotomy angle or a more proximal osteotomy may lead to excessive motion in some elbows [28]. In a recent study, the authors reported a less severe misalignment than expected if the ulna osteotomy exceeded the recommendations given by Caron and Fitzpatrick [29]. In our study, osteotomies of BODPUO executed too proximally were associated with excessive motion. However, according to recent observations, the excessive motion in our population could also be due to a limited obliquity of the osteotomy [34]. At the long-term follow-up (T24), %STS and OA scores were significantly lower in the BODPUO-ND group than in the BODPUO-D group, while the ROM was significantly higher. Surprisingly, in the BODPUO-D group, despite that the lameness score was not improved at T12 and T24, the arthralgia score was decreased. Considering the low numbers of cases, the subjective assessment of arthralgia, and the theoretical variability of patients’ response to conscious examination, this result is of difficult interpretation. Excluding the BODPUO-D group, the result of the CM, DUO and BODPUO-ND groups, in the present study, are consistent with the data reported in literature [28,30].
This study has several limitations. First of all, the older age and the more severe radiographic changes of the patients in the CM group make the statistical comparison of the data with the DUO and BODPUO group questionable. However, our data support the clinical importance of an early diagnosis and the potential benefits associated with dynamic ulnar osteotomy/ectomy, when case selection is appropriate. On the other hand, the present study highlights how conservative management may have a more limited clinical efficacy in older patients with severe radiographic changes associated with MCD.
A second limit is the lack of use of CT osteoabsorptiometry to objectively describe bone density distribution in subchondral bone at the level of the base of the medial coronoid process [46]. In this study CT osteoabsorptiometry was not used, but this limitation allowed to stress the utility of evaluating the %STS as an objective parameter that anyone with X-ray equipment can use [47].
In the present study, the position of the elbows was standardized, in order to allow repeatable measurements of %STS. However, the INC and the presence of osteophytes on the caudal aspect of the radial head may have affected the assessment of the most proximocaudal aspect of the radial head (a reference point to measure the %STS) [40]. The possibility that %STS may vary depending on the dog breed should be taken into consideration [46]. Therefore, it should be kept in mind that %STS is not a parameter usable to compare individual elbows of dogs of different breeds, but it could be an interesting parameter to assess and monitor the progression of the disease after surgical treatment.
Elbow incongruity was classified by the modified IEWG score during radiographic examination at T0, in order to define the severity of radiographic signs and establish the appropriate treatment. Nevertheless, the INC was not assessed in the short and long term, because the radiographic exam did not allow an accurate measurement. In fact, in the past decade, the golden standard for incongruity detection was CT, which provides images without overlapping [48,49].
The absence of a CT scan or arthroscopy did not allow characterization/grading of the severity of the disease in the CM group. The group consisted in older patients with severe radiographic evidence of OA or patients in which the owners declined surgical options. Assessing the outcome of conservative management in such a heterogenous population is difficult.
Moreover, it is widely recognized that keeping the patient’s body condition score at the low end of the normal range slows the progression of degenerative joint disease and the clinical signs associated with it [50]. All the enrolled dogs increased their BCS throughout the study, thereby the weight gain was a conditioning factor and it could be argued that part of the conservative management in the CM group was not adequately performed and that those patients could have had a better clinical outcome if weight control was implemented.
Several arthroscopic procedures (joint exploration, medial coronoid fragment removal and curettage of the medial compartment) were performed in the DUO and BODPUO groups, as required. The influence of these procedures in the final patients’ outcome is unknown.
A kinetic and kinematic evaluation of the patients before and after treatment may have objectively confirmed our pre and postoperative subjective evaluation of the lameness. Finally, long-term follow-up was not available for all the patients.
5. Conclusions
This study emphasized the beneficial effects of DUO and BODPUO in reducing lameness, arthralgia and extension of %STS in young patients affected by medial compartment disease. These results highlight the possible capacity of early surgical procedures to homogeneously distribute the intra-articular loads and to slow down and reduce the secondary changes. In particular, this was demonstrated for BODPUO [29], but further research should be conducted regarding the humero-ulnar joint kinematics after DUO surgery. Conservative management in older patients with severe radiographic evidence of elbow OA may be associated with a worse short- and long-term outcome.
Author Contributions
Conceptualization, A.P.P.; methodology, A.P.P.; software, D.S. and I.B.; validation, D.S.; formal analysis, S.S. and A.M.T.; investigation, A.P.P., F.G. and A.S.; resources, S.S. and D.S.; data curation, S.S.; writing—original draft preparation, D.S. and S.S.; writing—review and editing, D.S., S.S. and A.P.P.; visualization, F.G.; supervision, A.P.P.; project administration, A.P.P., A.S. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the fact that patients were treated following the current available guidelines for the treatment of medial compartment disease in dogs.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data present in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Ryssen, B.; Van Bree, H. Arthroscopic findings in 100 dogs with elbow lameness. Vet. Rec. 1997, 140, 360–362. [Google Scholar] [CrossRef]
- Morgan, J.P.; Wind, A.; Davidson, A.P. Bone dysplasias in the Labrador retriever: A radiographic study. J. Am. Anim. Hosp. Assoc. 1999, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Lavrijsen, I.C.; Heuven, H.C.; Voorhout, G.; Meji, B.P.; Theyse, L.F.; Leegwater, P.A.; Hasewinkel, H.A. Phenotypic and genetic evaluation of elbow dysplasia in Dutch Labrador Retrievers, Golden Retrievers and Bernese Mountain Dogs. Vet. J. 2012, 193, 486–492. [Google Scholar] [CrossRef]
- Wennemuth, J.; Tellhelm, B.; Eley, N.; Von Pückler, K. Computed tomography enhances diagnostic accuracy in challenging medial coronoid disease cases: An imaging study in dog breeding appeal cases. Vet. Comp. Orthop. Traumatol. 2020, 33, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, N.; Smith, T.J.; Evans, R.B.; Yeadon, R. Radiographic and arthroscopic findings in the elbow joints of 263 dogs with medial coronoid disease. Vet. Surg. 2009, 38, 213–223. [Google Scholar] [CrossRef]
- Fitzpatrick, N.; Smith, T.J.; Evans, R.B.; O’Riordan, J.; Yeadon, R. Subtotal coronoid ostectomy for treatment of medial coronoid disease in 263 dogs. Vet. Surg. 2009, 38, 233–245. [Google Scholar] [CrossRef]
- Bräuer, S.; Böttcher, P. Focal humero-ulnar impingement following subtotal coronoid ostectomy in six dogs with fragmented medial coronoid process. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2015, 43, 156–160. [Google Scholar] [CrossRef]
- Fitzpatrick, N.; Yeadon, R. Working algorithm for treatment decision making for developmental disease of the medial compartment of the elbow in dogs. Vet. Surg. 2009, 38, 285–300. [Google Scholar] [CrossRef]
- Coppieters, E.; Gielen, I.; Verhoeven, G.; Van Vynckt, D.; Van Ryssen, B. Erosion of the medial compartment of the canine elbow: Occurrence, diagnosis and currently available treatment options. Vet. Comp. Orthop. Traumatol. 2015, 28, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, N.P.; Griffon, D.J.; Ragetly, G.R.; Carrera, I.; Schaeffer, D.J. Short- and long-term outcomes after arthroscopic treatment of young large breed dogs with medial compartment disease of the elbow. Vet. Surg. 2014, 43, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Ondreka, N. Explanation of grading according to IEWG and discussion of cases. In Proceedings of the 29th Annual Meeting IEWG, Bangkok, Thailand, 17 May 2015. [Google Scholar]
- Krotscheck, U.; Böttcher, P. Veterinary Surgery Small Animal, 2th ed.; Tobias, K.M., Johnston, S.A., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2018; pp. 836–885. [Google Scholar]
- Meyer-Lindenberg, A.; Fehr, M.; Nolte, I. Shot- and long-term results after surgical treatment of an ununited anconeal process in the dog. Vet. Comp. Orthop. Traumatol. 2001, 14, 101–110. [Google Scholar] [CrossRef]
- House, M.R.; Marino, D.J.; Lesser, M.L. Effect of limb position on elbow congruity with CT evaluation. Vet. Surg. 2009, 38, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Samoy, Y.; Van Ryssen, B.; Gielen, I.; Walschot, N.; Van Bree, H. Review of the literature—Elbow incongruity in the dog. Vet. Comp. Orthop. Traumatol. 2006, 1, 1–8. [Google Scholar]
- Fitzpatrick, N.; Caron, A.; Solano, M.A. Bi-oblique dynamic proximal ulnar osteotomy in dogs: Reconstructed computed tomographic assessment of radioulnar congruence over 12 weeks. Vet. Surg. 2013, 42, 727–738. [Google Scholar] [CrossRef]
- Rohwedder, T.; Fischer, M.; Böttcher, P. In vivo fluoroscopic kinematography of dynamic radio-ulnar incongruence in dogs. Open Vet. J. 2017, 7, 221–228. [Google Scholar] [CrossRef][Green Version]
- Cook, J.L.; Schulz, K.S.; Karnes, G.J.; Franklin, S.P.; Canapp, S.O.; Lotsikas, P.J.; Fitzpatrick, N.; Wheeler, J.L.; Stiffler, K.S.; Gillick, M.; et al. Clinical outcomes associated with the initial use of the canine unicompartmental elbow (CUE) arthroplasty system ®. Can. Vet. J. 2015, 56, 971–977. [Google Scholar]
- Evans, R.B.; Gordon-Evans, W.J.; Conzemius, M.G. Comparison of three methods for the management of fragmented medial coronoid process in the dog—A systematic review and meta-analysis. Vet. Comp. Orthop. Traumatol. 2008, 21, 106–109. [Google Scholar] [CrossRef]
- Fitzpatrick, N.; Yeadon, R.; Smith, T.J. Early clinical experience with osteochondral autograft transfer for treatment of osteochondritis dissecans of the medial humeral condyle in dogs. Vet. Surg. 2009, 38, 246–260. [Google Scholar] [CrossRef]
- McConkey, M.J.; Valenzano, D.M.; Wei, A.; Li, T.; Thompson, M.S.; Mohammed, H.O.; Van der Meulen, M.C.H.; Krotscheck, U. Effect of the proximal abducting ulnar osteotomy on intra-articular pressure distribution and contact mechanics of congruent and incongruent canine elbows ex vivo. Vet. Surg. 2016, 45, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, N.; Yeadon, R.; Smith, T.; Shulz, K.L. Techniques of application and initial clinical experience with sliding humeral osteotomy for treatment of medial compartment disease of the canine elbow. Vet. Surg. 2009, 38, 261–278. [Google Scholar] [CrossRef]
- Bayer, K.; Winkels, P.; Andreoni, A.A.; Schmierer, P.; Rohwedder, T.; Pozzi, A.; Böttcher, P. Complications and short-to-midterm results in a case series of 52 CUE procedures using a modified caudo-medial approach. Open Vet. J. 2019, 9, 205–215. [Google Scholar] [CrossRef]
- Franklin, S.P.; Schulz, K.S.; Karnes, J.; Cook, J.L. Theory and development of a unicompartmental resurfacing system for treatment of medial compartment disease of the canine elbow. Vet. Surg. 2014, 43, 765–773. [Google Scholar] [CrossRef]
- Pettitt, R.A.; Tattersall, J.; Gemmill, T.; Butterworth, S.J.; O’Neill, T.J.; Langley- Hoobs, S.J.; Comerford, E.J.; Innes, J.F. Effect of surgical technique on radiographic fusion of the anconeus in the treatment of ununited anconeal process. J. Small Anim. Pract. 2009, 50, 545–548. [Google Scholar] [CrossRef]
- Wilson, D.M.; Goh, C.S.S.; Palmer, R.H. Arthroscopic Biceps Ulnar Release Procedure (BURP): Technique Description and In Vitro Assessment of the Association of Visual Control and Surgeon Experience to Regional Damage and Tenotomy Completeness. Vet. Surg. 2014, 43, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, J. Canine elbow dysplasia: Aetiopathogenesis and current treatment recommendations. Vet. J. 2013, 196, 12–19. [Google Scholar] [CrossRef]
- Caron, A.; Fitzpatrick, N. Bi-oblique dynamic proximal ulnar osteotomy: Surgical technique and clinical outcome in 86 dogs. Vet. Surg. 2016, 45, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Rohwedder, T.; Rabentrost, P.; Böttcher, P. Three-dimensional joint kinematics in a canine elbow joint with medial coronoid disease before and after bi-oblique dynamic proximal ulnar osteotomy. Vet. Comp. Orthop. Traumatol. 2019, 2, 44–49. [Google Scholar] [CrossRef]
- Vezzoni, A. Radiological evaluation before and after elbow surgery. In Proceedings of the 30th Annual Meeting of the IEWG, Verona, Italy, 23 June 2016; pp. 16–23. [Google Scholar]
- Vannini, R. Treatment Strategies in the Growing and Adult Dog with Elbow Disease. In Proceedings of the 29th Annual Meeting of the IEWG, Bangkok, Thailand, 17 May 2015; pp. 27–30. [Google Scholar]
- Might, K.R.; Hanzlik, K.A.; Case, J.B.; Duncan, C.G.; Egger, E.L.; Rooney, M.B.; Duerr, F.M. In vitro comparison of proximal ulnar osteotomy and distal ulnar osteotomy with release of the interosseous ligament in a canine model. Vet. Surg. 2011, 40, 321–326. [Google Scholar] [CrossRef]
- Palmer, R.H. Surgical treatment of canine elbow dysplasia (MCPD, UAP, OCD, EI). In Proceedings of the 33th Annual Meeting of the IEWG, Singapore, 24 September 2018; 2018; pp. 14–19. [Google Scholar]
- Böttcher, P.; Bräuer, S.; Werner, H. Estimation of Joint Incongruence in Dysplastic Canine before and after dynamic proximal ulnar osteotomy. Vet. Surg. 2013, 42, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, P.B.; Johnson, A.L.; Budsberg, S.C.; Lincoln, J.D.; Toombs, J.P.; Whitehair, J.G.; Lents, E.L. Randomized controlled trial of the efficacy of carprofen, a nonsteroidal anti-inflammatory drug, in the treatment of osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 1995, 206, 807–811. [Google Scholar] [PubMed]
- Freund, K.A.; Kieves, N.R.; Hart, J.L.; Foster, S.A.; Duerr, F.M. Assessment of novel digital and smartphone goniometers for measurement of canine stifle joint angles. Am. J. Vet. Res. 2016, 77, 749–755. [Google Scholar] [CrossRef]
- Foster, S. Canine Lameness. In The Rehabilitation Examination, 1st ed.; Wiley Blackwell: Fort Collins, CO, USA, 2020; pp. 67–84. [Google Scholar]
- Lang, J.; Busato, A.; Baumgartner, D.; Flückiger, M.; Weber, U.T. Comparison of two classification protocols in the evaluation of elbow dysplasia in the dog. J. Small Anim. Pract. 1998, 39, 169–174. [Google Scholar] [CrossRef]
- Smith, T.J.; Fitzpatrick, N.; Evans, R.B.; Pead, M.J. Measurement of ulnar subtrochlear sclerosis using a percentage scale in Labrador Retrievers with minimal radiographic signs of periarticular osteophytosis. Vet. Surg. 2009, 38, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Draffan, D.; Carrera, I.; Carmicheal, S.; Heller, J.; Hammond, G. Radiographic analysis of trochlear notch sclerosis in the diagnosis of osteoarthritis secondary to medial coronoid disease. Vet. Comp. Orthop. Traumatol. 2009, 22, 7–15. [Google Scholar]
- Dempsey, L.M.; Maddox, T.W.; Comerford, E.J.; Pettitt, R.A.; Tomlinson, A.W. A Comparison of owner-assessed long-term outcome of arthroscopic intervention versus conservative management of dogs with medial coronoid process disease. Vet. Comp. Orthop. Traumatol. 2019, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vezzoni, A. Aftercare following surgery. In Proceedings of the 17th ESVOT Congress, Venice, Italy, 2–4 October 2015; pp. 317–320. [Google Scholar]
- Budsberg, S.C. Long-term temporal evaluation of ground reaction forces during development of experimentally induced osteoarthritis in dogs. Am. J. Vet. Res. 2001, 62, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Burton, N.J.; Owen, M.R.; Kirk, L.S.; Toscano, M.J.; Colborne, R. Conservative versus arthroscopic management for medial coronoid process disease in dogs: A prospective gait evaluation. Vet. Surg. 2011, 40, 972–980. [Google Scholar] [CrossRef]
- Villamonte-Chevalier, A.; Dingemanse, W.; Broeckx, B.J.G.; Van Caelenberg, A.; Agut, A.; Duchateau, L.; Van Bree, H.; Gielen, I. Bone density of elbow joints in Labrador retrievers and Golden retrievers: Comparison of healthy joints and joints with medial coronoid disease. Vet. J. 2016, 216, 1–7. [Google Scholar] [CrossRef]
- Cook, C.R.; Cook, J.L. Diagnostic imaging of the canine elbow dysplasia: A review. Vet. Surg. 2009, 38, 144–153. [Google Scholar] [CrossRef]
- Alves-Pimenta, S.; Ginja, M.M.; Colaço, B. Role of elbow incongruity in canine elbow dysplasia: Advances in diagnostics and biomechanics. Vet. Comp. Orthop. Traumatol. 2019, 32, 87–96. [Google Scholar] [CrossRef]
- Gemmill, T.J.; Hammond, G.; Mellor, D.; Sullivan, M.; Bennett, D.; Carmichael, S. Use of reconstructed computed tomography for the assessment of joint spaces in the canine elbow. J. Small Anim. Pract. 2006, 47, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Nolte, I.; Wefstaedt, P. Quantitative Radiographic Evaluation of Elbow Incongruity in Labrador and Golden Retrievers with Confirmed Medial Coronoid Disease. Vet. Comp. Orthop. Traumatol. 2019, 32, 10–17. [Google Scholar] [CrossRef] [PubMed]
- How, K.L. Aftercare following surgery. In Proceedings of the 30th Annual Meeting of the IEWG, Bangkok, Thailand, 23 June 2016; p. 18. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).