Environmental Contamination by Echinococcus spp. Eggs as a Risk for Human Health in Educational Farms of Sardinia, Italy
Abstract
:1. Introduction
2. Materials and Methods
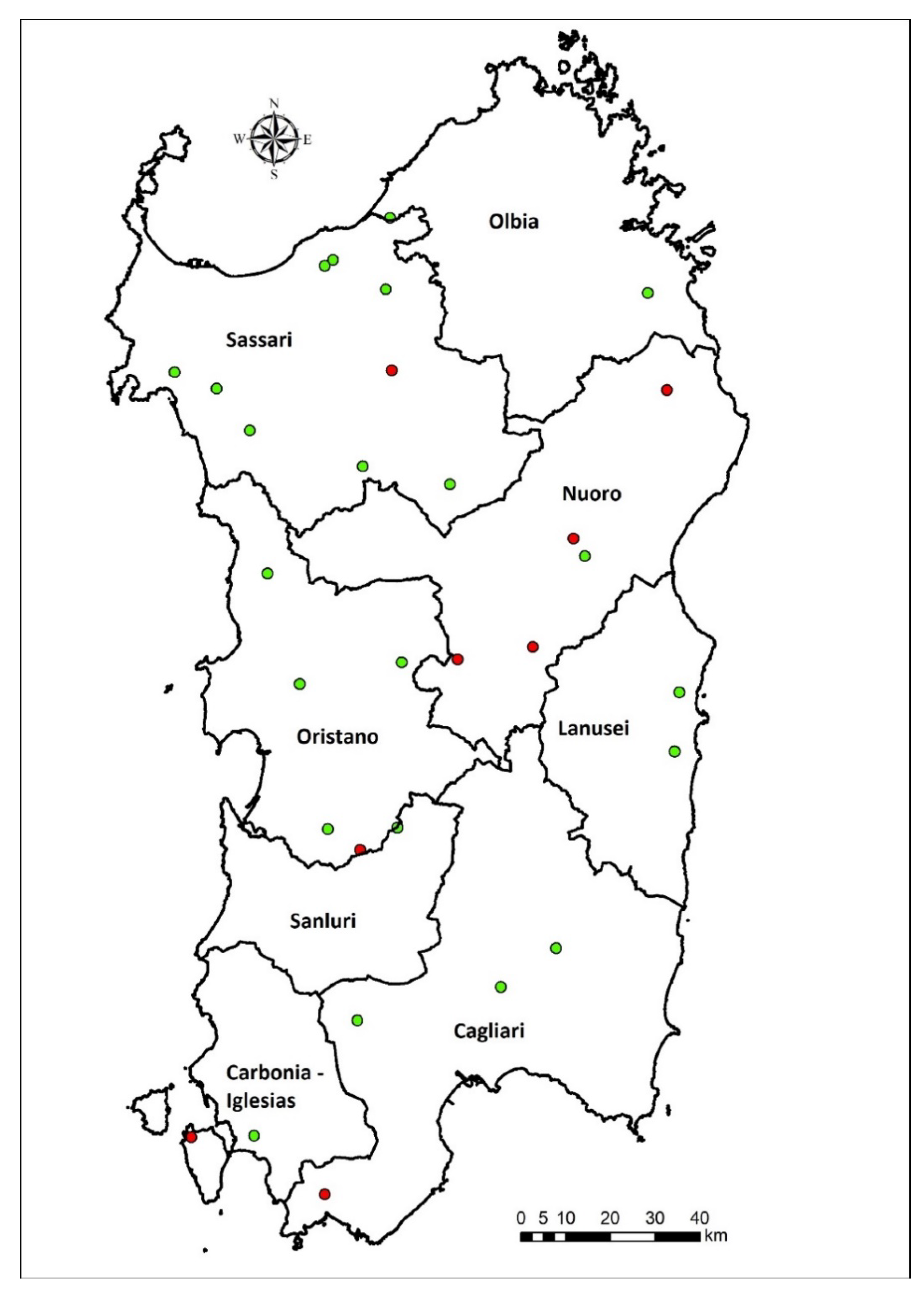
2.1. Study Area and Contest Analysis
2.2. Recruitment of the Education Farms
2.3. Samples Collections
- Reception area used to greet the guests and to provide them a waiting area;
- Educational path equipped to carry out training activities for people interested to go deeper into rural areas;
- Areas used for recreational activities.
2.4. Sample Processing
2.4.1. Soil
2.4.2. Water
2.4.3. Vegetables
2.4.4. Faecal Samples
2.5. Molecular Detection and Characterisation of Cestode Eggs
2.5.1. Multiplex PCR for Discrimination of E.g. Sensu Lato and Echinococcus multilocularis from Other Cestodes
2.5.2. CoproPCR Assay for E. granulosus s.l. Detection
2.6. Data Processing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romig, T.; Deplazesx, P.; Jenkins, D.; Giraudoux, P.; Massolo, A.; Craig, P.S.; Wassermann, M.; Takahashi, K.; de la Rue, M. Ecology and Life Cycle Patterns of Echinococcus Species. Adv. Parasitol. 2017, 95, 213–292. [Google Scholar] [PubMed]
- Maksimov, P.; Bergmann, H.; Wassermann, M.; Romig, T.; Gottstein, B.; Casulli, A.; Conraths, F.J. Species Detection within the Echinococcus granulosus sensu lato Complex by Novel Probe-Based Real-Time PCRs. Pathogens 2020, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Vuitton, D.A.; McManus, D.P.; Rogan, M.T.; Romig, T.; Gottstein, B.; Naidich, A.; Tuxun, T.; Wen, H.; Da Silva, A.M.; Avcioglu, A.; et al. International consensus on terminology to be used in the field of echinococcoses. Parasite 2020, 27, 41. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Rojas, C.A.; Romig, T.; Lightowlers, M.W. Echinococcus granulosus sensu lato genotypes infecting humans—Review of current knowledge. Int. J. Parasitol. 2014, 44, 9–18. [Google Scholar] [CrossRef]
- McManus, D.P.; Thompson, R.C.A. Molecular epidemiology of cystic echinococcosis. Parasitology 2003, 127, S37–S51. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Deplazes, P.; Casulli, A. Reinventing the Wheel of Echinococcus granulosus sensu lato Transmission to Humans. Trends Parasitol. 2020, 36, 427–434. [Google Scholar] [CrossRef]
- Chaâbane-Banaoues, R.; Oudni-M’rad, M.; M’rad, S.; Amani, H.; Mezhoud, H.; Babba, H. A novel PCR-RFLP assay for molecular characterization of Echinococcus granulosus sensu lato and closely related species in developing countries. Parasitol. Res. 2016, 115, 3817–3824. [Google Scholar] [CrossRef]
- Agudelo Higuita, N.I.; Brunetti, E.; McCloskey, C. Cystic Echinococcosis. J. Clin. Microbiol. 2016, 54, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Conchedda, M.; Seu, V.; Capra, S.; Caredda, A.; Pani, S.P.; Lochi, P.G.; Bortoletti, G. A study of morphological aspects of cystic echinococcosis in sheep in Sardinia. Acta Trop. 2016, 159, 200–210. [Google Scholar] [CrossRef]
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, S.; Cringoli, G.; et al. Global Distribution of Alveolar and Cystic Echinococcosis. Adv. Parasitol. 2017, 95, 315–493. [Google Scholar] [CrossRef] [Green Version]
- Craig, P.S.; Larrieu, E. Control of Cystic Echinococcosis/ Hydatidosis: 1863–2002. In Advances in Parasitology. Control of Human Parasitic Diseases; Molyneux, D.H., Ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 443–508. [Google Scholar]
- Otero-Abad, B.; Torgerson, P.R. A Systematic Review of the Epidemiology of Echinococcosis in Domestic and Wild Animals. PLoS Negl. Trop. Dis. 2013, 7, e2249. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zartarian, V.; Moya, J.; Freeman, N.; Beamer, P.; Black, K.; Tulve, N.; Shalat, S. A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal. 2007, 27, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Gemmell, M.A.; Meslin, F.-X.; Pawlowski, Z.S. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; World Organisation for Animal Health: Paris, France, 2001. [Google Scholar]
- Collender, P.A.; Kirby, A.E.; Addiss, D.G.; Freeman, M.C.; Remais, J.V. Methods for quantification of soil-transmitted helminths in environmental media: Current techniques and recent advances. Trends Parasitol. 2015, 31, 625–639. [Google Scholar] [CrossRef] [Green Version]
- Eckert, J.; Deplazes, P. Biological, Epidemiological, and Clinical Aspects of Echinococcosis, a Zoonosis of Increasing Concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathis, A.; Deplazes, P.; Eckert, J. An improved test system for PCR-based specific detection of Echinococcus multilocularis eggs. J. Helminthol. 1996, 70, 219–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubert, D.; Villena, I. Detection of Toxoplasma gondii oocysts in water: Proposition of a strategy and evaluation in Champagne-Ardenne Region, France. Mem. Inst. Oswaldo Cruz 2009, 104, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Abougrain, A.K.; Nahaisi, M.H.; Madi, N.S.; Saied, M.M.; Ghenghesh, K.S. Parasitological contamination in salad vegetables in Tripoli-Libya. Food Control 2010, 21, 760–762. [Google Scholar] [CrossRef]
- Federer, K.; Armua-Fernandez, M.T.; Gori, F.; Hoby, S.; Wenker, C.; Deplazes, P. Detection of taeniid (Taenia spp., Echinococcus spp.) eggs contaminating vegetables and fruits sold in European markets and the risk for metacestode infections in captive primates. Int. J. Parasitol. Parasites Wildl. 2016, 5, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Dryden, M.W.; Payne, P.A.; Ridley, R.; Smith, V. Comparison of common faecal flotation techniques for the recovery of parasite eggs and oocysts. Vet. Ther. 2005, 6, 15–28. [Google Scholar]
- Trachsel, D.; Deplazes, P.; Mathis, A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 2007, 134, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Lett, W.S.; Boufana, B.; Lahmar, S.; Bradshaw, H.; Walters, T.M.H.; Brouwer, A.; Fraser, A.R.; Maskell, D.; Craig, P.S. Canine echinococcosis screening in foxhound hunts in England and Wales using coproantigen ELISA and coproPCR. Parasitol. Open 2018, 4, e17. [Google Scholar] [CrossRef] [Green Version]
- Rolesu, S.; Loi, F.; Cappai, S.; Coccollone, A.; Cataldi, M.; Usala, P.; Podda, A.; Deliperi, S.; Oppia, P.; Natale, A.; et al. Description and typology of dairy sheep farm management profiles in Sardinia. Small Rumin. Res. 2018, 164, 39–47. [Google Scholar] [CrossRef]
- Mitchell, A. Geographic patterns and relationships. In The ESRI Guide to GIS Analysis; ESRI: Redlands, CA, USA, 1999; Volume 1. [Google Scholar]
- Possenti, A.; Manzano-Román, R.; Sánchez-Ovejero, C.; Boufana, B.; La Torre, G.; Siles-Lucas, M.; Casulli, A. Potential Risk Factors Associated with Human Cystic Echinococcosis: Systematic Review and Meta-analysis. PLoS Negl. Trop. Dis. 2016, 10, e0005114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varcasia, A.; Tanda, B.; Giobbe, M.; Solinas, C.; Pipia, A.P.; Malgor, R.; Carmona, C.; Garippa, G.; Scala, A. Cystic echinococcosis in Sardinia: Farmers’ knowledge and dog infection in sheep farms. Vet. Parasitol. 2011, 181, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Berchialla, P.; Masu, G.; Masala, G.; Scaramozzino, P.; Carvelli, A.; Caligiuri, V.; Santi, A.; Bona, M.C.; Maresca, C.; et al. Prevalence estimation of Italian ovine cystic echinococcosis in slaughterhouses: A retrospective Bayesian data analysis, 2010–2015. PLoS ONE 2019, 14, e0214224. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Canova, S.; Rosenzvit, M.; Guarnera, E. Identification of Echinococcus granulosus eggs. Diagn. Microbiol. Infect. Dis. 2002, 44, 29–34. [Google Scholar] [CrossRef]
- Brundu, D.; Piseddu, T.; Stegel, G.; Masu, G.; Ledda, S.; Masala, G. Retrospective study of human cystic echinococcosis in Italy based on the analysis of hospital discharge records between 2001 and 2012. Acta Trop. 2014, 140, 91–96. [Google Scholar] [CrossRef]
- Piseddu, T.; Brundu, D.; Stegel, G.; Loi, F.; Rolesu, S.; Masu, G.; Ledda, S.; Masala, G. The disease burden of human cystic echinococcosis based on HDRs from 2001 to 2014 in Italy. PLoS Negl. Trop. Dis. 2017, 11, e0005771. [Google Scholar] [CrossRef] [Green Version]
- Torgerson, P.R.; Keller, K.; Magnotta, M.; Ragland, N. The global burden of alveolar echinococcosis. PLoS Negl. Trop. Dis. 2010, 4, e722. [Google Scholar] [CrossRef]
- Wang, Q.; Vuitton, D.A.; Yang, W.; Qiu, J.; Giraudoux, P.; Craig, P.S.; Raoul, F.; Schantz, P.M. Socioeconomic and behavior risk factors of human alveolar echinococcosis in Tibetan communities in Sichuan, People’s Republic of China. Am. J. Trop. Med. Hyg. 2006, 74, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Nordin, A.; Nyberg, K.; Vinnerås, B. Inactivation of Ascaris eggs in source-separated urine and feces by ammonia at ambient temperatures. Appl. Environ. Microbiol. 2009, 75, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Düwel, D. The prevalence of Toxocara eggs in the sand in children’s playgrounds in Frankfurt/M. Ann. Trop. Med. Parasitol. 1984, 78, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Glickman, L.T.; Schantz, P.M. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol. Rev. 1981, 3, 230–250. [Google Scholar] [CrossRef] [PubMed]
- Borg, O.A.; Woodruff, A.W. Prevalence of infective ova of Toxocara species in public places. BMJ 1973, 4, 470–472. [Google Scholar] [CrossRef] [Green Version]
| Matrices | Sample Group | Frequency (Number, %) |
|---|---|---|
| Water | Well | 15 (43%) |
| Cistern | 8 (24%) | |
| Public supply | 10 (28%) | |
| Stream | 2 (5%) | |
| Total | 35 (30%) | |
| Soil | Reception point | 12 (36%) |
| Educational path | 16 (49%) | |
| Recreational point | 5 (15%) | |
| Total | 33 (28%) | |
| Vegetables | Production garden | 18 (78%) |
| Educational garden | 5 (22%) | |
| Total | 23 (20%) | |
| Dog Faeces | Identified dogs | 8 (32%) |
| Unidentified dogs | 17 (68%) | |
| Total | 25 (22%) | |
| Overall samples | 116 |
| Parasite | Gene Target | Primers Pairs | Primer Sequences | Amplicon Size |
|---|---|---|---|---|
| E. multilocularis | NADH dehydrogenase subunit 1 (nad1) | Cest1 | 5′-TGCTGATTTGTTAAAGTTAGTGATC-3′ | 395 bp |
| Cest2 | 5′-CATAAATCAATGGAAACAACAACAAG-3′ | |||
| E. granulosus | Ribosomal RNA (rrnS) subunit | Cest4 | 5′-GTTTTTGTGTGTTACATTAATAAGGGTG-3′ | 117 bp |
| Cest5 | GCGGTGTGTACMTGAGCTAAAC-3′ | |||
| Taenia spp. | Ribosomal RNA (rrnS) subunit | Cest3 | 5′-YGAYTCTTTTTAGGGGAAGGTGTG-3′ | 267 bp |
| Cest5 | 5′-GCGGTGTGTACMTGAGCTAAAC-3′ |
| ASL | No. of Sheep Farms | Sheep Farms Density by Km2 | Sheep Density by Km2 | No. of Sheep | EF Sampled |
|---|---|---|---|---|---|
| 01-Sassari | 3821 (20.6%) | 0.77 (0.22) | 189 (75) | 803,695 (20.6%) | 9 (30%) |
| 02-Olbia | 1513 (8.1%) | 0.27 (0.41) | 41 (19) | 146,504 (4.8%) | 2 (7%) |
| 03-Nuoro | 4400 (23.7%) | 0.95 (0.19) | 191 (78) | 765,678 (25.0%) | 6 (20%) |
| 04-Lanusei | 1218 (6.6%) | 0.84 (0.21) | 39 (27) | 73,198 (2.4%) | 2 (7%) |
| 05-Oristano | 2602 (14.0%) | 0.81 (0.15) | 158 (69) | 485,477 (15.9%) | 5 (16%) |
| 06-Sanluri | 1134 (6.1%) | 0.58 (0.26) | 139 (78) | 213,028 (7.0%) | 1 (3%) |
| 07-Carbonia-Iglesias | 1170 (6.3%) | 0.72 (0.33) | 110 (51) | 140,612 (4.6%) | 2 (7%) |
| 08-Cagliari | 2729 (14.7%) | 0.83 (0.17) | 116 (58) | 429,930 (14.1%) | 4 (13%) |
| Total | 18587 | 0.77 (0.23) | 126 (70) | 3,058,122 | 30 |
| Soil (n = 33) | Water (n = 35) | Vegetables (n = 23) | Faeces (n = 25) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RR | EP | RA | W | C | PS | S | PG | EG | ID | UD |
| 12 Neg | 16 Neg | 5 Neg | 15 Neg | 1 Pos Taenia spp. 7 Neg | 10 Neg | 2 Neg | 18 Neg | 5 Neg | 2 Pos E.g. 6 Neg | 6 Pos E.g. 11 Neg |
| Variables | Positive EF (n = 8) | Negative EF (n = 22) | Overall (n = 30) |
|---|---|---|---|
| Altimetry (MASL) | 250 [75–675] | 175 [100–350] | 200 [100–400] |
| Distance from farms (m) | 363 (203) | 640 (419) | 566 (390) |
| No. farms located in 2 km of radius | 9.6 (4.1) | 10.0 (6.1) | 9.9 (5.5) |
| Fenced | 3 (20%) | 12 (80%) | 15 (50%) |
| Species | |||
| Dogs | 8, 4 [3–5] | 16, 3 [0–5] | 24, 3 [1–5] |
| Cats | 4, 4 [2–5] | 11, 3 [3–6] | 15, 3 [3–6] |
| Sheep | 5, 400 [185–412] | 9, 100 [43–120] | 14, 110 [43–412] |
| Goats | 3, 20 [3–50] | 5, 5 [1–40] | 8, 12 [2–45] |
| Pigs | 4, 15 [6–34] | 10, 8 [6–10] | 14, 8 [6–24] |
| Poultry | 4, 8 [4–12] | 7, 15 [8–50] | 11, 10 [6–20] |
| Horses | 4, 4 [3–6] | 4, 1 [1–4] | 8, 3 [2–5] |
| Donkeys/Mules | 2, 2 [2–2] | 3, 3 [2–4] | 5, 2 [2,3] |
| Other (cattle, deer, muflon, wild boar) | 2, 15 [4–26] | 8, 6 [5–16] | 10, 6 [5–20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, E.; Masu, G.; Chisu, V.; Cappai, S.; Masala, G.; Loi, F.; Piseddu, T. Environmental Contamination by Echinococcus spp. Eggs as a Risk for Human Health in Educational Farms of Sardinia, Italy. Vet. Sci. 2022, 9, 143. https://doi.org/10.3390/vetsci9030143
Serra E, Masu G, Chisu V, Cappai S, Masala G, Loi F, Piseddu T. Environmental Contamination by Echinococcus spp. Eggs as a Risk for Human Health in Educational Farms of Sardinia, Italy. Veterinary Sciences. 2022; 9(3):143. https://doi.org/10.3390/vetsci9030143
Chicago/Turabian StyleSerra, Elisa, Gabriella Masu, Valentina Chisu, Stefano Cappai, Giovanna Masala, Federica Loi, and Toni Piseddu. 2022. "Environmental Contamination by Echinococcus spp. Eggs as a Risk for Human Health in Educational Farms of Sardinia, Italy" Veterinary Sciences 9, no. 3: 143. https://doi.org/10.3390/vetsci9030143
APA StyleSerra, E., Masu, G., Chisu, V., Cappai, S., Masala, G., Loi, F., & Piseddu, T. (2022). Environmental Contamination by Echinococcus spp. Eggs as a Risk for Human Health in Educational Farms of Sardinia, Italy. Veterinary Sciences, 9(3), 143. https://doi.org/10.3390/vetsci9030143






