Pharmacokinetics of the Anti-Inflammatory Drug Meloxicam after Single 1.5 mg/kg Intramuscular Administration to Undulate Skates (Raja undulata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Meloxicam Quantification
2.4. Data Analysis
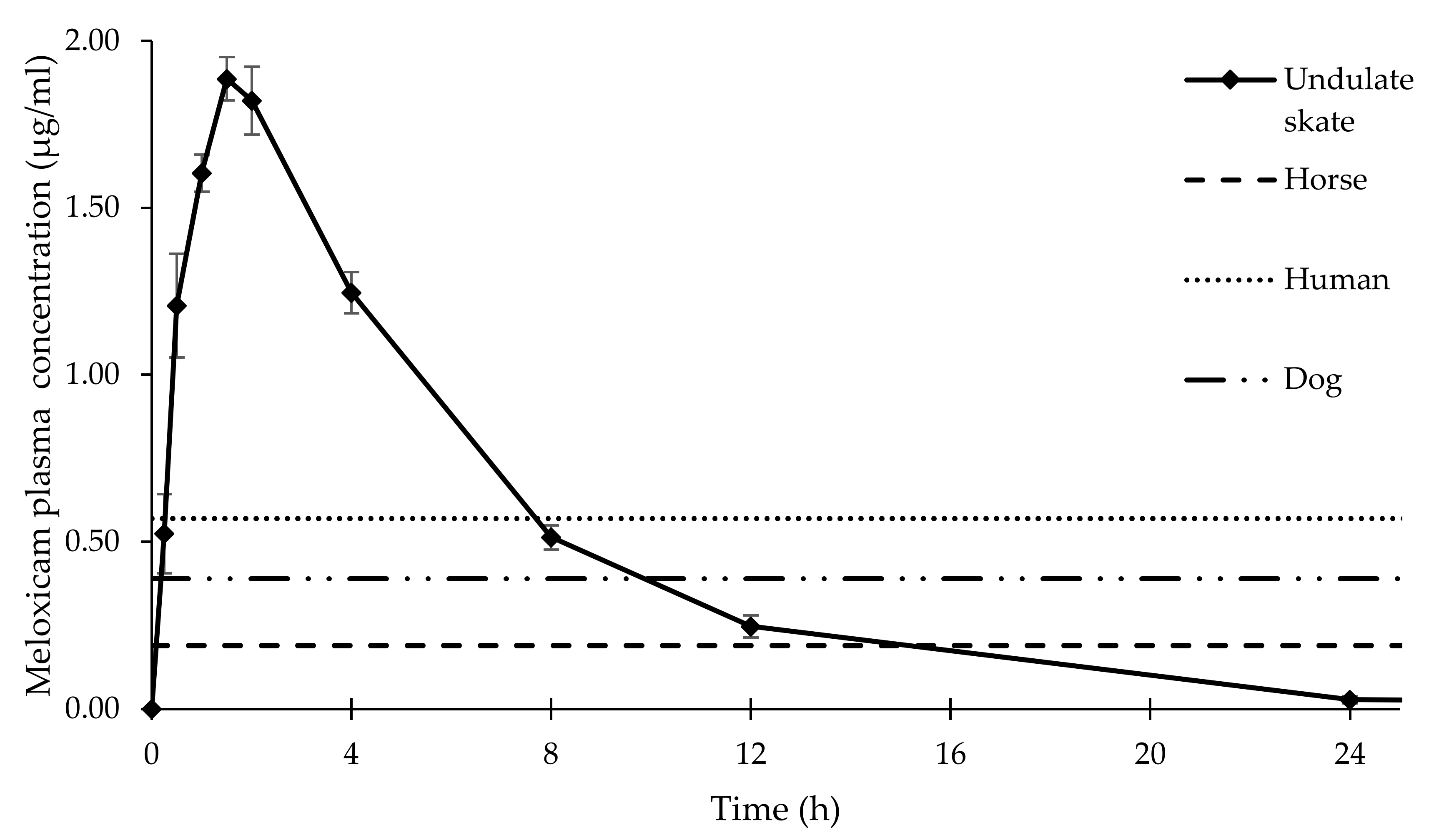
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpenter, J.W.; Marion, C.J. Exotic Animal Formulary, 5th ed.; Elsevier Inc.: St. Louis, MO, USA, 2017; p. 1104. ISBN 9780323498036. [Google Scholar]
- Schattenkirchner, M. Meloxicam: A Selective COX-2 Inhibitor Non-Steroidal Anti-Inflammatory Drug. Expert Opin. Investig. Drugs 1997, 6, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, A.; Encinas, T.; Ardiaca, M.; Gilabert, J.A.; Bonvehí, C.; Orós, J. Pharmacokinetics of Meloxicam during Multiple Oral or Intramuscular Dose Administration to African Grey Parrots (Psittacus Erithacus). Am. J. Vet. Res. 2019, 80, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chesné, C.; Guyomard, C.; Guillouzo, A.; Schmid, J.; Ludwig, E.; Sauter, T. Metabolism of Meloxicam in Human Liver Involves Cytochromes P4502C9 and 3A4. Xenobiotica 1998, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Chen, C.H.; Taylor, M.W. Pharmacokinetics of Meloxicam in Rabbits after Single and Repeat Oral Dosing. Comp. Med. 2006, 56, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M. Cytochrome P450 Drug Metabolism and Protein Induction and Inhibition in Fish Liver Microsomes. Master’s Thesis, McMaster University, Hamilton, ON, Canada, 2009; p. 134. Available online: http://hdl.handle.net/11375/9496 (accessed on 17 February 2020).
- Hutchinson, T.H.; Madden, J.C.; Naidoo, V.; Walker, C.H. Comparative Metabolism as a Key Driver of Wildlife Species Sensitivity to Human and Veterinary Pharmaceuticals. Philos. Trans. R. Soc. B Biol. Sci. 2013, 369, 20130583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribalta, C.; Solé, M. In Vitro Interaction of Emerging Contaminants with the Cytochrome P450 System of Mediterranean Deep-Sea Fish. Environ. Sci. Technol. 2014, 48, 12327–12335. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S. Drug Metabolism and Pharmacokinetics in Drug Discovery. Curr. Opin. Drug Discov. Dev. 2003, 6, 66–80. [Google Scholar] [CrossRef]
- Lees, P.; Landoni, M.F.; Giraundel, J.; Toutain, P.L. Pharmacodynamics and Pharmacokinetics of Nonsteroidal Anti-Inflammatory Drugs in Species of Veterinary Interest. J. Vet. Pharmacol. Ther. 2004, 27, 479–490. [Google Scholar] [CrossRef]
- Stamper, M.A.; Miller, S.M.; Berzins, I.K. Pharmacology in Elasmobranchs. In Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays, and Their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Eds.; Ohio Biological Survey: Columbus, OH, USA, 2004; pp. 447–466. ISBN 0867271523. [Google Scholar]
- Corum, O.; Terzi, E.; Durna Corum, D.; Uney, K. Pharmacokinetics and Bioavailability of Meloxicam in Rainbow Trout (Oncorhynchus Mykiss) Broodstock Following Intravascular, Intramuscular, and Oral Administrations. J. Vet. Pharmacol. Ther. 2021, 45, 213–219. [Google Scholar] [CrossRef]
- Martin, M.; Smith, S.; Kleinhenz, M.; Magnin, G.; Lin, Z.; Kuhn, D.; Montgomery, S.; Coetzee, J. Comparative Pharmacokinetics and Tissue Concentrations of Flunixin Meglumine and Meloxicam in Tilapia (Oreochromis spp.). Fishes 2021, 6, 68. [Google Scholar] [CrossRef]
- Fredholm, D.V.; Mylniczenko, N.D.; Kukanich, B. Pharmacokinetic Evaluation of Meloxicam after Intravenous and Intramuscular Administration in Nile Tilapia (Oreochromis niloticus). J. Zoo Wildl. Med. 2016, 47, 736–742. [Google Scholar] [CrossRef]
- Morón-Elorza, P.; Rojo-Solís, C.; Álvaro-Álvarez, T.; Valls-Torres, M.; García-Párraga, D.; Encinas, T. Pharmacokinetics of Meloxicam after Single 1.5 Mg/Kg Intramuscular Administration to Nursehound Sharks (Scyliorhinus stellaris) and Its Effects on Hematology and Plasma Biochemistry. J. Zoo Wildl. Med. 2022, 53. In press; accepted for publication 5 February 2022. [Google Scholar] [CrossRef]
- Morón-Elorza, P.; Rojo-Solís, C.; Álvaro-Álvarez, T.; Valls-Torres, M.; García-Párraga, D.; Encinas, T. Pharmacokinetic Studies in Elasmobranchs: Meloxicam Administered at 0.5 Mg/Kg Using Intravenous, Intramuscular and Oral Routes to Nursehound Sharks (Scyliorhinus stellaris). Front. Vet. Sci. 2022, 9, 845555. [Google Scholar] [CrossRef]
- Mylniczenko, N. Appendix 1- Elasmobranch Formulary. In Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and Their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Murray, M., Ezcurra, J., Eds.; Ohio Biological Survey: Columbus, OH, USA, 2018. [Google Scholar]
- Kane, L.P.; O’Connor, M.R.; Papich, M.G. Pharmacokinetic of a Single Dose of Intramuscular and Oral Meloxicam in Yellow Stingrays (Urobatis jamaicensis). J. Zoo Wildl. Med. 2022, 53, 153–158. [Google Scholar] [CrossRef]
- Garner, M.M. A Retrospective Study of Disease in Elasmobranchs. Vet. Pathol. 2013, 50, 377–389. [Google Scholar] [CrossRef]
- Stamper, A.M. Elasmobranchs (Sharks, Rays, and Skates). In Zoo Animal and Wildlife Immobilization and Anesthesia, 2nd ed.; West, G., Heard, D., Caulkett, N., Eds.; Wiley Blackwell: Victoria, TX, USA, 2014; pp. 197–201. [Google Scholar]
- Zang, L.; Shimada, Y.; Nishimura, Y.; Tanaka, T.; Nishimura, N. A Novel, Reliable Method for Repeated Blood Collection from Aquarium Fish. Zebrafish 2013, 10, 425–432. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Raby, G.D.; Teffer, A.K.; Jeffries, K.M.; Danylchuk, A.J.; Eliason, E.J.; Hasler, C.T.; Clark, T.D.; Cooke, S.J. Best Practices for Non-Lethal Blood Sampling of Fish via the Caudal Vasculature. J. Fish Biol. 2020, 97, 4–15. [Google Scholar] [CrossRef]
- Beretta, C.; Garavaglia, G.; Cavalli, M. COX-1 and COX-2 Inhibition in Horse Blood by Phenylbutazone, Flunixin, Carprofen and Meloxicam: An in Vitro Analysis. Pharmacol. Res. 2005, 52, 302–306. [Google Scholar] [CrossRef]
- Montoya, L.; Ambros, L.; Kreil, V.; Bonafine, R.; Albarellos, G.; Hallu, R.; Soraci, A. A Pharmacokinetic Comparison of Meloxicam and Ketoprofen Following Oral Administration to Healthy Dogs. Vet. Res. Commun. 2004, 28, 415–428. [Google Scholar] [CrossRef]
- Bae, J.W.; Kim, M.J.; Jang, C.G.; Lee, S.Y. Determination of Meloxicam in Human Plasma Using a HPLC Method with UV Detection and Its Application to a Pharmacokinetic Study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 859, 69–73. [Google Scholar] [CrossRef]
- Larouche, C.B.; Limoges, M.J.; Lair, S. Absence of Acute Toxicity of a Single Intramuscular Injection of Meloxicam in Goldfish (Carassius auratus auratus): A Randomized Controlled Trial. J. Zoo Wildl. Med. 2018, 49, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; Cester, C.C. Pharmacokinetic-Pharmacodynamic Relationships and Dose Response to Meloxicam in Horses with Induced Arthritis in the Right Carpal Joint. Am. J. Vet. Res. 2004, 65, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.; Hill, N.J.; Carrasco, S.E.; Patterson, M.M. Pharmacokinetics and Safety of Intramuscular Meloxicam in Zebra Finches (Taeniopygia guttata). J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 589. [Google Scholar] [CrossRef] [PubMed]
- Lai, O.R.; Di Bello, A.; Soloperto, S.; Freggi, D.; Marzano, G.; Cavaliere, L.; Crescenzo, G. Pharmacokinetic Behavior of Meloxicam in Loggerhead Sea Turtles (Caretta caretta) after Intramuscular and Intravenous Administration. J. Wildl. Med. 2015, 51, 509–512. [Google Scholar] [CrossRef]
- Samuelsen, O.B. Pharmacokinetics of Quinolones in Fish: A Review. Aquaculture 2006, 255, 55–75. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; p. 787. ISBN 9781119174844. [Google Scholar]
- Gorbi, S.; Pellegrini, D.; Tedesco, S.; Regoli, F. Antioxidant Efficiency and Detoxification Enzymes in Spotted Dogfish Scyliorhinus canicula. Mar. Environ. Res. 2004, 58, 293–297. [Google Scholar] [CrossRef]
- Stamper, M.A. Immobilizatuon of Elasmobranchs. In The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and Their Relatives; Smith, M.D., Warmolts, D., Thoney, D., Hueter, R., Eds.; Ohio Biological Survey: Columbus, OH, USA, 2004; pp. 281–295. [Google Scholar]
- Bernal, D.; Carlson, J.; Goldman, K.J.; Lowe, C.G. Energetics, Metabolism and Endothermy in Sharks and Rays. In Biology of Sharks and Their Relatives; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 212–237. [Google Scholar]
- Kulka, D.W.; Anderson, B.; Herman, K.; Derrick, D.; Pacoureau, N.; Dulvy, N.K. Leucoraja erinacea. The IUCN Red List of Threatened Species. Available online: https://doi.org/10.2305/IUCN.UK.2020-3.RLTS.T161418A124481430.en (accessed on 15 December 2021).
- Lacy, E.R.; Reale, E. The Elasmobranch Kidney. I. Gross Anatomy and General Distribution of the Nephrons. Anat. Embryol. 1985, 173, 23–34. [Google Scholar] [CrossRef]
- Churchill, P.C.; Malvin, R.L.; Churchill, M.C. Lack of Renal Effects of DOCA, ACTH, Spironolactone, and Angiotensin II in Squalus Acanthias. J. Exp. Zool. 1985, 234, 17–22. [Google Scholar] [CrossRef]
- Greenwell, M.G.; Sherrill, J.; Clayton, L.A. Osmoregulation in Fish: Mechanisms and Clinical Implications. Vete. Clin. Exot. Anim. Pract. 2003, 6, 169–189. [Google Scholar] [CrossRef]
- Wood, C.; Kajimura, M.; Bucking, C.; Walsh, P. Osmoregulation, Ionoregulation and Acid-Base Regulation by the Gastrointestinal Tract after Feeding in the Elasmobranch (Squalus acanthias). J. Exp. Biol. 2007, 210, 1335–1349. [Google Scholar] [CrossRef] [Green Version]
- Ballantyne, J.S. Some of the Most Interesting Things We Know, and Don’t Know, about the Biochemistry and Physiology of Elasmobranch Fishes (Sharks, Skates and Rays). Comp. Biochem. Physiol. Biochem. Mol. Biol. 2016, 199, 21–28. [Google Scholar] [CrossRef]
- Jeunesse, E.C.; Bargues, I.A.; Toutain, C.E.; Lacroix, M.Z.; Letellier, I.M.; Giraudel, J.M.; Toutain, P.L. Paw Inflammation Model in Dogs for Preclinical Pharmacokinetic/Pharmacodynamic Investigations of Nonsteroidal Anti-Inflammatory Drugs. J. Pharmacol. Exp. Ther. 2011, 338, 548–558. [Google Scholar] [CrossRef]
- Guo, F.; Li, Y.; Yang, D.; Jiang, X.; Ren, J.; Miao, Y.; Ding, F.; Yu, Z. Comparative Pharmacokinetics of Meloxicam Oil Suspension in Pigs at Different Dosages Following Intramuscular Administration. Res. Vet. Sci. 2021, 139, 172–176. [Google Scholar] [CrossRef]

| Parameter (Unit) | MEAN | SEM |
|---|---|---|
| Tmax (h) | 1.50 | 0.24 |
| Cmax (μg/mL) | 1.84 | 0.31 |
| t1/2β (h) | 3.55 | 0.65 |
| AUCt (h·µg/mL) | 11.43 | 2.04 |
| AUCinf (h·µg/mL) | 11.63 | 2.08 |
| AUCt/inf (h·µg/mL) | 0.98 | 0.16 |
| MRT (h) | 5.37 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morón-Elorza, P.; Cañizares-Cooz, D.; Rojo-Solis, C.; Álvaro-Álvarez, T.; Valls-Torres, M.; García-Párraga, D.; Encinas, T. Pharmacokinetics of the Anti-Inflammatory Drug Meloxicam after Single 1.5 mg/kg Intramuscular Administration to Undulate Skates (Raja undulata). Vet. Sci. 2022, 9, 216. https://doi.org/10.3390/vetsci9050216
Morón-Elorza P, Cañizares-Cooz D, Rojo-Solis C, Álvaro-Álvarez T, Valls-Torres M, García-Párraga D, Encinas T. Pharmacokinetics of the Anti-Inflammatory Drug Meloxicam after Single 1.5 mg/kg Intramuscular Administration to Undulate Skates (Raja undulata). Veterinary Sciences. 2022; 9(5):216. https://doi.org/10.3390/vetsci9050216
Chicago/Turabian StyleMorón-Elorza, Pablo, Daniela Cañizares-Cooz, Carlos Rojo-Solis, Teresa Álvaro-Álvarez, Mónica Valls-Torres, Daniel García-Párraga, and Teresa Encinas. 2022. "Pharmacokinetics of the Anti-Inflammatory Drug Meloxicam after Single 1.5 mg/kg Intramuscular Administration to Undulate Skates (Raja undulata)" Veterinary Sciences 9, no. 5: 216. https://doi.org/10.3390/vetsci9050216
APA StyleMorón-Elorza, P., Cañizares-Cooz, D., Rojo-Solis, C., Álvaro-Álvarez, T., Valls-Torres, M., García-Párraga, D., & Encinas, T. (2022). Pharmacokinetics of the Anti-Inflammatory Drug Meloxicam after Single 1.5 mg/kg Intramuscular Administration to Undulate Skates (Raja undulata). Veterinary Sciences, 9(5), 216. https://doi.org/10.3390/vetsci9050216






