Primary Broiler Hepatocytes for Establishment of a Steatosis Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Primary Hepatocytes from Broiler Chickens
2.2. Optimal Conditions for Hepatocyte Steatosis Model Induced by Fat Emulsion
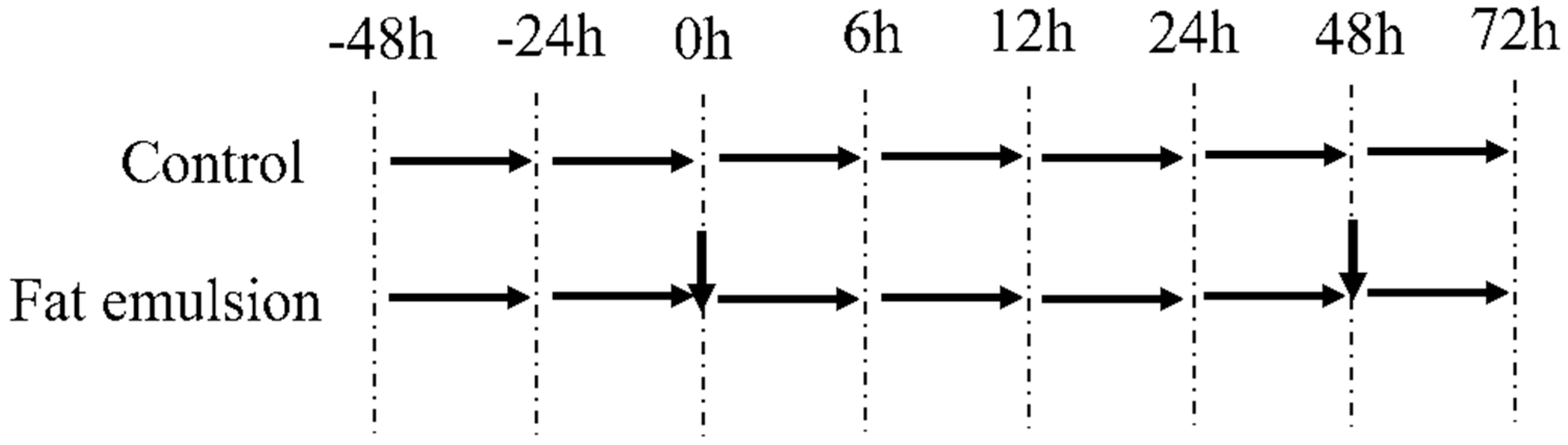
2.3. Establishing a Primary Hepatocyte Model of Steatosis in Broiler Chickens
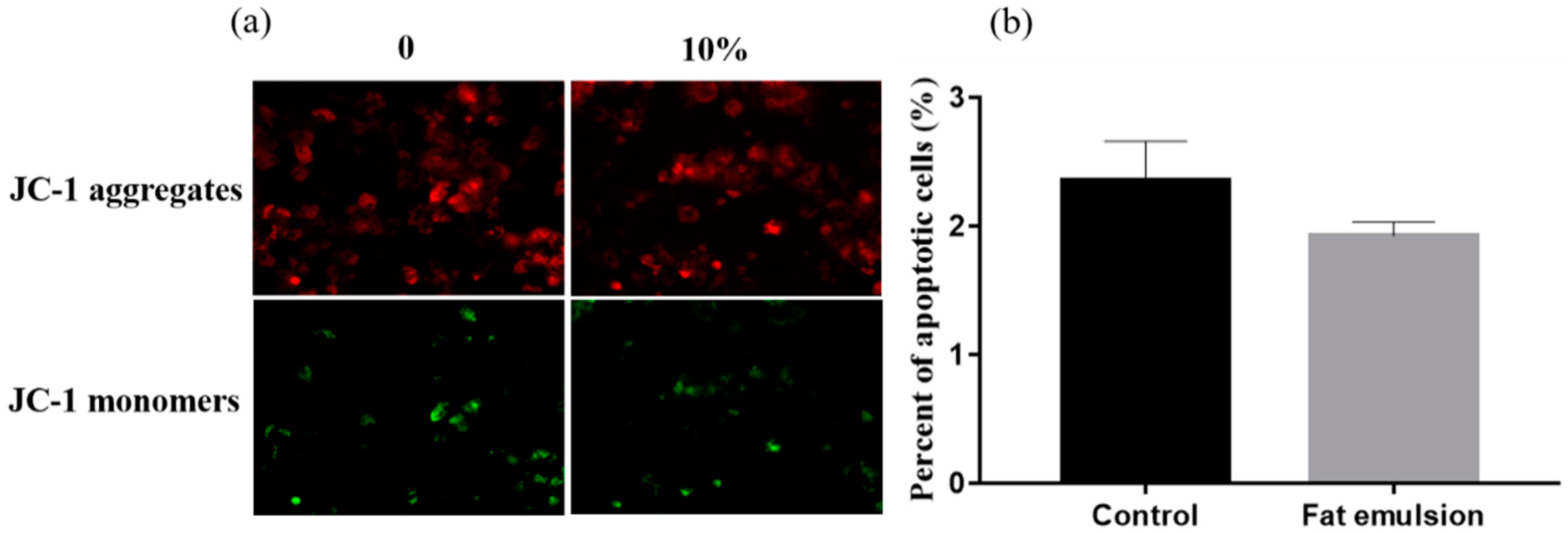
2.4. Effect of Fat Emulsion on Mitochondrial Membrane Potential in Hepatocytes
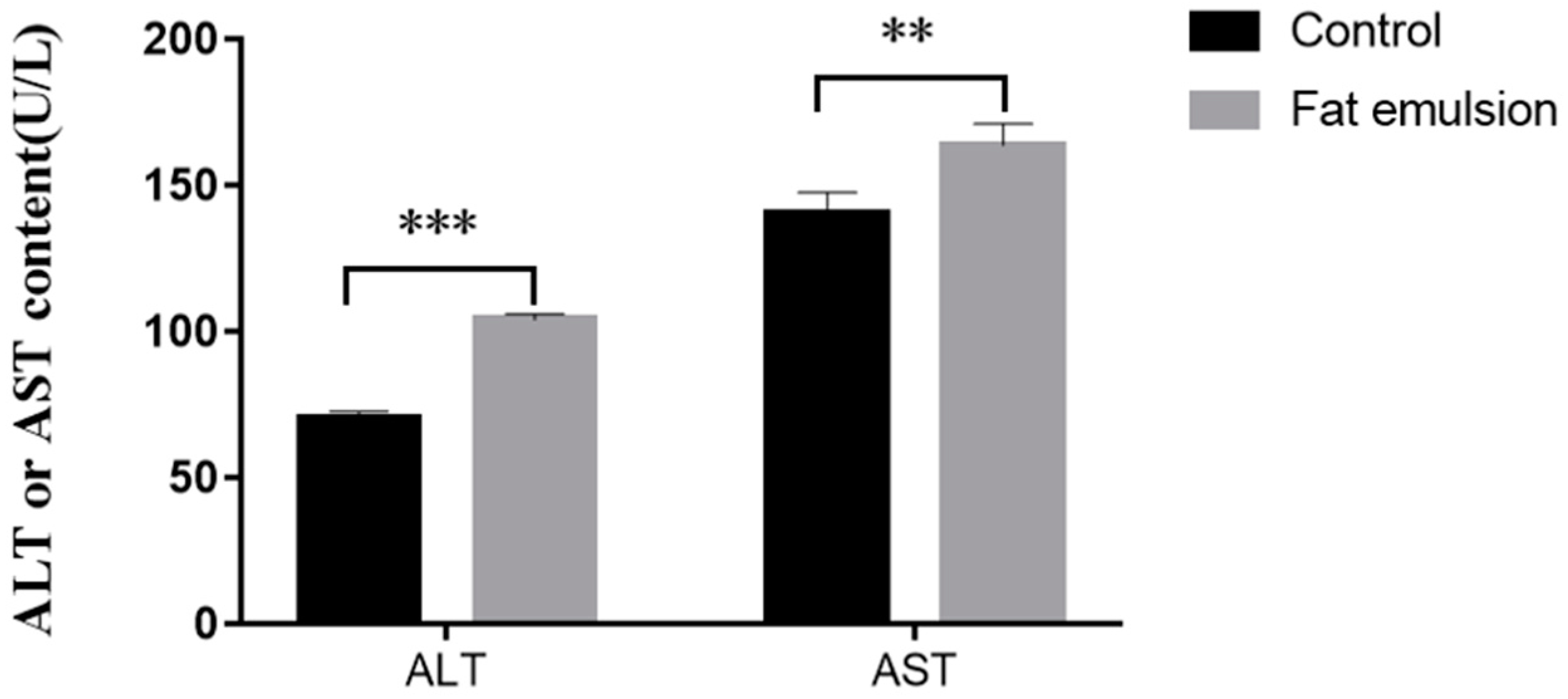
2.5. Effect of Fat Emulsion on Release of ALT and AST by Hepatocytes
2.6. Effect of Fat Emulsion on TG Content in Hepatocytes
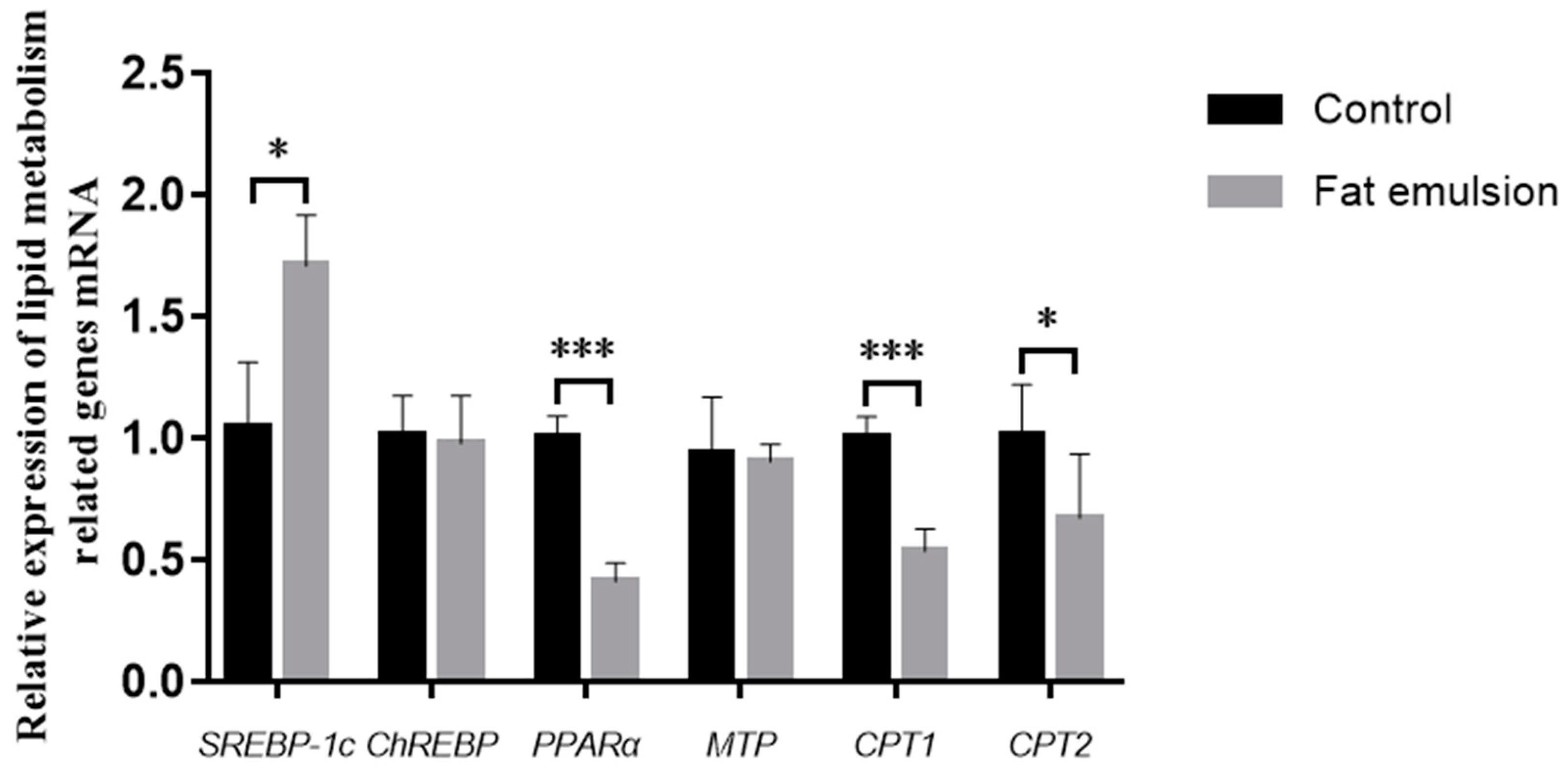
2.7. Effect of Fat Emulsion on Expression of Lipid Metabolism Genes
2.8. Statistical Analysis
3. Results
3.1. Optimal Conditions for Induction of Steatosis in Broiler Primary Hepatocytes by Fat Emulsion
3.2. Effect of Fat Emulsion on Hepatocyte Mitochondrial Membrane Potential
3.3. Effect of Fat Emulsion on Release of ALT and AST from Hepatocytes
3.4. Effect of Fat Emulsion on TG Level in Hepatocytes
3.5. Effect of Fat Emulsion on Expression of Lipid Metabolism Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Shi, Y.; Huang, C.; Huang, C.; Xu, P.; Zhou, C.; Liu, P.; Hu, R.; Zhuang, Y.; Li, G.; et al. Activation of AMP-activated protein kinase signaling pathway ameliorates steatosis in laying hen hepatocytes. Poult. Sci. 2020, 100, 100805. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, F.; Tang, H. Based on network pharmacology method to discovered the targets and therapeutic mechanism of Paederia scandens against nonalcoholic fatty liver disease in chicken. Poult. Sci. 2021, 100, 101042. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mao, H.; Peng, G.; Zeng, Q.; Wei, Q.; Ruan, J.; Huang, J. Effect of JAK-STAT pathway in regulation of fatty liver hemorrhagic syndrome in chickens. Anim. Biosci. 2021, 34, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Chu, X.; Yao, Y.; Cao, J.; Li, Q.; Ma, H.-T. (−)-Hydroxycitric acid alleviates oleic acid induced steatosis, oxidative stress and inflammation in primary chicken hepatocytes by regulating AMPK mediated ROS levels. J. Agric. Food Chem. 2020, 68, 11229–11241. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Qiu, K.; Zhao, Q.; Wang, J.; Qi, G.-H.; Wu, S.-G.; Zhang, H.-J. Effects of Pyrroloquinoline Quinone on Lipid Metabolism and Anti-Oxidative Capacity in a High-Fat-Diet Metabolic Dysfunction-Associated Fatty Liver Disease Chick Model. Int. J. Mol. Sci. 2021, 22, 1458. [Google Scholar] [CrossRef]
- Li, L.; Stanton, J.D.; Tolson, A.H.; Luo, Y.; Wang, H. Bioactive Terpenoids and Flavonoids from Ginkgo Biloba Extract Induce the Expression of Hepatic Drug-Metabolizing Enzymes Through Pregnane X Receptor, Constitutive Androstane Receptor, and Aryl hydrocarbon Receptor-Mediated Pathways. Pharm. Res. 2008, 26, 872–882. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.-X.; Liu, Q.; Tang, Y.-J.; Wang, W.-J.; Zheng, Q.-S.; Tian, H.-J.; Yao, D.-S.; Liu, L.; Peng, J.-H.; Zhao, Y.; et al. A Recipe Composed of Chinese Herbal Active Components Regulates Hepatic Lipid Metabolism of NAFLD In Vivo and In Vitro. BioMed Res. Int. 2016, 2016, 1–12. [Google Scholar]
- Xie, C.; Chen, Z.; Zhang, C.; Xu, X.; Jin, J.; Zhan, W.; Han, T.; Wang, J. Dihydromyricetin ameliorates oleic acid-induced lipid accumulation in L02 and HepG2 cells by inhibiting lipogenesis and oxidative stress. Life Sci. 2016, 157, 131–139. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, H.; Wang, L.; Song, X.; Zhang, J.; Liu, W.; Ge, Y.; Sun, Y.; Yu, X.; Wang, Z.; et al. Tumor suppressor ZHX2 inhibits NAFLD-HCC progression via blocking LPL-mediated lipid uptake. Cell Death Differ. 2019, 27, 1693–1708. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.-J.; Wang, Y.-C.; Quan, W.; Wang, J.-W.; Zhang, W.-G. Hemodynamic changes in hepatic sinusoids of hepatic steatosis mice. World J. Gastroenterol. 2019, 25, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, X.; Zhang, P.; Guo, Y.; Lin, H.; Diao, X.; Liu, Y.; Dong, C.; Hu, Y.; Chen, S.; et al. Dynamic mechanical analysis to assess viscoelasticity of liver tissue in a rat model of nonalcoholic fatty liver disease. Med. Eng. Phys. 2017, 44, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yuan, Y.; Wang, Q.; Li, X.; Men, J.; Lin, M. The Chinese medicine Chai Hu Li Zhong Tang protects against non-alcoholic fatty liver disease by activating AMPKα. Biosci. Rep. 2018, 38, BSR20180644. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, C. Effect of ω-3 polyunsaturated fatty acid-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy: A meta-analysis of randomized control trials in China. Medicine 2018, 97, e0472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.M.; Liu, G.W.; Su, W.; Huang, J.L.; Xie, G.H.; Wang, Z. Isolation and primary culture of calf hepatocytes. J. Cell Biol. 2007, 29, 880–884. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Flees, J.; Rajaei-Sharifabadi, H.; Greene, E.; Beer, L.; Hargis, B.M.; Ellestad, L.; Porter, T.; Donoghue, A.; Bottje, W.G.; Dridi, S. Effect of Morinda citrifolia (Noni)-Enriched Diet on Hepatic Heat Shock Protein and Lipid Metabolism-Related Genes in Heat Stressed Broiler Chickens. Front. Physiol. 2017, 8, 919. [Google Scholar] [CrossRef] [Green Version]
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian Liver: The Forgotten Organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yébenes, J.C.; Campins, L.; de Lagran, I.M.; Bordeje, L.; Lorencio, C.; Grau, T.; Montejo, J.C.; Bodí, M.; Serra-Prat, M.; Working Group on Nutrition and Metabolism of the Spanish Society of Critical Care. Nutritrauma: A Key Concept for Minimising the Harmful Effects of the Administration of Medical Nutrition Therapy. Nutrients 2019, 11, 1775. [Google Scholar] [CrossRef] [Green Version]
- Thanabalan, A.; Moats, J.; Kiarie, E.G. Effects of feeding broiler breeder hens a coextruded full-fat flaxseed and pulses mixture without or with multienzyme supplement. Poult. Sci. 2020, 99, 2616–2623. [Google Scholar] [CrossRef]
- Peng, G.; Huang, E.; Ruan, J.; Huang, L.; Liang, H.; Wei, Q.; Xie, X.; Zeng, Q.; Huang, J. Effects of a high energy and low protein diet on hepatic and plasma characteristics and Cidea and Cidec mRNA expression in liver and adipose tissue of laying hens with fatty liver hemorrhagic syndrome. Anim. Sci. J. 2018, 90, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Sheikhahmadi, A.; Li, X.; Buyse, J.; Lin, H.; Song, Z. Effects of dietary energy level on appetite and central adenosine monophosphate-activated protein kinase (AMPK) in broilers. J. Anim. Sci. 2019, 97, 4488–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Pliego, L.E.; Martínez-Carrillo, B.E.; Reséndiz-Albor, A.A.; Valdés-Ramos, R. Effect on Adipose Tissue of Diabetic Mice Supplemented with n-3 Fatty Acids Extracted from Microalgae. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Konda, P.Y.; Poondla, V.; Jaiswal, K.K.; Dasari, S.; Uyyala, R.; Surtineni, V.P.; Egi, J.Y.; Masilamani, A.J.A.; Bestha, L.; Konanki, S.; et al. Pathophysiology of high fat diet induced obesity: Impact of probiotic banana juice on obesity associated complications and hepatosteatosis. Sci. Rep. 2020, 10, 16894. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, R.; Wang, W.; Mao, X.; Yu, J. Lipid regulation effects of Polygoni Multiflori Radix, its processed products and its major substances on steatosis human liver cell line L02. J. Ethnopharmacol. 2012, 139, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Wang, M.; Guo, Y.; Wen, H.; Chen, X.; Chen, S.; Lin, H. Temperature dependent of viscoelasticity measurement on fat emulsion phantom using acoustic radiation force elasticity imaging method. Technol. Health Care 2018, 26, 449–458. [Google Scholar] [CrossRef]
- You, J.; Raghunathan, V.K.; Son, K.J.; Patel, D.; Haque, A.; Murphy, C.J.; Revzin, A. Impact of Nanotopography, Heparin Hydrogel Microstructures, and Encapsulated Fibroblasts on Phenotype of Primary Hepatocytes. ACS Appl. Mater. Interfaces 2014, 7, 12299–12308. [Google Scholar] [CrossRef]
- Jeong, D.; Han, C.; Kang, I.; Park, H.T.; Kim, J.; Ryu, H.; Gho, Y.S.; Park, J. Effect of Concentrated Fibroblast-Conditioned Media on In Vitro Maintenance of Rat Primary Hepatocyte. PLoS ONE 2016, 11, e0148846. [Google Scholar] [CrossRef] [Green Version]
- Wallace, K.; Fairhall, E.A.; Charlton, K.A.; Wright, M.C. AR42J-B-13 cell: An expandable progenitor to generate an unlimited supply of functional hepatocytes. Toxicology 2010, 278, 277–287. [Google Scholar] [CrossRef]
- Guo, R.; Tang, W.; Yuan, Q.; Hui, L.; Wang, X.; Xie, X. Chemical Cocktails Enable Hepatic Reprogramming of Mouse Fibroblasts with a Single Transcription Factor. Stem Cell Rep. 2017, 9, 499–512. [Google Scholar] [CrossRef] [Green Version]
- Marolt, T.P.; Kramar, B.; Rozman, K.B.; Šuput, D.; Milisav, I. Aripiprazole reduces liver cell division. PLoS ONE 2020, 15, e0240754. [Google Scholar]
- Tahan, A.C.; Tahan, V. Placental Amniotic Epithelial Cells and Their Therapeutic Potential in Liver Diseases. Front. Med. 2014, 1, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Lin, L.; Lin, H.; Qu, C.; Yan, L.; Ni, J. Interpretation the Hepatotoxicity Based on Pharmacokinetics Investigated Through Oral Administrated Different Extraction Parts of Polygonum multiflorum on Rats. Front. Pharmacol. 2018, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Zhao, Z.; Lu, H.; Ke, B.; Ye, X.; Wu, B.; Ye, J. NF- B/HDAC1/SREBP1c pathway mediates the inflammation signal in progression of hepatic steatosis. Acta Pharm. Sin. B 2020, 10, 825–836. [Google Scholar] [CrossRef]
- Wang, C.; Duan, X.; Sun, X.; Liu, Z.; Sun, P.; Yang, X.; Sun, H.; Liu, K.; Meng, Q. Protective effects of glycyrrhizic acid from edible botanical glycyrrhiza glabra against non-alcoholic steatohepatitis in mice. Food Funct. 2016, 7, 3716–3723. [Google Scholar] [CrossRef]
- Valtolina, C.; Robben, J.H.; E van Wolferen, M.; Kruitwagen, H.S.; Corbee, R.J.; Favier, R.P.; Penning, L.C. Gene expressions of de novo hepatic lipogenesis in feline hepatic lipidosis. J. Feline Med. Surg. 2019, 22, 500–505. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, U.M.; Sanchez, E.R.; Kumarasamy, S. COUP-TFII revisited: Its role in metabolic gene regulation. Steroids 2018, 141, 63–69. [Google Scholar] [CrossRef]
- Kwon, Y.; Jeong, S.J. Relative Skeletal Muscle Mass Is an Important Factor in Non-Alcoholic Fatty Liver Disease in Non-Obese Children and Adolescents. J. Clin. Med. 2020, 9, 3355. [Google Scholar] [CrossRef]
- Saki, S.; Saki, N.; Poustchi, H.; Malekzadeh, R. Assessment of Genetic Aspects of Non-alcoholic Fatty Liver and Premature Cardiovascular Events. Middle East J. Dig. Dis. 2020, 12, 65–88. [Google Scholar] [CrossRef]
- Welty, F.K. Hypobetalipoproteinemia and abetalipoproteinemia: Liver disease and cardiovascular disease. Curr. Opin. Lipidol. 2020, 31, 49–55. [Google Scholar] [CrossRef]
- Chen, Z.; Li, C.; Yang, C.; Zhao, R.; Mao, X.; Yu, J. Lipid Regulation Effects of Raw and Processed Notoginseng Radix Et Rhizome on Steatotic Hepatocyte L02 Cell. BioMed Res. Int. 2016, 2016, 2919034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Gao, X.; Xu, D.; Li, M.; Gao, Z.; Tang, Z.; Mhlambi, N.; Wang, W.; Fan, W.; Shi, X.; et al. Protective effect of the new prepared Atractylodes macrocephala Koidz polysaccharide on fatty liver hemorrhagic syndrome in laying hens. Poult. Sci. 2020, 100, 938–948. [Google Scholar] [CrossRef] [PubMed]

| Gene | Accession Number | Primer Sequence (5′→3′) | Length (bp) |
|---|---|---|---|
| PPARα | XM_025150258.2 | F: 5′ CATTTGCTGTGGAGCTGAAGTT 3′ R: 5′ TTCCGGCATAGAATCCCACTT 3′ | 131 bp |
| SREBP-1c | XM_015294109.3 | F: 5′ GAGACCATCTACAGCTCCGC 3′ R: 5′ TCCGAAAAGCACCTTCCCTC 3′ | 155 bp |
| ChREBP | NM_001110841.1 | F: 5′ ACAGCTCAATCAACCTGTGC 3′ R: 5′ GTATGGTGGAAGGGGCAGTG 3′ | 184 bp |
| CPT1 | NM_001012898.1 | F: 5′ AACCCTTGACACAACTGGCT 3′ R: 5′ GTGACGATAAGGGCAACCCA 3′ | 96 bp |
| CPT2 | NM_001031287.2 | F: 5′ CGCGTGACGGGCCAAC 3′ R: 5′ GTTTGGGAACAGGCAGTCTGG 3′ | 167 bp |
| MTP | NM_001109784.2 | F: 5′ TTGGCTCTCCTTTCAGGCATT3′ R: 5′ AGCCATGGATTCAGGACACC3′ | 225 bp |
| GAPDH | NM_204305.1 | F:5′ TCGGAGTCAACGGATTTGGC3′ R: 5′ CCGTTCTCAGCCTTGACAGT3′ | 178 bp |
| Incubation (Hours) | Fat Emulsion (%) | Cell Viability (%) | Incubation (Hours) | Fat Emulsion (%) | Cell Viability (%) |
|---|---|---|---|---|---|
| 6 h | 0 | 117.8 ± 0.046 ab | 48 h | 0 | 43.6 ± 0.073 ghi |
| 2.5 | 121.0 ± 0.046 ab | 2.5 | 41.8 ± 0.052 hi | ||
| 5 | 125.0 ± 0.06 ab | 5 | 51.2 ± 0.052 fghi | ||
| 10 | 134.4 ± 0.046 a | 10 | 66.6 ± 0.052 cdef | ||
| 20 | 45.6 ± 0.046 fghi | 20 | 38.3 ± 0.052 i | ||
| 12 h | 0 | 54.9 ± 0.046 efghi | 72 h | 0 | 31.0 ± 0.073 i |
| 2.5 | 73.9 ± 0.046 cde | 2.5 | 34.5 ± 0.052 i | ||
| 5 | 87.8 ± 0.06 c | 5 | 47.3 ± 0.06 fghi | ||
| 10 | 110.1 ± 0.046 b | 10 | 65.8 ± 0.073 cdefg | ||
| 20 | 40.1 ± 0.06 hi | 20 | 38.3 ± 0.073 i | ||
| 24 h | 0 | 44.7 ± 0.052 fghi | |||
| 2.5 | 62.7 ± 0.06 defgh | ||||
| 5 | 66.9 ± 0.073 cdef | ||||
| 10 | 77.7 ± 0.073 cd | ||||
| 20 | 57.1 ± 0.052 defghi |
| Incubation (Hours) | Fat Emulsion (%) | Cell Viability (%) |
|---|---|---|
| 6 | 107.4 ± 0.345 a | |
| 12 | 75.1 ± 0.257 ab | |
| 24 | 59.0 ± 0.120 b | |
| 48 | 48.8 ± 0.111 b | |
| 72 | 42.3 ± 0.121 b | |
| 0 | 72.6 ± 0.3356 ab | |
| 2.5 | 74.8 ± 0.320 ab | |
| 5 | 79.8 ± 0.288 ab | |
| 10 | 98.0 ± 0.267 a | |
| 20 | 55.8 ± 0.246 b | |
| p-value | ||
| Incubation time | <0.0001 | |
| Fat emulsion level | <0.0001 | |
| Interaction | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Meng, S.; Li, C.; Yang, Z.; Wang, G.; Wang, X.; Ma, Y. Primary Broiler Hepatocytes for Establishment of a Steatosis Model. Vet. Sci. 2022, 9, 316. https://doi.org/10.3390/vetsci9070316
Zhang C, Meng S, Li C, Yang Z, Wang G, Wang X, Ma Y. Primary Broiler Hepatocytes for Establishment of a Steatosis Model. Veterinary Sciences. 2022; 9(7):316. https://doi.org/10.3390/vetsci9070316
Chicago/Turabian StyleZhang, Cai, Sudan Meng, Chenxu Li, Zijun Yang, Guoyong Wang, Xueying Wang, and Yanbo Ma. 2022. "Primary Broiler Hepatocytes for Establishment of a Steatosis Model" Veterinary Sciences 9, no. 7: 316. https://doi.org/10.3390/vetsci9070316
APA StyleZhang, C., Meng, S., Li, C., Yang, Z., Wang, G., Wang, X., & Ma, Y. (2022). Primary Broiler Hepatocytes for Establishment of a Steatosis Model. Veterinary Sciences, 9(7), 316. https://doi.org/10.3390/vetsci9070316






