Time-Restricted Feeding Improved Vascular Endothelial Function in a High-Fat Diet-Induced Obesity Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of High-Fat Diet
2.2. Animals and Experimental Protocols
- Group 1:
- Rats fed a standard diet for 6 weeks, then continued for 6 weeks on a standard diet (NR)
- Group 2:
- Rats fed a standard diet for 6 weeks, then 6 weeks of TRF with a standard diet (NR + TRFSD)
- Group 3:
- Rats fed a HFD for 6 weeks, then continued for 6 weeks on HFD (OR)
- Group 4:
- Rats fed a HFD for 6 weeks, then 6 weeks of TRF with a HFD (OR + TRFHFD)
- Group 5:
- Rats fed a HFD for 6 weeks, then 6 weeks TRF with a standard diet (OR + TRFSD)
2.3. Biochemical Measurements of Serum Lipid Profile and Atherogenic Index
2.4. Anthropometric Measurements
- (i)
- BMI = body weight (g)/length2 (cm2)
- (ii)
- Lee’s index = cube root of body weight (g)/nose to anus length (cm)
2.5. Serum Insulin Level and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)
2.6. Macrovascular Function Studies
2.7. Immunoblotting
2.8. Statistical Analysis
3. Results
3.1. Effect of TRF on Body Weight, Fasting Blood Glucose, Serum Lipid Profile, and Atherogenic Index in the Obese Rat Model
3.2. Anthropometric Measurements
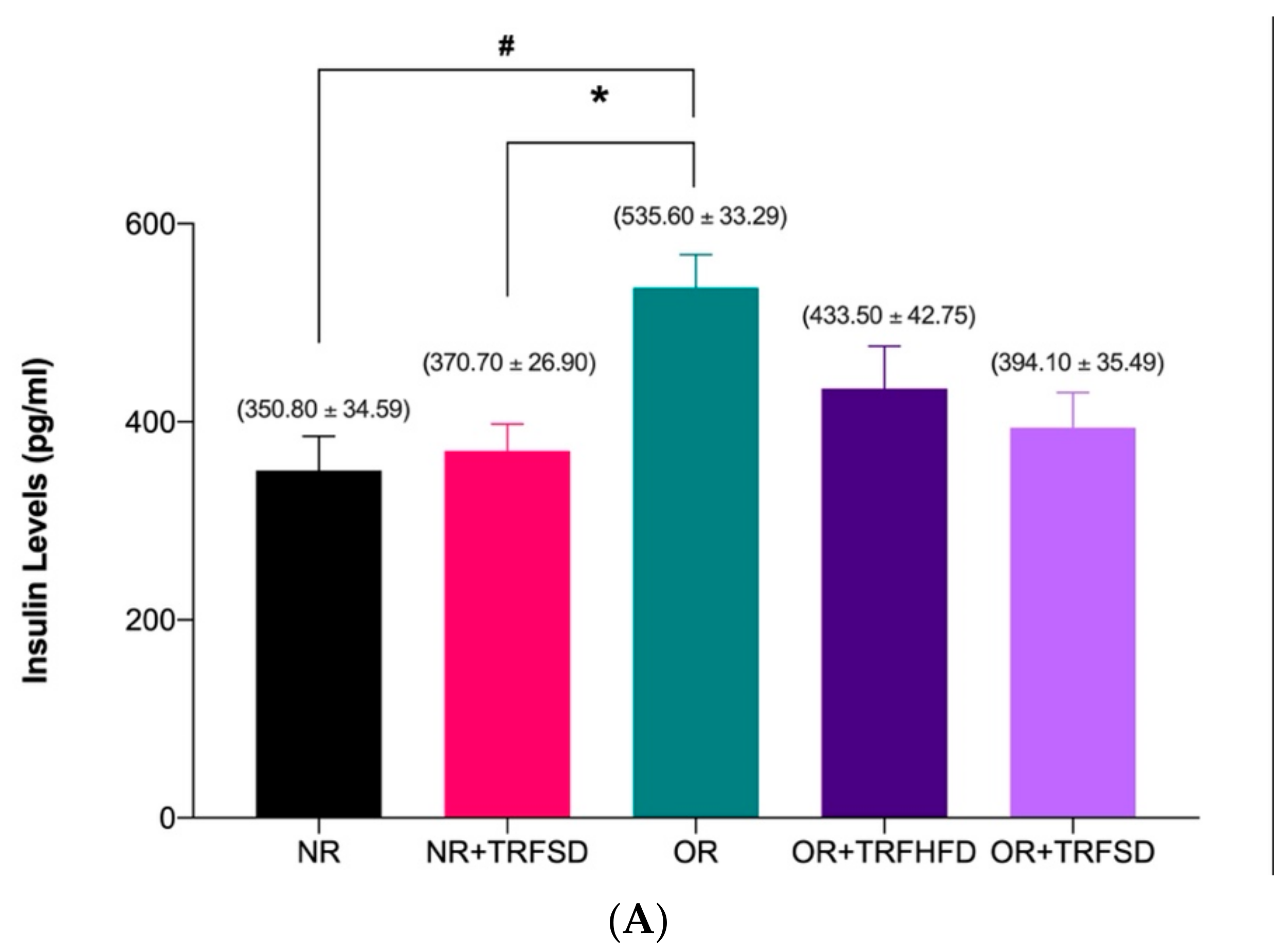
3.3. Effect of TRF on Insulin Levels and HOMA-IR
3.4. Vascular Function Study
3.4.1. Contractile Response to KCl
3.4.2. Effect of TRF on Vascular Relaxations in Normal and Obese Rats
3.4.3. Effect of TRF on Vascular Contractions in Normal and Obese Rats
3.5. Immunoblotting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, 139–598. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Su, J.; Yan, Y.; Zhao, Q.; Ma, J.; Zhu, M.; He, X.; Zhang, B.; Xu, H.; Yang, X.; et al. Intermittent Fasting Inhibits High-Fat Diet–Induced Atherosclerosis by Ameliorating Hypercholesterolemia and Reducing Monocyte Chemoattraction. Front. Pharmacol. 2021, 12, 719750. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural Anti-Obesity Agents and Their Therapeutic Role in Management of Obesity: A Future Trend Perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Xu, J. Vascular Endothelial Regulation of Obesity-Associated Insulin Resistance. Front. Cardiovasc. Med. 2017, 4, 51. [Google Scholar] [CrossRef]
- Abbate, J.M.; Macrì, F.; Arfuso, F.; Iaria, C.; Capparucci, F.; Anfuso, C.; Ieni, A.; Cicero, L.; Briguglio, G.; Lanteri, G. Anti-Atherogenic Effect of 10% Supplementation of Anchovy (Engraulis encrasicolus) Waste Protein Hydrolysates in Apoe-Deficient Mice. Nutrients 2021, 13, 2137. [Google Scholar] [CrossRef]
- Giannetto, A.; Esposito, E.; Lanza, M.; Oliva, S.; Riolo, K.; di Pietro, S.; Abbate, J.M.; Briguglio, G.; Cassata, G.; Cicero, L.; et al. Protein Hydrolysates from Anchovy (Engraulis encrasicolus) Waste: In Vitro and in Vivo Biological Activities. Mar. Drugs 2020, 18, 86. [Google Scholar] [CrossRef]
- Malinowski, B.; Zalewska, K.; Węsierska, A.; Sokołowska, M.M.; Socha, M.; Liczner, G.; Pawlak-Osińska, K.; Wiciński, M. Intermittent Fasting in Cardiovascular Disorders—An Overview. Nutrients 2019, 11, 673. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar] [CrossRef]
- Belkacemi, L.; Selselet-Attou, G.; Bulur, N.; Louchami, K.; Sener, A.; Malaisse, W.J. Intermittent Fasting Modulation of the Diabetic Syndrome in Sand Rats. III. Post-Mortem Investigations. Int. J. Mol. Med. 2011, 27, 95–102. [Google Scholar] [CrossRef][Green Version]
- Belkacemi, L.; Selselet-Attou, G.; Hupkens, E.; Nguidjoe, E.; Louchami, K.; Sener, A.; Malaisse, W.J. Intermittent Fasting Modulation of the Diabetic Syndrome in Streptozotocin-Injected Rats. Int. J. Endocrinol. 2012, 2012, 962012. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Chou, W.; Sears, D.D.; Patterson, R.E.; Webster, N.J.G.; Ellies, L.G. Time-Restricted Feeding Improves Insulin Resistance and Hepatic Steatosis in a Mouse Model of Postmenopausal Obesity. Metab. Clin. Exp. 2016, 65, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.J.; Smith, J.T.; Narbaiza, J.; Mueez, F.; Bustle, L.B.; Qureshi, S.; Fieseler, C.; Legan, S.J. Restricting Feeding to the Active Phase in Middle-Aged Mice Attenuates Adverse Metabolic Effects of a High-Fat Diet. Physiol. Behav. 2016, 167, 1–9. [Google Scholar] [CrossRef] [PubMed]
- García-Luna, C.; Soberanes-Chávez, P.; de Gortari, P. Prepuberal Light Phase Feeding Induces Neuroendocrine Alterations in Adult Rats. J. Endocrinol. 2017, 232, 15–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Olsen, M.K.; Choi, M.H.; Kulseng, B.; Zhao, C.M.; Chen, D. Time-Restricted Feeding on Weekdays Restricts Weight Gain: A Study Using Rat Models of High-Fat Diet-Induced Obesity. Physiol. Behav. 2017, 173, 298–304. [Google Scholar] [CrossRef]
- Park, S.; Yoo, K.M.; Hyun, J.S.; Kang, S. Intermittent Fasting Reduces Body Fat but Exacerbates Hepatic Insulin Resistance in Young Rats Regardless of High Protein and Fat Diets. J. Nutr. Biochem. 2017, 40, 14–22. [Google Scholar] [CrossRef]
- Sundaram, S.; Yan, L. Time-Restricted Feeding Reduces Adiposity in Mice Fed a High-Fat Diet. Nutr. Res. 2016, 36, 603–611. [Google Scholar] [CrossRef]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans That Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of Eight Weeks of Time-Restricted Feeding (16/8) on Basal Metabolism, Maximal Strength, Body Composition, Inflammation, and Cardiovascular Risk Factors in Resistance-Trained Males. J. Transl. Med. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial Dysfunction—A Major Mediator of Diabetic Vascular Disease. Biochim. Biophys. Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2011. [Google Scholar] [CrossRef]
- Azemi, A.K.; Mokhtar, S.S.; Hou, L.J.; Sharif, S.E.T.; Rasool, A.H.G. Model for Type 2 Diabetes Exhibits Changes in Vascular Function and Structure Due to Vascular Oxidative Stress and Inflammation. Biotech. Histochem. 2020, 96, 498–506. [Google Scholar] [CrossRef]
- Azemi, A.K.; Mokhtar, S.S.; Sharif, S.E.T.; Rasool, A.H.G. Clinacanthus Nutans Attenuates Atherosclerosis Progression in Rats with Type 2 Diabetes by Reducing Vascular Oxidative Stress and Inflammation. Pharm. Biol. 2021, 59, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Samat, S.; Kanyan Enchang, F.; Nor Hussein, F.; Wan Ismail, W.I. Four-Week Consumption of Malaysian Honey Reduces Excess Weight Gain and Improves Obesity-Related Parameters in High Fat Diet Induced Obese Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 1342150. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Goh, Y.M.; Mohtarrudin, N.; Loh, S.P. Germinated Brown Rice Ameliorates Obesity in High-Fat Diet Induced Obese Rats. BMC Complement. Altern. Med. 2016, 16, 140. [Google Scholar] [CrossRef]
- Malafaia, A.B.; Nassif, P.A.N.; Ribas, C.A.P.M.; Ariede, B.L.; Sue, K.N.; Cruz, M.A. Obesity Induction with High Fat Sucrose in Rats. Arq. Bras. Cir. Dig. 2013, 26, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Sarega, N.; Imam, M.U.; Esa, N.M.; Zawawi, N.; Ismail, M. Effects of Phenolic-Rich Extracts of Clinacanthus Nutans on High Fat and High Cholesterol Diet-Induced Insulin Resistance. BMC Complement. Altern. Med. 2016, 16, 88. [Google Scholar] [CrossRef]
- Roza, N.A.V.; Possignolo, L.F.; Palanch, A.C.; Gontijo, J.A.R. Effect of Long-Term High-Fat Diet Intake on Peripheral Insulin Sensibility, Blood Pressure, and Renal Function in Female Rats. Food Nutr. Res. 2016, 60, 28536. [Google Scholar] [CrossRef]
- Azemi, A.K.; Mokhtar, S.S.; Rasool, A.H.G. Clinacanthus Nutans Leaves Extract Reverts Endothelial Dysfunction in Type 2 Diabetes Rats by Improving Protein Expression of ENOS. Oxid. Med. Cell. Longev. 2020, 2020, 7572892. [Google Scholar] [CrossRef]
- Wee, C.L.; Mokhtar, S.S.; Banga Singh, K.K.; Rasool, A.H.G. Vitamin D Deficiency Attenuates Endothelial Function by Reducing Antioxidant Activity and Vascular ENOS Expression in the Rat Microcirculation. Microvasc. Res. 2021, 138, 104227. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab. 2014, 20, 991–1105. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.W.; Oliveira, D.C.; Hastreiter, A.; Silva, G.B.; de Oliveira Beltran, J.S.; Rogero, M.M.; Fock, R.A.; Borelli, P. Short-Term High-Fat Diet Affects Macrophages Inflammatory Response, Early Signs of a Long-Term Problem. Braz. J. Pharm. Sci. 2019, 55, 1–12. [Google Scholar] [CrossRef]
- Kostrycki, I.M.; Wildner, G.; Donato, Y.H.; dos Santos, A.B.; Beber, L.C.C.; Frizzo, M.N.; Ludwig, M.S.; Keane, K.N.; Cruzat, V.; Rhoden, C.R.; et al. Effects of High-Fat Diet on EHSP72 and Extra-to-Intracellular HSP70 Levels in Mice Submitted to Exercise under Exposure to Fine Particulate Matter. J. Diabetes Res. 2019, 2019, 4858740. [Google Scholar] [CrossRef]
- Kilany, O.E.; Abdelrazek, H.M.A.; Aldayel, T.S.; Abdo, S.; Mahmoud, M.M.A. Anti-Obesity Potential of Moringa Olifera Seed Extract and Lycopene on High Fat Diet Induced Obesity in Male Sprauge Dawely Rats. Saudi J. Biol. Sci 2020, 27, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hanzawa, F.; Umeki, M.; Ikeda, S.; Mochizuki, S.; Oda, H. Time-Restricted Feeding Suppresses Excess Sucrose-Induced Plasma and Liver Lipid Accumulation in Rats. PLoS ONE 2018, 13, e0201261. [Google Scholar] [CrossRef]
- Aouichat, S.; Chayah, M.; Bouguerra-Aouichat, S.; Agil, A. Time-Restricted Feeding Improves Body Weight Gain, Lipid Profiles, and Atherogenic Indices in Cafeteria-Diet-Fed Rats: Role of Browning of Inguinal White Adipose Tissue. Nutrients 2020, 12, 2185. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-Hour Time Restricted Feeding on Body Weight and Metabolic Disease Risk Factors in Obese Adults: A Pilot Study. Nutr. Health Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- De Oliveira Maranhão Pureza, I.R.; da Silva Junior, A.E.; Silva Praxedes, D.R.; Lessa Vasconcelos, L.G.; de Lima Macena, M.; Vieira de Melo, I.S.; de Menezes Toledo Florêncio, T.M.; Bueno, N.B. Effects of Time-Restricted Feeding on Body Weight, Body Composition and Vital Signs in Low-Income Women with Obesity: A 12-Month Randomized Clinical Trial. Clin. Nutr. 2021, 40, 759–766. [Google Scholar] [CrossRef]
- Antoni, R.; Robertson, T.M.; Robertson, M.D.; Johnston, J.D. A Pilot Feasibility Study Exploring the Effects of a Moderate Time-Restricted Feeding Intervention on Energy Intake, Adiposity and Metabolic Physiology in Free-Living Human Subjects. J. Nutr. Sci. 2018, 7, e22. [Google Scholar] [CrossRef]
- Madkhali, H.A. Morin Attenuates High-Fat Diet Induced-Obesity Related Vascular Endothelial Dysfunction in Wistar Albino Rats. Saudi Pharm. J. 2020, 28, 300–307. [Google Scholar] [CrossRef]
- Nurmasitoh, T.; Utami, S.Y.; Kusumawardani, E.; Najmuddin, A.A.; Fidianingsih, I. Intermittent Fasting Decreases Oxidative Stress Parameters in Wistar Rats (Rattus norvegicus). Universa Med. 2018, 37, 31–38. [Google Scholar] [CrossRef]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Magnan, C.; Migrenne, S.; Chua, S.C.; Dunn-Meynell, A.A. F-DIO Obesity-Prone Rat Is Insulin Resistant before Obesity Onset. Am. J. Physiol. Regul. Integ. Comp. Physiol. 2005, 289, 704–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, H.; Qin, Y.L.; Shi, Z.Y.; Chen, J.H.; Zeng, M.J.; Zhou, W.; Chen, R.Q.; Chen, Z.Y. Effects of Alternate-Day Fasting on Body Weight and Dyslipidaemia in Patients with Non-Alcoholic Fatty Liver Disease: A Randomised Controlled Trial. BMC Gastroenterol. 2019, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men with Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Radford, B.E.; Hawley, J.A. A Delayed Morning and Earlier Evening Time-Restricted Feeding Protocol for Improving Glycemic Control and Dietary Adherence in Men with Overweight/Obesity: A Randomized Controlled Trial. Nutrients 2020, 12, 505. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate Day Fasting and Endurance Exercise Combine to Reduce Body Weight and Favorably Alter Plasma Lipids in Obese Humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef]
- Fulton, D.; Gratton, J.P.; McCabe, T.J.; Fontana, J.; Fujio, Y.; Walsh, K.; Franke, T.F.; Papapetropoulos, A.; Sessa, W.C. Regulation of Endothelium-Derived Nitric Oxide Production by the Protein Kinase Akt. Nature 1999, 399, 597–601. [Google Scholar] [CrossRef]
- Molnar, J.; Yu, S.; Mzhavia, N.; Pau, C.; Chereshnev, I.; Dansky, H.M. Diabetes Induces Endothelial Dysfunction but Does Not Increase Neointimal Formation in High-Fat Diet Fed C57BL/6J Mice. Circ. Res. 2005, 96, 1178–1184. [Google Scholar] [CrossRef]
- Abeyrathna, P.; Su, Y. The Critical Role of Akt in Cardiovascular Function. Vasc. Pharmacol. 2015, 74, 38–48. [Google Scholar] [CrossRef]
- Brouet, A.; Sonveaux, P.; Dessy, C.; Balligand, J.L.; Feron, O. Hsp90 Ensures the Transition from the Early Ca2+-Dependent to the Late Phosphorylation-Dependent Activation of the Endothelial Nitric-Oxide Synthase in Vascular Endothelial Growth Factor-Exposed Endothelial Cells. J. Biol. Chem. 2001, 276, 32663–32669. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.; Fulton, D.; Chen, Y.; Fairchild, T.A.; McCabe, T.J.; Fujita, N.; Tsuruo, T.; Sessa, W.C. Domain Mapping Studies Reveal That the M Domain of Hsp90 Serves as a Molecular Scaffold to Regulate Akt-Dependent Phosphorylation of Endothelial Nitric Oxide Synthase and NO Release. Circ. Res. 2002, 90, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Fujio, Y.; Kureishi, Y.; Rudic, R.D.; Daumerie, G.; Fulton, D.; Sessa, W.C.; Walsh, K. Acute Modulation of Endothelial Akt/PKB Activity Alters Nitric Oxide-Dependent Vasomotor Activity in Vivo. J. Clin. Investig. 2000, 106, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Q.; Venugopal, J.; Wang, J.; Kleiman, K.; Guo, C.; Eitzman, D.T. Obesity-Induced Endothelial Dysfunction Is Prevented by Neutrophil Extracellular Trap Inhibition. Sci. Rep. 2018, 8, 4881. [Google Scholar] [CrossRef]
- Chikopela, T.; Heimburger, D.C.; Kaluba, L.; Hamambulu, P.; Simfukwe, N.; Mutale, W.; Koethe, J.R.; Goma, F. Endothelial Dysfunction and Body Mass Index: Is There a Role for Plasma Peroxynitrite? Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 4. [Google Scholar] [CrossRef]
- Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Nna, V.U.; Romli, A.C.; Ghazali, W.S.W.; Mohamed, M. Bee Bread Ameliorates Vascular Inflammation and Impaired Vasorelaxation in Obesity-Induced Vascular Damage Rat Model: The Role of Enos/No/Cgmp-Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 4225. [Google Scholar] [CrossRef]
- Mughal, R.S.; Bridge, K.; Buza, I.; Slaaby, R.; Worm, J.; Klitgaard-Povlsen, G.; Hvid, H.; Schiødt, M.; Cubbon, R.; Yuldasheva, N.; et al. Effects of Obesity on Insulin: Insulin-like Growth Factor 1 Hybrid Receptor Expression and Akt Phosphorylation in Conduit and Resistance Arteries. Diabetes Vasc. Disease Res. 2019, 16, 160–170. [Google Scholar] [CrossRef]
- Costa, R.M.; Neves, K.B.; Mestriner, F.L.; Louzada-Junior, P.; Bruder-Nascimento, T.; Tostes, R.C. TNF-α Induces Vascular Insulin Resistance via Positive Modulation of PTEN and Decreased Akt/ENOS/NO Signaling in High Fat Diet-Fed Mice. Cardiovasc. Diabetol. 2016, 15, 119. [Google Scholar] [CrossRef]
- Sousa, A.S.; Sponton, A.C.S.; Trifone, C.B.; Delbin, M.A. Aerobic Exercise Training Prevents Perivascular Adipose Tissue-Induced Endothelial Dysfunction in Thoracic Aorta of Obese Mice. Front. Physiol. 2019, 10, 1009. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Nameni, G.; Hajiluian, G.; Mesgari-Abbasi, M. Cardiac Tissue Oxidative Stress and Inflammation after Vitamin D Administrations in High Fat- Diet Induced Obese Rats. BMC Cardiovasc. Disord. 2017, 17, 161. [Google Scholar] [CrossRef]
- Chung, A.P.Y.S.; Gurtu, S.; Chakravarthi, S.; Moorthy, M.; Palanisamy, U.D. Geraniin Protects High-Fat Diet-Induced Oxidative Stress in Sprague Dawley Rats. Front. Nutr. 2018, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Blair, A.; Shaul, P.W.; Yuhanna, I.S.; Conrad, P.A.; Smart, E.J. Oxidized Low Density Lipoprotein Displaces Endothelial Nitric-Oxide Synthase (ENOS) from Plasmalemmal Caveolae and Impairs ENOS Activation. J. Biol. Chem. 1999, 274, 32512–32519. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hein, T.W.; Zhang, C.; Zawieja, D.C.; Liao, J.C.; Kuo, L. Oxidized Low-Density Lipoprotein Inhibits Nitric Oxide-Mediated Coronary Arteriolar Dilation by up-Regulating Endothelial Arginase I. Microcirculation 2011, 18, 36–45. [Google Scholar] [CrossRef]
- Muniyappa, R.; Sowers, J.R. Role of Insulin Resistance in Endothelial Dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef]
- Rabkin, S.W. The Effect of Calcium Ions and the Calcium Ionophore A23187 on Choline Uptake and Phosphatidylcholine Biosynthesis in Chick Embryo Hearts. Basic Res. Cardiol. 1988, 83, 664–671. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Tang, E.H. Endothelium-Dependent Contractions: When a Good Guy Turns Bad! J. Physiol. 2008, 586, 5295–5304. [Google Scholar] [CrossRef]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular Actions of Insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Matsumoto, T.; Noguchi, E.; Ishida, K.; Kobayashi, T.; Yamada, N.; Kamata, K. Metformin Normalizes Endothelial Function by Suppressing Vasoconstrictor Prostanoids in Mesenteric Arteries from OLETF Rats, a Model of Type 2 Diabetes. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1165–H1176. [Google Scholar] [CrossRef]






| Parameter, Unit | g/100 g |
|---|---|
| Protein | 6.10 |
| Total fat | 39.00 |
| Total carbohydrate | 46.50 |
| Ash | 3.90 |
| Moisture | 4.50 |
| Energy, kcal/100 g | 561 (2356 kJ) |
| Title | NR | NR + TRFSD | OR | OR + TRFHFD | OR + TRFSD |
|---|---|---|---|---|---|
| Initial body weight (g) (Day 0) | 315.30 ± 4.34 | 305.80 ± 6.25 | 326.0 ± 3.70 | 311.20 ± 11.49 | 309.80 ± 12.52 |
| Body weight before TRF (g) (Week 6) | 386.80 ± 8.43 a | 411.60 ± 2.99 a | 453.00 ± 12.36 ##, a | 428.80 ± 11.06 a | 414.30 ± 16.43 a |
| Final body weight after TRF (g) (Week 12) | 417.70 ± 5.26 a,b | 406.60 ± 6.92 ****,a | 524.80 ± 20.52 ###,a,b | 395.00 ± 17.01 ****,a | 380.80 ± 4.49 ****,a |
| Final FBG (mmol/L) | 4.40 ± 0.14 | 4.50 ± 0.29 | 4.63 ± 0.16 | 4.61 ± 0.27 | 4.67 ± 0.19 |
| Total cholesterol (TC) | 3.49 ± 0.24 | 4.13 ± 0.18 | 4.53 ± 0.07 # | 4.10 ± 0.22 | 3.38 ± 0.34 * |
| Triglycerides (TG) | 0.60 ± 0.01 | 0.64 ± 0.07 ** | 1.37 ± 0.24 ## | 0.75 ± 0.10 * | 0.56 ± 0.08 ** |
| High-density lipoprotein cholesterol (HDL-C) | 0.70 ± 0.05 | 0.68 ± 0.06 * | 0.47 ± 0.04 # | 0.62 ± 0.04 | 0.55 ± 0.02 |
| Low-density lipoprotein cholesterol (LDL-C) | 0.06 ± 0.02 | 0.09 ± 0.05 ** | 0.50 ± 0.12 ## | 0.17 ± 0.09 * | 0.10 ± 0.06 ** |
| Atherogenic index (AI) | 0.18 ± 0.08 | 0.14 ± 0.07 ** | 0.70 ± 0.16 ## | 0.01 ± 0.01 *** | 0.19 ± 0.11 ** |
| Title | NR | NR + TRFSD | OR | OR + TRFHFD | OR + TRFSD |
|---|---|---|---|---|---|
| Body length (cm) | 25.23 ± 0.15 | 25.36 ± 0.21 | 25.14 ± 0.09 | 24.64 ± 0.30 | 24.57 ± 0.35 |
| BMI | 0.66 ± 0.01 | 0.64 ± 0.01 **** | 0.80 ± 0.03 #### | 0.68 ± 0.03 *** | 0.66 ± 0.01 **** |
| Lee’s index | 0.81 ± 0.01 | 0.80 ± 0.01 **** | 0.90 ± 0.01 #### | 0.82 ± 0.02 *** | 0.81 ± 0.01 *** |
| Title | NR | NR + TRFSD | OR | OR + TRFHFD | OR + TRFSD |
|---|---|---|---|---|---|
| Acetylcholine Emax (%) | 95.27 ± 6.78 | 92.89 ± 2.92 * | 69.24 ± 1.83 ## | 107.50 ± 4.72 **** | 92.27 ± 2.96 * |
| Sodium nitroprusside Emax (%) | 137.30 ± 9.76 | 127.90 ± 7.27 | 111.30 ± 4.94 | 137.80 ± 8.33 | 130.50 ± 15.84 |
| Title | NR | NR + TRFSD | OR | OR + TRFHFD | OR + TRFSD |
|---|---|---|---|---|---|
| Calcium ionophore Emax (%) | 13.10 ± 3.68 | 15.40 ± 1.57 * | 29.96 ± 4.51 ## | 17.04 ± 1.39 * | 9.45 ± 2.67 *** |
| Phenylephrine Emax (%) | 91.64 ± 3.48 | 86.45 ± 11.80 | 109.80 ± 8.60 | 78.16 ± 9.29 | 85.10 ± 7.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azemi, A.K.; Siti-Sarah, A.R.; Mokhtar, S.S.; Rasool, A.H.G. Time-Restricted Feeding Improved Vascular Endothelial Function in a High-Fat Diet-Induced Obesity Rat Model. Vet. Sci. 2022, 9, 217. https://doi.org/10.3390/vetsci9050217
Azemi AK, Siti-Sarah AR, Mokhtar SS, Rasool AHG. Time-Restricted Feeding Improved Vascular Endothelial Function in a High-Fat Diet-Induced Obesity Rat Model. Veterinary Sciences. 2022; 9(5):217. https://doi.org/10.3390/vetsci9050217
Chicago/Turabian StyleAzemi, Ahmad Khusairi, Abdul Rahim Siti-Sarah, Siti Safiah Mokhtar, and Aida Hanum Ghulam Rasool. 2022. "Time-Restricted Feeding Improved Vascular Endothelial Function in a High-Fat Diet-Induced Obesity Rat Model" Veterinary Sciences 9, no. 5: 217. https://doi.org/10.3390/vetsci9050217
APA StyleAzemi, A. K., Siti-Sarah, A. R., Mokhtar, S. S., & Rasool, A. H. G. (2022). Time-Restricted Feeding Improved Vascular Endothelial Function in a High-Fat Diet-Induced Obesity Rat Model. Veterinary Sciences, 9(5), 217. https://doi.org/10.3390/vetsci9050217







