Seronegative Myasthenia Gravis with Concomitant SARS-CoV-2 Infection in a Dog
Abstract
:Simple Summary
Abstract
1. Introduction
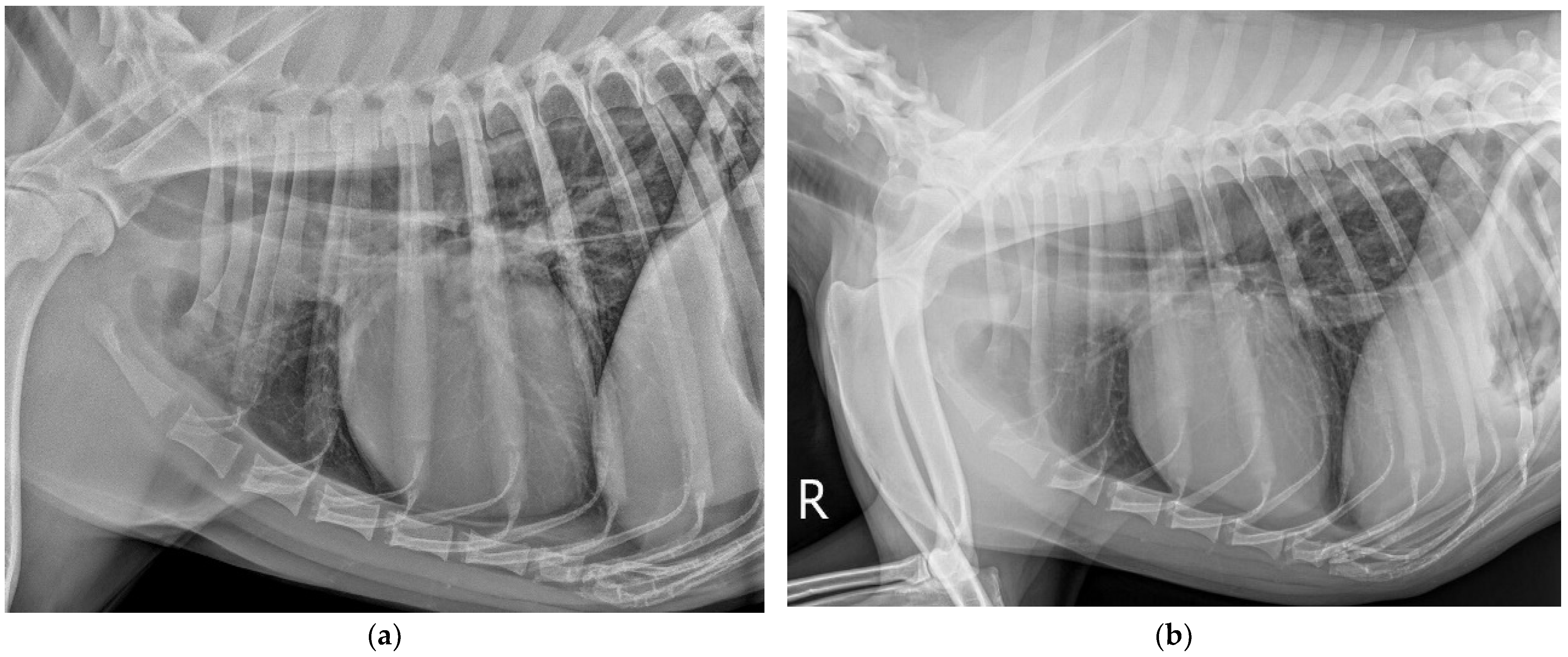
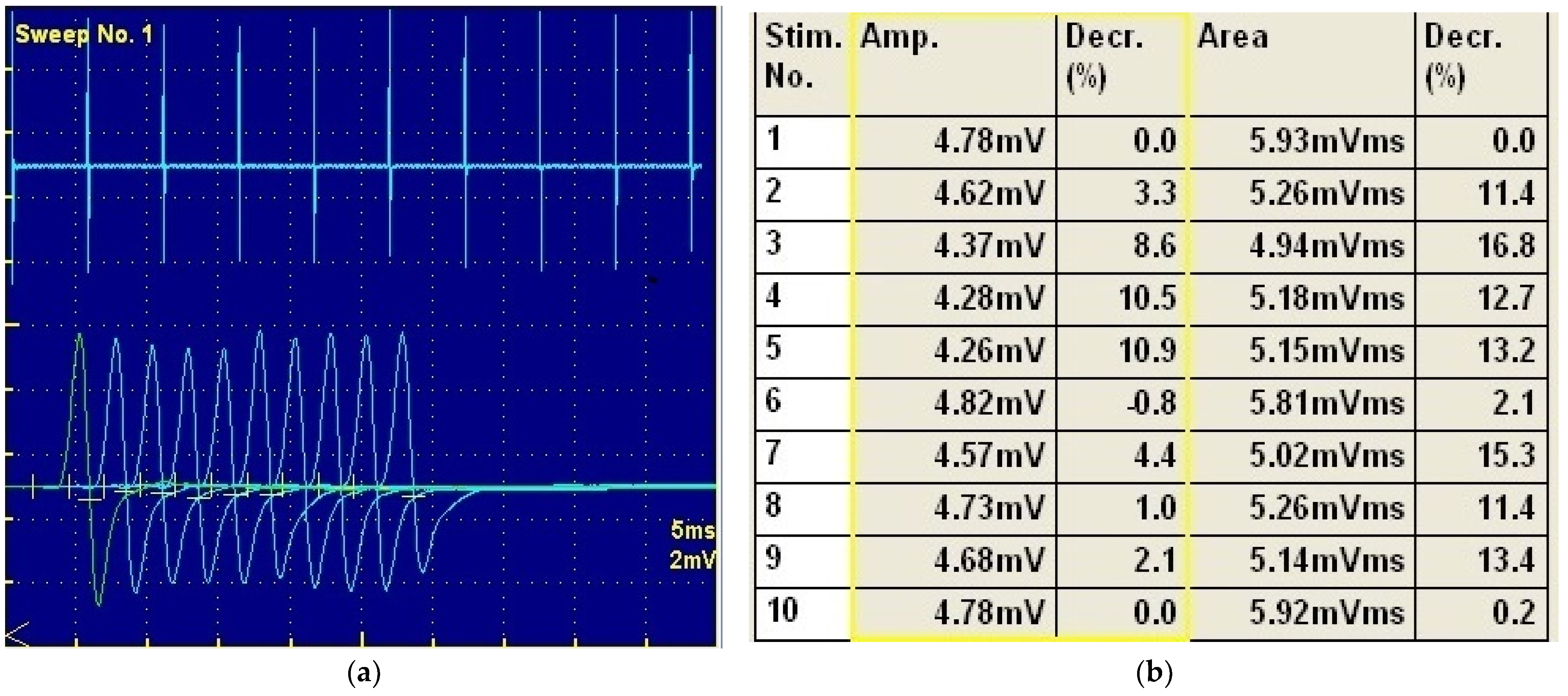
2. Case Description
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mignan, T.; Targett, M.; Lowrie, M. Classification of Myasthenia Gravis and Congenital Myasthenic Syndromes in Dogs and Cats. J. Vet. Intern. Med. 2020, 34, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Shelton, G.D. Myasthenia Gravis and Disorders of Neuromuscular Transmission. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 189–206. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Solcan, G. Acute Idiopathic Polyradiculoneuritis Concurrent with Acquired Myasthenia Gravis in a West Highland White Terrier Dog. BMC Vet. Res. 2016, 12, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelton, G.D. Routine and Specialized Laboratory Testing for the Diagnosis of Neuromuscular Diseases in Dogs and Cats. Vet. Clin. Pathol. 2010, 39, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, P.; Bernasconi, P.; Mantegazza, R. Autoimmune Mechanisms in Myasthenia Gravis. Curr. Opin. Neurol. 2012, 25, 621–629. [Google Scholar] [CrossRef]
- Hung, W.-L.; Lin, Y.-H.; Wang, P.-Y.; Chang, M.-H. HIV-Associated Myasthenia Gravis and Impacts of HAART: One Case Report and a Brief Review. Clin. Neurol. Neurosurg. 2011, 113, 672–674. [Google Scholar] [CrossRef]
- Csuka, D.; Banati, M.; Rozsa, C.; Füst, G.; Illes, Z. High Anti-EBNA-1 IgG Levels Are Associated with Early-Onset Myasthenia Gravis. Eur. J. Neurol. 2012, 19, 842–846. [Google Scholar] [CrossRef]
- Cavalcante, P.; Maggi, L.; Colleoni, L.; Caldara, R.; Motta, T.; Giardina, C.; Antozzi, C.; Berrih-Aknin, S.; Bernasconi, P.; Mantegazza, R. Inflammation and Epstein-Barr Virus Infection Are Common Features of Myasthenia Gravis Thymus: Possible Roles in Pathogenesis. Autoimmun. Dis. 2011, 2011, 213092. [Google Scholar] [CrossRef] [Green Version]
- Forgash, J.T.; Chang, Y.-M.; Mittelman, N.S.; Petesch, S.; Benedicenti, L.; Galban, E.; Hammond, J.J.; Glass, E.N.; Barker, J.R.; Shelton, G.D.; et al. Clinical Features and Outcome of Acquired Myasthenia Gravis in 94 Dogs. J. Vet. Intern. Med. 2021, 35, 2315–2326. [Google Scholar] [CrossRef]
- Brown, S.; Atkins, C.; Bagley, R.; Carr, A.; Cowgill, L.; Davidson, M.; Egner, B.; Elliott, J.; Henik, R.; Labato, M.; et al. Guidelines for the Identification, Evaluation, and Management of Systemic Hypertension in Dogs and Cats. J. Vet. Intern. Med. 2007, 21, 542–558. [Google Scholar] [CrossRef]
- Gunther, M.; Jaffey, J.A.; Evans, J.; Paige, C. Case Report: Persistent Moderate-to-Severe Creatine Kinase Enzyme Activity Elevation in a Subclinical Dog. Front. Vet. Sci. 2021, 8, 1245. [Google Scholar] [CrossRef] [PubMed]
- Furlanello, T.; Simonato, G.; Caldin, M.; De Lorenzi, D.; Lubas, G.; Bernardini, D.; Solano-Gallego, L. Validation of an Automated Spectrophotometric Assay for the Determination of Cholinesterase Activity in Canine Serum. Vet. Res. Commun. 2006, 30, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Sit, T.H.C.; Brackman, C.J.; Ip, S.M.; Tam, K.W.S.; Law, P.Y.T.; To, E.M.W.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of Dogs with SARS-CoV-2. Nature 2020, 586, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Salajegheh Tazerji, S.; Magalhães Duarte, P.; Rahimi, P.; Shahabinejad, F.; Dhakal, S.; Singh Malik, Y.; Shehata, A.A.; Lama, J.; Klein, J.; Safdar, M.; et al. Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) to Animals: An Updated Review. J. Transl. Med. 2020, 18, 358. [Google Scholar] [CrossRef]
- Lee, D.-H.; Helal, Z.H.; Kim, J.; Hunt, A.; Barbieri, A.; Tocco, N.; Frasca, S.; Kerr, K.; Hyeon, J.-Y.; Chung, D.H.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in a Dog in Connecticut in February 2021. Viruses 2021, 13, 2141. [Google Scholar] [CrossRef]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of Ferrets, Cats, Dogs, and Other Domesticated Animals to SARS–Coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Decaro, N.; Vaccari, G.; Lorusso, A.; Lorusso, E.; De Sabato, L.; Patterson, E.I.; Di Bartolo, I.; Hughes, G.L.; Teodori, L.; Desario, C.; et al. Possible Human-to-Dog Transmission of SARS-CoV-2, Italy, 2020. Emerg. Infect. Dis. 2021, 27, 1981–1984. [Google Scholar] [CrossRef]
- Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Gordy, P.W.; Nehring, M.; Byas, A.D.; VandeWoude, S.; Ragan, I.K.; Maison, R.M.; Bowen, R.A. Experimental Infection of Domestic Dogs and Cats with SARS-CoV-2: Pathogenesis, Transmission, and Response to Reexposure in Cats. Proc. Natl. Acad. Sci. USA 2020, 117, 26382–26388. [Google Scholar] [CrossRef]
- Medkour, H.; Catheland, S.; Boucraut-Baralon, C.; Laidoudi, Y.; Sereme, Y.; Pingret, J.; Million, M.; Houhamdi, L.; Levasseur, A.; Cabassu, J.; et al. First Evidence of Human-to-dog Transmission of SARS-CoV-2 B.1.160 Variant in France. Transbound. Emerg. Dis. 2021, 1–8. [Google Scholar] [CrossRef]
- Grobben, M.; van der Straten, K.; Brouwer, P.J.; Brinkkemper, M.; Maisonnasse, P.; Dereuddre-Bosquet, N.; Appelman, B.; Lavell, A.A.; van Vught, L.A.; Burger, J.A.; et al. Cross-Reactive Antibodies after SARS-CoV-2 Infection and Vaccination. eLife 2021, 10, e70330. [Google Scholar] [CrossRef]
- Cridge, H.; Little, A.; José-López, R.; Pancotto, T.; Michaels, J.R.; Menchetti, M.; Suñol, A.; Lipsitz, D.; Beasley, M.J. The Clinical Utility of Neostigmine Administration in the Diagnosis of Acquired Myasthenia Gravis. J. Vet. Emerg. Crit. Care 2021, 31, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Sriwastava, S.; Tandon, M.; Kataria, S.; Daimee, M.; Sultan, S. New Onset of Ocular Myasthenia Gravis in a Patient with COVID-19: A Novel Case Report and Literature Review. J. Neurol. 2021, 268, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Okhovat, A.A.; Ziaadini, B.; Haghi Ashtiani, B.; Nafissi, S.; Fatehi, F. Myasthenia Gravis Associated with Novel Coronavirus 2019 Infection: A Report of Three Cases. Clin. Neurol. Neurosurg. 2021, 208, 106834. [Google Scholar] [CrossRef]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following MRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Rogozinski, S.; Puppe, W.; Framme, C.; Höglinger, G.; Hufendiek, K.; Wegner, F. Postinfectious Onset of Myasthenia Gravis in a COVID-19 Patient. Front. Neurol. 2020, 11, 576153. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.; Pougnier, C. A Case of COVID-19 Vaccine Associated New Diagnosis Myasthenia Gravis. J. Prim. Care Community Health 2021, 12, 21501327211051932. [Google Scholar] [CrossRef]
- Paliwal, V.K.; Garg, R.K.; Gupta, A.; Tejan, N. Neuromuscular Presentations in Patients with COVID-19. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2020, 41, 3039–3056. [Google Scholar] [CrossRef]
- Mobasheri, L.; Nasirpour, M.H.; Masoumi, E.; Azarnaminy, A.F.; Jafari, M.; Esmaeili, S.-A. SARS-CoV-2 Triggering Autoimmune Diseases. Cytokine 2022, 154, 155873. [Google Scholar] [CrossRef]
- Guernier, V.; Milinovich, G.J.; Bezerra Santos, M.A.; Haworth, M.; Coleman, G.; Soares Magalhaes, R.J. Use of Big Data in the Surveillance of Veterinary Diseases: Early Detection of Tick Paralysis in Companion Animals. Parasit. Vectors 2016, 9, 303. [Google Scholar] [CrossRef] [Green Version]
- Malik, R.; Farrow, B.R. Tick Paralysis in North America and Australia. Vet. Clin. N. Am. Small Anim. Pract. 1991, 21, 157–171. [Google Scholar] [CrossRef]
- Mosabah, A.A.A.E.; Morsy, T.A. Tick Paralysis: First Zoonosis Record in Egypt. J. Egypt. Soc. Parasitol. 2012, 42, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Littman, M.P.; Gerber, B.; Goldstein, R.E.; Labato, M.A.; Lappin, M.R.; Moore, G.E. ACVIM Consensus Update on Lyme Borreliosis in Dogs and Cats. J. Vet. Intern. Med. 2018, 32, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Speck, S.; Reiner, B.; Streich, W.J.; Reusch, C.; Wittenbrink, M.M. Canine Borreliosis: A Laboratory Diagnostic Trial. Vet. Microbiol. 2007, 120, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Shelton, G.D. Myasthenia Gravis: Lessons from the Past 10 Years. J. Small Anim. Pract. 1998, 39, 368–372. [Google Scholar] [CrossRef]
- Garden, O.A.; Kidd, L.; Mexas, A.M.; Chang, Y.-M.; Jeffery, U.; Blois, S.L.; Fogle, J.E.; MacNeill, A.L.; Lubas, G.; Birkenheuer, A.; et al. ACVIM Consensus Statement on the Diagnosis of Immune-Mediated Hemolytic Anemia in Dogs and Cats. J. Vet. Intern. Med. 2019, 33, 313–334. [Google Scholar] [CrossRef]
- Viitanen, S.J.; Lappalainen, A.K.; Christensen, M.B.; Sankari, S.; Rajamäki, M.M. The Utility of Acute-Phase Proteins in the Assessment of Treatment Response in Dogs With Bacterial Pneumonia. J. Vet. Intern. Med. 2017, 31, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Canonne, A.M.; Menard, M.; Maurey, C.; Benchrekroun, G.; Fernandes Rodrigues, N.; Billen, F.; Clercx, C. Comparison of C-Reactive Protein Concentrations in Dogs with Bordetella Bronchiseptica Infection and Aspiration Bronchopneumonia. J. Vet. Intern. Med. 2021, 35, 1519–1524. [Google Scholar] [CrossRef]
| Anti-SARS-CoV-2 Specific Antibodies | IgG (U/mL) | IgA (U/mL) | IgM (U/mL) |
|---|---|---|---|
| Anti-SARS-CoV-2—nucleocapsid (N) protein | 8.521 | 8.490 | 142.050 |
| Anti-SARS-CoV-2–spike S1 subunit receptor binding domain RBD | 17.638 | 7.858 | 144.000 |
| Anti-SARS-CoV-2—S2 unit of spike (Spike S2) | 24.968 | 7.679 | 131.563 |
| Anti-SARS-CoV-2—envelope (E) protein | 19.730 | 6.704 | 132.002 |
| Anti-ACE2—(antibodies of angiotensin—converting enzyme 2) | 19.287 | 7.564 | 139.756 |
| Anti-PL pro—(papain-like protease) | 9.9710 | 9.538 | 163.163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musteata, M.; Borcea, D.-G.; Despa, A.; Ștefănescu, R.; Ivănescu, L.; Hrițcu, L.D.; Baisan, R.A.; Lăcătuș, R.; Solcan, G. Seronegative Myasthenia Gravis with Concomitant SARS-CoV-2 Infection in a Dog. Vet. Sci. 2022, 9, 318. https://doi.org/10.3390/vetsci9070318
Musteata M, Borcea D-G, Despa A, Ștefănescu R, Ivănescu L, Hrițcu LD, Baisan RA, Lăcătuș R, Solcan G. Seronegative Myasthenia Gravis with Concomitant SARS-CoV-2 Infection in a Dog. Veterinary Sciences. 2022; 9(7):318. https://doi.org/10.3390/vetsci9070318
Chicago/Turabian StyleMusteata, Mihai, Denis-Gabriel Borcea, Andreea Despa, Raluca Ștefănescu, Larisa Ivănescu, Luminița Diana Hrițcu, Radu Andrei Baisan, Radu Lăcătuș, and Gheorghe Solcan. 2022. "Seronegative Myasthenia Gravis with Concomitant SARS-CoV-2 Infection in a Dog" Veterinary Sciences 9, no. 7: 318. https://doi.org/10.3390/vetsci9070318
APA StyleMusteata, M., Borcea, D.-G., Despa, A., Ștefănescu, R., Ivănescu, L., Hrițcu, L. D., Baisan, R. A., Lăcătuș, R., & Solcan, G. (2022). Seronegative Myasthenia Gravis with Concomitant SARS-CoV-2 Infection in a Dog. Veterinary Sciences, 9(7), 318. https://doi.org/10.3390/vetsci9070318






