Clinical and Magnetic Resonance Imaging (MRI) Features, Tumour Localisation, and Survival of Dogs with Presumptive Brain Gliomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Medical Records
2.2. MRI and Tumour Localisation
2.3. Statistical Analysis
3. Results
3.1. Demographics
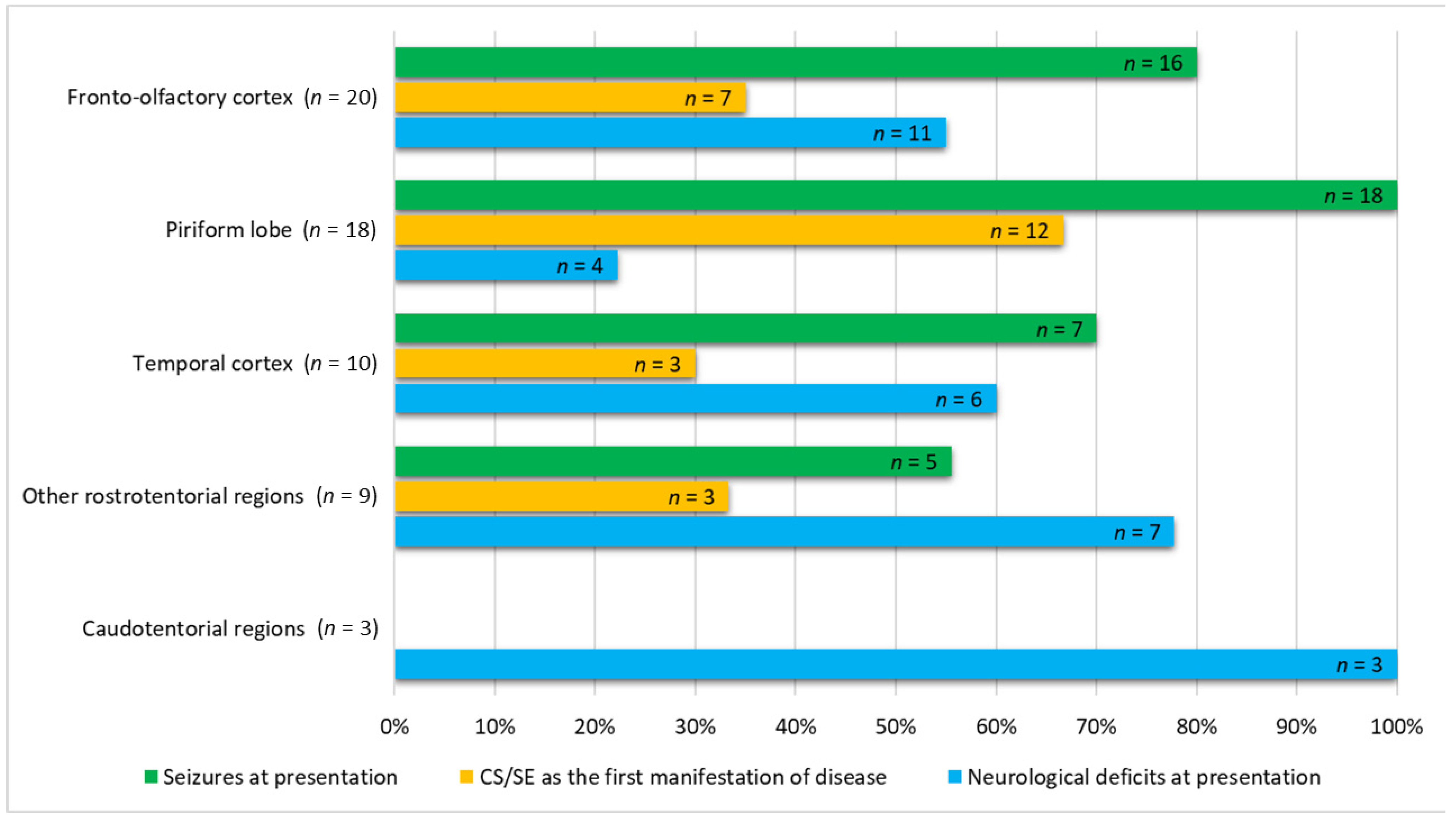
3.2. Clinical Presentation and Clinicopathologic Findings
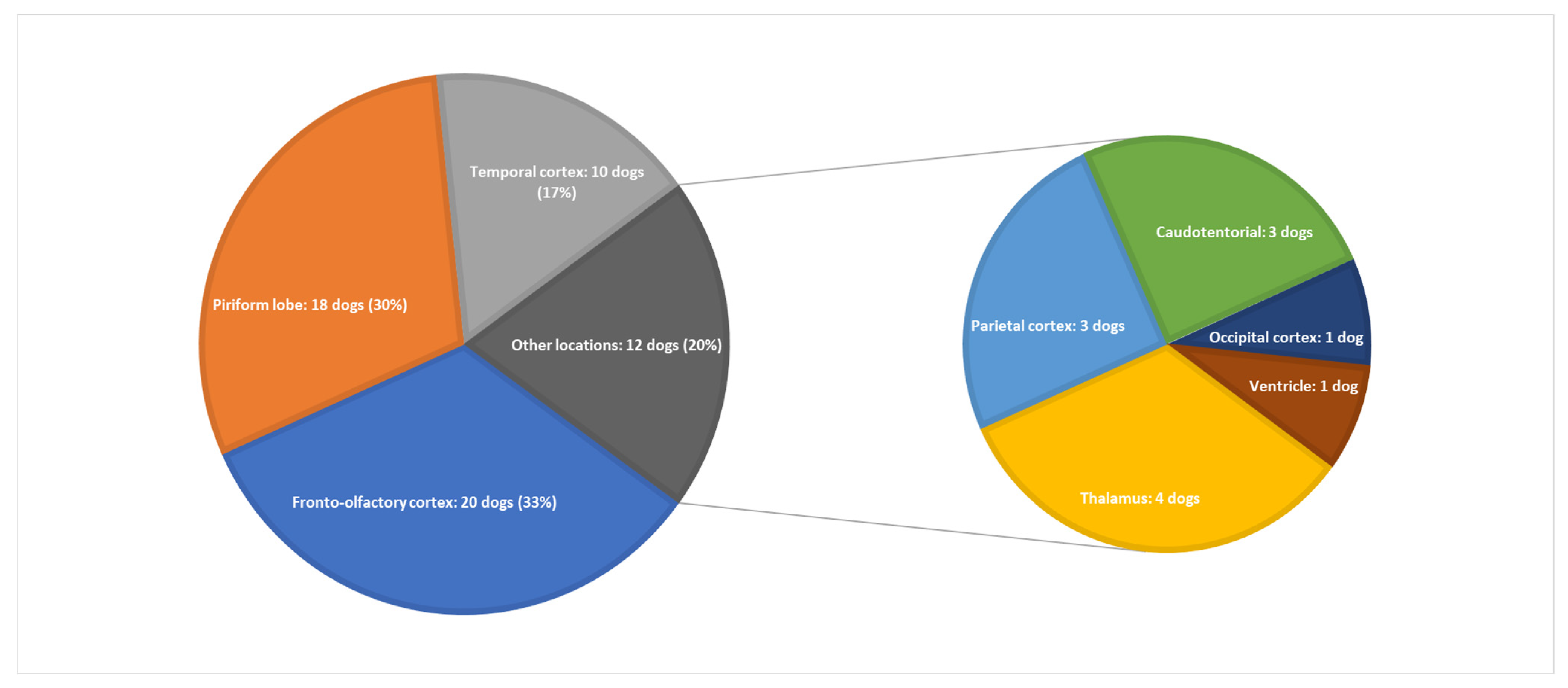
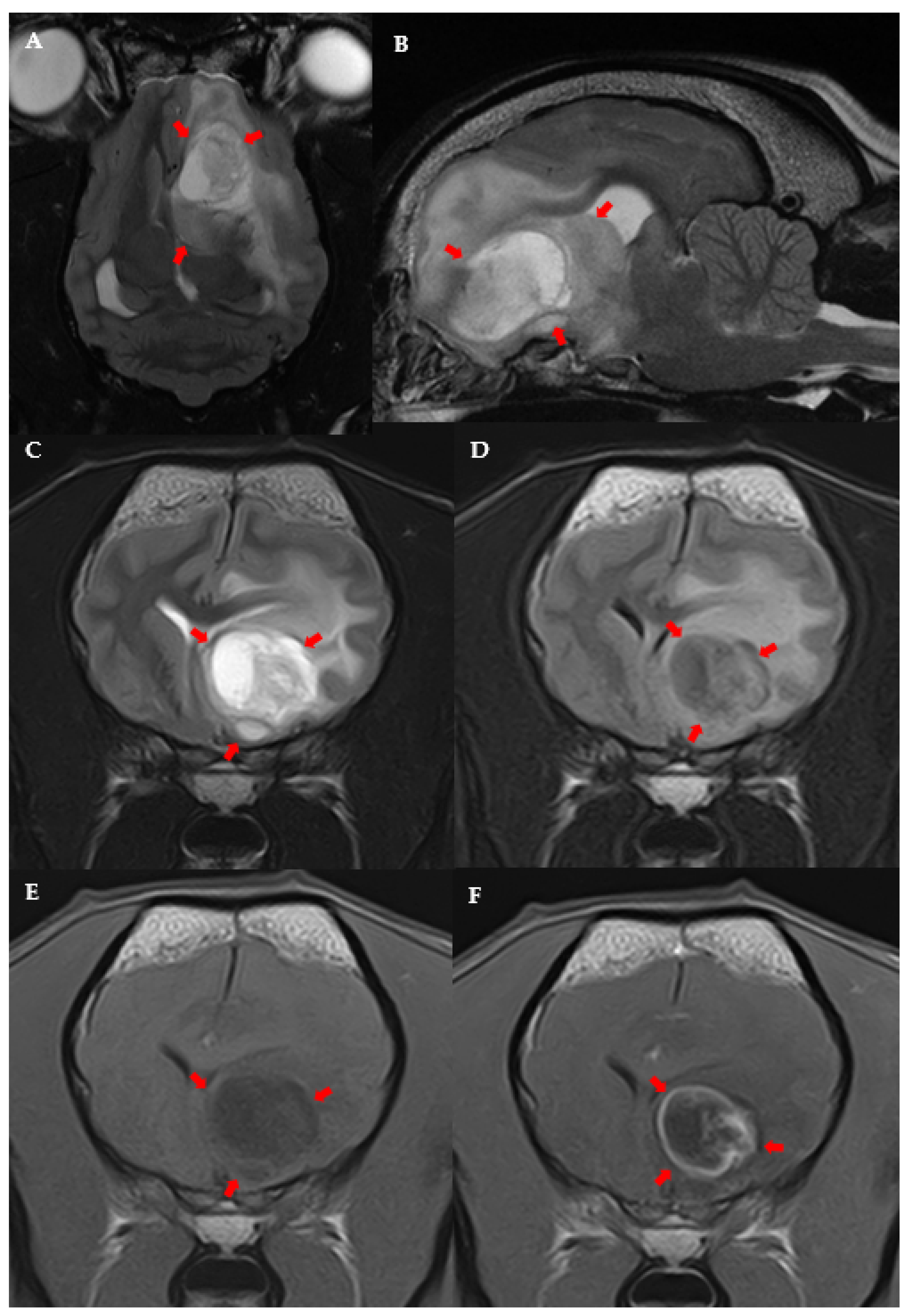
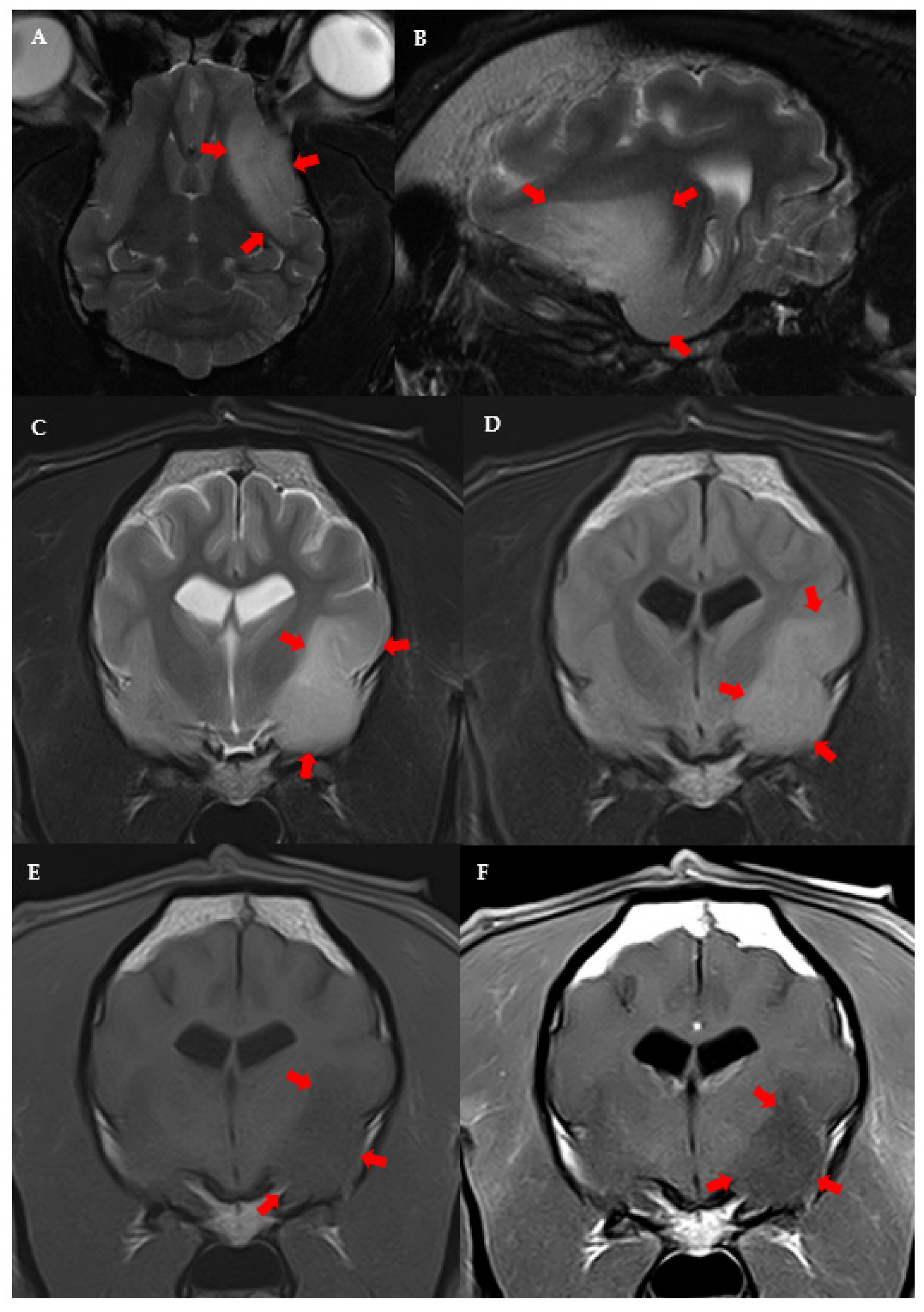
3.3. MRI Features and Localisation of Suspected Glioma
3.4. Association among Suspected Glioma Localisation, Demographic Data, Clinical Features, and MRI Features
3.5. Treatment
3.6. Survival Time and Cause of Death
4. Discussion
4.1. Clinical and MRI Features and Tumour Location
4.2. Survival Time
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SE | Status epilepticus |
| CE | Contrast enhancement |
| CI | Confidence interval |
| CS | Cluster seizures |
| CSF | Cerebrospinal fluid |
| CT | Computed tomography |
| ES | Epileptic seizures |
| FLAIR | Fluid-attenuated inversion recovery |
| HR | Hazard ratio |
| IDH | Isocitrate dehydrogenase |
| IQR | Interquartile range |
| MST | Median overall survival time |
| MRI | Magnetic Resonance Imaging |
| OR | Odds ratio |
| OS | Overall survival time |
| T1W | T1-weighted |
| T2W | T2-weighted |
| T2* | T2-weighted gradient echo |
References
- Hayes, H.M.; Priester, W.A.; Pendergrass, T.W. Occurrence of nervous-tissue tumors in cattle, horses, cats and dogs. Int. J. Cancer 1975, 15, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Song, R.B.; Vite, C.H.; Bradley, C.W.; Cross, J.R. Postmortem Evaluation of 435 Cases of Intracranial Neoplasia in Dogs and Relationship of Neoplasm with Breed, Age, and Body Weight. J. Vet. Intern. Med. 2013, 27, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.M.; Shofer, F.S.; Van Winkle, T.J.; Massicotte, C. Canine intracranial primary neoplasia: 173 Cases (1986–2003). J. Vet. Intern. Med. 2006, 20, 669–675. [Google Scholar]
- Truvé, K.; Dickinson, P.; Xiong, A.; York, D.; Jayashankar, K.; Pielberg, G.; Koltookian, M.; Muren, E.; Fuxelius, H.-H.; Weishaupt, H.; et al. Utilizing the Dog Genome in the Search for Novel Candidate Genes Involved in Glioma Development—Genome Wide Association Mapping followed by Targeted Massive Parallel Sequencing Identifies a Strongly Associated Locus. PLoS Genet. 2016, 12, e1006000. [Google Scholar] [CrossRef]
- Dolera, M.; Malfassi, L.; Bianchi, C.; Carrara, N.; Finesso, S.; Marcarini, S.; Mazza, G.; Pavesi, S.; Sala, M.; Urso, G. Frameless stereotactic radiotherapy alone and combined with temozolomide for presumed canine gliomas. Vet. Comp. Oncol. 2018, 16, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Debreuque, M.; De Fornel, P.; David, I.; Delisle, F.; Ducerveau, M.N.; Devauchelle, P.; Thibaud, J.L. Definitive-intent uniform megavoltage fractioned radiotherapy protocol for presumed canine intracranial gliomas: Retrospective analysis of survival and prognostic factors in 38 cases (2013–2019). BMC Vet. Res. 2020, 16, 412. [Google Scholar] [CrossRef]
- José-López, R.; Gutierrez-Quintana, R.; de la Fuente, C.; Manzanilla, E.G.; Suñol, A.; Pi Castro, D.; Anor, S.; Sanchez-Masian, D.; Fernandez-Flores, F.; Ricci, E.; et al. Clinical features, diagnosis, and survival analysis of dogs with glioma. J. Vet. Intern. Med. 2021, 35, 1902–1917. [Google Scholar] [CrossRef]
- Koehler, J.W.; Miller, A.D.; Miller, C.R.; Porter, B.; Aldape, K.; Beck, J.; Brat, D.; Cornax, I.; Corps, K.; Frank, C.; et al. A revised diagnostic classification of canine glioma: Towards validation of the canine glioma patient as a naturally occurring preclinical model for human glioma. J. Neuropathol. Exp. Neurol. 2018, 77, 1039–1054. [Google Scholar] [CrossRef]
- Bentley, R.T.; Ober, C.P.; Anderson, K.L.; Feeney, D.A.; Naughton, J.F.; Ohlfest, J.R.; O’Sullivan, M.G.; Miller, M.A.; Constable, P.D.; Pluhar, G.E. Canine intracranial gliomas: Relationship between magnetic resonance imaging criteria and tumor type and grade. Vet. J. 2013, 198, 3921–3932. [Google Scholar] [CrossRef] [Green Version]
- Young, B.D.; Levine, J.M.; Porter, B.F.; Chen-Allen, A.V.; Rossmeisl, J.H.; Platt, S.R.; Kent, M.; Fosgate, G.T.; Schatzberg, S.J. Magnetic resonance imaging features of intracranial astrocytomas and oligodendrogliomas in dogs. Vet. Radiol. Ultrasound 2011, 52, 132–141. [Google Scholar] [CrossRef]
- Suñol, A.; Mascort, J.; Font, C.; Bastante, A.R.; Pumarola, M.; Feliu-Pascual, A.L. Long-term follow-up of surgical resection alone for primary intracranial rostrotentorial tumors in dogs: 29 cases (2002–2013). Open Vet. J. 2017, 7, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moirano, S.J.; Dewey, C.W.; Wright, K.Z.; Cohen, P.W. Survival times in dogs with presumptive intracranial gliomas treated with oral lomustine: A comparative retrospective study (2008–2017). Vet. Comp. Oncol. 2018, 16, 459–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moirano, S.J.; Dewey, C.W.; Haney, S.; Yang, J. Efficacy of frameless stereotactic radiotherapy for the treatment of presumptive canine intracranial gliomas: A retrospective analysis (2014–2017). Vet. Comp. Oncol. 2020, 18, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.H.; Jones, J.C.; Zimmerman, K.L.; Robertson, J.L. Survival time following hospital discharge in dogs with palliatively treated primary brain tumors. J. Am. Vet. Med. Assoc. 2013, 242, 193–198. [Google Scholar] [CrossRef]
- Van Breemen, M.S.; Wilms, E.B.; Vecht, C.J. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet Neurol. 2007, 6, 421–430. [Google Scholar] [CrossRef]
- Akgun, M.Y.; Cetintas, S.C.; Kemerdere, R.; Yeni, S.N.; Tanriverdi, T. Are low-grade gliomas of mesial temporal area alone? Surg. Neurol. Int. 2019, 10, 170. [Google Scholar] [CrossRef]
- Vecht, C.J.; Kerkhof, M.; Duran-Pena, A. Seizure Prognosis in Brain Tumors: New Insights and Evidence-Based Management. Oncologist 2014, 19, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Pallud, J.; Audureau, E.; Blonski, M.; Sanai, N.; Bauchet, L.; Fontaine, D.; Mandonnet, E.; Dezamis, E.; Psimaras, D.; Guyotat, J.; et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain 2014, 137, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Kemerdere, R.; Yuksel, O.; Kacira, T.; Yeni, N.; Ozkara, C.; Tanriverdi, T.; Uzan, M.; Ozyurt, E. Low-grade temporal gliomas: Surgical strategy and long-term seizure outcome. Clin. Neurol. Neurosurg. 2014, 126, 196–200. [Google Scholar] [CrossRef]
- Young, J.C.; Vaughan, D.N.; Nasser, H.M.; Jackson, G.D. Anatomical imaging of the piriform cortex in epilepsy. Exp. Neurol. 2019, 320, 113013. [Google Scholar] [CrossRef]
- Czerwik, A.; Płonek, M.; Podgórski, P.; Wrzosek, M. Comparison of electroencephalographic findings with hippocampal magnetic resonance imaging volumetry in dogs with idiopathic epilepsy. J. Vet. Intern. Med. 2018, 32, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Hetch, S. Brain Neoplasia. In Diagnostic MRI in Dogs and Cats, 1st ed.; Mai, W., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 216–218. [Google Scholar]
- Berendt, M.; Farquhar, R.G.; Mandigers, P.J.J.; Pakozdy, A.; Bhatti, S.F.M.; De Risio, L.; Fischer, A.; Long, S.; Matiasek, K.; Munana, K.; et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet. Res. 2015, 11, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, F.; Deviers, A.; Dally, C.; Mogicato, G.; Delverdier, M.; Cauzinille, L.; Gnirs, K.; Anor, S.; de la Fuente, C.; Fondevila, D.; et al. Presence of neural progenitors in spontaneous canine gliomas: A histopathological and immunohistochemical study of 20 cases. Vet. J. 2016, 209, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K.L.; Ruth, J.D.; Pancotto, T.E.; Were, S.R.; Rossmeisl, J.H. Computed tomography and magnetic resonance imaging are equivalent in mensuration and similarly inaccurate in grade and type predictability of canine intracranial gliomas. Front. Vet. Sci. 2017, 4, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, C.A.; Holmes, S.P.; Young, B.D.; Chen, A.V.; Platt, S.R.; Savage, M.Y.; Schatzberg, S.J.; Fosgate, G.T.; Levine, J.M. Magnetic Resonance Imaging for the differentiation of Neoplastic Inflammatory, and cerebrovascular brain disease in dogs. J. Vet. Intern. Med. 2012, 26, 589–597. [Google Scholar] [CrossRef]
- Maschio, M. Brain Tumor-Related Epilepsy. Curr. Neuropharmacol. 2012, 10, 124–133. [Google Scholar] [CrossRef]
- Heidner, G.L.; Kornegay, J.N.; Page, R.L.; Dodge, R.K.; Thrall, D.E. Analysis of Survival in a Retrospective Study of 86 Dogs with Brain Tumors. J. Vet. Intern. Med. 1991, 5, 219–226. [Google Scholar] [CrossRef]
- Schwart, M.; Lamb, C.R.; Brodbelt, D.C.; Volk, H.A. Canine intracranial neoplasia: Clinical risk factors for development of epileptic seizures. Small Anim. Pract. 2011, 52, 632–637. [Google Scholar] [CrossRef]
- Parker, R.L.; Hsu, F.; Du, J.; Shinn, R.L.; Drury, A.G.; Roberston, J.L.; Cecere, T.E.; Arendse, A.U.; Rossmeisl, J.H. Incidence, risk factors, and outcomes for early postoperative seizures in dogs with rostrotentorial brain tumors after intracranial surgery. J. Vet. Intern. Med. 2022, 36, 694–701. [Google Scholar] [CrossRef]
- Liebel, F.X.; Smith, P.M. Cental nervous system neoplasia. FOCUS 2014, 36, 24–29. [Google Scholar]
- Pallud, J.; McKhann, G.M. Diffuse Low-Grade Glioma-Related Epilepsy. Neurosurg. Clin. N. Am. 2019, 30, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Pallud, J.; Capelle, L.; Huberfeld, G. Tumors and tumoral epilepsy. Tumoral epileptogenicity: How does it happen? Epilepsia 2013, 54, 30–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2021 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2021, 23, 1231–1251. [Google Scholar]
- Nagashima, N.; Tanaka, K.; Sasayama, T.; Irino, Y.; Sato, N.; Takeuchi, Y.; Kyotani, K.; Mukasa, A.; Mizukawa, K.; Sakata, J.; et al. Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro Oncol. 2016, 18, 1559–1568. [Google Scholar] [PubMed] [Green Version]
- De Risio, L.; Bhatti, S.; Muñana, K.; Penderis, J.; Stein, V.; Tipold, A.; Berendt, M.; Farqhuar, R.; Fischer, A.; Long, S.; et al. International veterinary epilepsy task force consensus proposal: Diagnostic approach to epilepsy in dogs. BMC Vet. Res. 2015, 11, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, M.P.; Bagley, R.S.; Harrington, M.L.; Gavin, P.R. Intracranial tumors. Vet. Clin. N. Am. Small Anim. Pract. 1996, 26, 759–777. [Google Scholar] [CrossRef]
- Piredda, S.; Gale, K. A crucial epileptogenic site in the deep prepiriform cortex. Nature 1985, 317, 623–625. [Google Scholar] [CrossRef]
- Curia, G.; Lucchi, C.; Vinet, J.; Gualtieri, F.; Marinelli, C.; Torsello, A.; Constantino, L.; Biagini, G. Pathophysiogenesis of Mesial Temporal Lobe Epilepsy: Is Prevention of Damage Antiepileptogenic? Curr. Med. Chem. 2014, 21, 663–688. [Google Scholar] [CrossRef] [Green Version]
- Blumcke, I.; Spreafico, R.; Haaker, G.; Coras, R.; Kobow, K.; Bien, C.G.; Pfafflin, M.; Elger, C.; Widman, G.; Schramm, J.; et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 2017, 377, 1648–1656. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.M.G.; Insausti, R.; Artacho-Pérula, E.; Salmenperä, T.; Kälviäinen, R.; Pitkänen, A. MR volumetric analysis of the piriform cortex and cortical amygdala in drug-refractory temporal lobe epilepsy. Am. J. Neuroradiol. 2005, 26, 319–332. [Google Scholar]
- Vaughan, D.N.; Jackson, G.D. The piriform cortex and human focal epilepsy. Front. Neurol. 2014, 5, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miodonski, R. The structure of the posterior piriform cortex in the dog. Acta Anat. 1974, 88, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Menn, O.; Meisner, C.; Steinbach, J.; Hermisson, M.; Tatagiba, M.; Weller, M. Pharmacotherapy of epileptic seizures in glioma patients: Who, when, why and how long? Onkologie 2005, 28, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.T.; Rhoton, A.L.; De Oliveira, E.; Cardoso, A.C.C.; Tedeschi, H.; Baccanelli, M.; Marino, R., Jr. Microsurgical anatomy of the temporal lobe: Part 1: Mesial temporal lobe anatomy and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery 1999, 45, 549–592. [Google Scholar] [CrossRef] [PubMed]
- Asada, R.; Hamamoto, Y.; Yu, Y.; Mizuno, S.; Chambers, J.K.; Uchida, K.; Hasegawa, D. Ventrolateral temporal lobectomy in normal dogs as a counterpart to human anterior temporal lobectomy: A preliminary study on the surgical procedure and complications. J. Vet. Med. Sci. 2021, 83, 1513–1520. [Google Scholar] [CrossRef]
- Shihab, N.; Summers, B.A.; Benigni, L.; Mcevoy, A.W.; Volk, H.A. Novel approach to temporal lobectomy for removal of a cavernous hemangioma in a dog. Vet. Surg. 2014, 43, 877–881. [Google Scholar] [CrossRef]
- De Risio, L.S.P. Canine and Feline Epilepsy: Diagnosis and Management; CABI: Boston, MA, USA, 2014; 101p.
- Jia, H.; Pustovyy, O.M.; Waggoner, P.; Beyers, R.J.; Schumacher, J.; Wildey, C.; Barrett, J.; Morrison, E.; Salibi, N.; Denney, T.S.; et al. Functional MRI of the olfactory system in conscious dogs. PLoS ONE 2014, 9, e86362. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, S.R.M.; Rossmeisl, J.H.; Russell, J.; Holmes, M.A.; Wessmann, A.; Morris, J.; Dobson, J.M.; Vanhaesebrouck, A.E. Effect of radiotherapy on freedom from seizures in dogs with brain tumors. J. Vet. Intern. Med. 2020, 34, 821–827. [Google Scholar] [CrossRef]


| Neurological Findings | Description |
|---|---|
| Absence | Only seizures. |
| Mild/Moderate | At least one of the following: mildly altered mentation, head tilt, ambulatory status, any type of ataxia, proprioceptive deficits, and cranial nerve deficits. |
| Severe | At least one of the following: severe obtundation, stupor or coma, or non-ambulatory tetraparesis. |
| Post-ictal | At least one of the following: altered mental status, compulsive behaviour, tetraparesis or paraparesis, bilateral symmetrical proprioceptive deficits, or bilateral menace response deficits. |
| Glioma-Related Death of Known Cause | Tumour Location | |||
|---|---|---|---|---|
| Fronto-Olfactory Cortex (n = 14) | Piriform Lobe (n = 11) | Temporal Cortex (n = 4) | Other Rostrotentorial (n = 3) | |
| No improvement—euthanasia within 48 h | 2 (14) | 0 (0) | 1 (25) | 0 |
| Progression of neurological signs | 9 (64) | 1 (9) | 2 (50) | 1 (33) |
| Increased intensity of seizures | 3 (22) | 10 (91) | 1 (25) | 2 (67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pons-Sorolla, M.; Dominguez, E.; Czopowicz, M.; Suñol, A.; Maeso Ordás, C.; Morales Moliner, C.; Pérez Soteras, M.; Montoliu, P. Clinical and Magnetic Resonance Imaging (MRI) Features, Tumour Localisation, and Survival of Dogs with Presumptive Brain Gliomas. Vet. Sci. 2022, 9, 257. https://doi.org/10.3390/vetsci9060257
Pons-Sorolla M, Dominguez E, Czopowicz M, Suñol A, Maeso Ordás C, Morales Moliner C, Pérez Soteras M, Montoliu P. Clinical and Magnetic Resonance Imaging (MRI) Features, Tumour Localisation, and Survival of Dogs with Presumptive Brain Gliomas. Veterinary Sciences. 2022; 9(6):257. https://doi.org/10.3390/vetsci9060257
Chicago/Turabian StylePons-Sorolla, Marta, Elisabet Dominguez, Michał Czopowicz, Anna Suñol, Christian Maeso Ordás, Carles Morales Moliner, Marc Pérez Soteras, and Patrícia Montoliu. 2022. "Clinical and Magnetic Resonance Imaging (MRI) Features, Tumour Localisation, and Survival of Dogs with Presumptive Brain Gliomas" Veterinary Sciences 9, no. 6: 257. https://doi.org/10.3390/vetsci9060257
APA StylePons-Sorolla, M., Dominguez, E., Czopowicz, M., Suñol, A., Maeso Ordás, C., Morales Moliner, C., Pérez Soteras, M., & Montoliu, P. (2022). Clinical and Magnetic Resonance Imaging (MRI) Features, Tumour Localisation, and Survival of Dogs with Presumptive Brain Gliomas. Veterinary Sciences, 9(6), 257. https://doi.org/10.3390/vetsci9060257







