1. Introduction
Epidemiological studies on congenital heart diseases in dogs have been conducted globally since the early 1960s [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11] In almost all studies, the most common congenital heart diseases observed were patent ductus arteriosus (PDA), pulmonic stenosis, and subaortic stenosis [
1,
2,
3,
4,
5,
6,
7,
8,
12]. PDA can occur in any breed; however, small or medium breeds appear to be overrepresented [
13,
14]. PDA is also more often seen in females than in males [
14].
Left-to-right shunting PDA can lead to pathological pulmonary overcirculation and left-sided volume overload, potentially resulting in congestive heart failure [
2,
15,
16]. If PDA occlusion is not performed, 64% of affected dogs may die within one year [
15].
Treatment of PDA involves permanent attenuation of the ductal flow, either through surgical ligation or minimally invasive transcatheter occlusion with devices such as the Amplatz canine ductal occluder (ACDO), vascular plugs, or embolization coils released via an arterial or venous approach [
17,
18,
19,
20,
21]. Among the minimally invasive treatment approaches, transcatheter occlusion with ACDO devices has been reported as the treatment of choice for dogs with left-to-right shunting PDA [
17,
19,
21]. This provides a minimally invasive controlled catheter-based delivery system, easy deployment, low complication rate, a broad range of sizes of the ADCO device and effective closing of a wide range of patients and PDA dimensions [
18,
21]. However, the ACDO delivery system size and the need to use this device following a transarterial approach may limit its use in small dogs, especially when presenting large-sized PDAs [
21].
Using Amplatzer vascular plugs II (AVP II) for interventional closure of PDA through a transvenous approach has recently been reported [
22,
23]. AVP II is a self-expandable occlusion device made of nitinol wire mesh with lateral retention discs and a central component with the same diameter in a symmetric cylindrical shape. Due to its symmetrical shape, this device can also be used through a transvenous vascular approach using relatively small catheters [
22,
23].
Placing an introducer vascular access sheath in the femoral vein is safe and effective for PDA occlusion using the AVP II [
22,
23]. Similarly, as with the arterial vascular access, transvenous vascular access through the femoral vein can be performed either by a surgical cut-down or percutaneous puncture of the femoral vein using the Seldinger technique [
24]. The removal of the introducer vascular sheath from the percutaneous femoral vein access may rarely cause a significant hemorrhage and hematoma. This could be managed with compression or prevented by a surgical cut-down with subsequent femoral vein ligation. On the other hand, surgical cut-down of the femoral vein can prolong the procedure time and can be technically difficult in small dogs in comparison to transjugular venous access [
25,
26].
Right jugular venotomy in human medicine is described as an easier and faster vascular venous access via either the cut-down technique or the percutaneous Seldinger technique compared with the transfemoral approach, and it enables adequate compression to be applied to decrease the risk of hemorrhage and complications [
27]. Furthermore, the British Cardiac Society recommends that the right internal jugular vein is the best route in most human patients, particularly during procedures conducted by inexperienced operators. This is because it provides the most direct route to the right ventricle, a high success rate, a shorter procedure duration, and few complications [
28,
29].
To the best of our knowledge, there are no studies that describe the transjugular occlusion of the PDA with AVP II in dogs and no direct comparisons of outcomes between AVP II implantation with the femoral vein approach and the jugular vein approach have been reported. The aim of this case series was thus to describe a new venous approach through the right jugular vein for PDA closure with AVP II in dogs. This study did not intend to compare the results obtained by transjugular occlusion with AVP II with a control group treated with transcatheter occlusion by ACDO devices.
2. Materials and Methods
The procedures described in this study were performed following veterinary good clinical practice guidelines and written informed consent was obtained from the owners to perform the PDA closure. Data regarding dogs that underwent a minimally invasive PDA occlusion with the AVP II (
Figure 1) via the transjugular approach were retrospectively reviewed.
The left-to-right shunting PDA was suspected by auscultation of a characteristic continuous murmur at the left axillary area and confirmed by transthoracic echocardiography. All cases also underwent complete hematobiochemical analysis, thoracic radiographs, and electrocardiography. The PDA was visualized from the right parasternal short axis view and from the left parasternal cranial views. Maximal ductal measurements, including the minimal ductal diameter (MDD) and the ampulla diameter (AD), were obtained from the echocardiographic view that provided the highest echocardiographic quality. Measurements were performed by an experienced operator in veterinary interventional procedures (L.V.) and by a board-certified cardiologist (O.D.). The MDD was measured at the level of the ductal opening into the pulmonary artery and the AD was measured perpendicular to the ductal wall at the midway through its long axis. The dimension of the occlusion device was based on the type and size of the duct which was at least twice as large as the minimal ductal diameter and at least 20% larger than the maximal echocardiographic AD measurement [
22].
Premedication included methadone (0.2 mg/kg, intramuscularly [IM]). Anesthesia was induced with fentanyl (3 mcg/kg intravenously [IV]), midazolam (0.2 mg/kg IV) and propofol (3 mg/kg IV). An appropriate plane of anesthesia was maintained, with isoflurane and oxygen delivered via a precision vaporizer and a non-rebreathing circuit. For the procedure, all dogs were placed in left lateral recumbency on the fluoroscopy table. The right jugular vein was surgically isolated by the cut down technique and a 7 or an 8 Fr introducer vascular sheath (PINNACLE
® R/O II HIFLO Introducer Sheath 7 Fr × 4 cm × 0.038″ or PINNACLE
® PERIPHERAL Introducer Sheath 7 Fr/8 Fr × 10 cm × 0.035″ Terumo Interventional Systems, Tokyo, Japan; Super Sheath™ Introducer Sheath 8 Fr/9 Fr × 11 cm × 0.038″, distributed by Boston Scientific Corporation, Natick, MA, USA, manufactured by Togo Medikit Co., Ltd., Hyūga, Tokyo, Japan) was placed into the right jugular vein. A 4 Fr Berenstein guiding catheter (distributed by Infiniti Medical, Huddersfield, UK and manufactured by Abbott Medical, Plymouth, MN, USA) or 5 Fr multipurpose catheter (Beacon Tip 5 Fr × 0.038″ × 100 cm, Cook Medical Inc, Bloomington, IN, USA) was then inserted into the jugular vein along with a preplaced straight tip soft guidewire (0.035″ Terumo Glidewire, 0.035″ × 150 cm, Straight). It was passed through the right ventricle, the pulmonary artery and the PDA in a retrograde manner until the tip of the guiding catheter or multipurpose catheter was located in the descending aorta under fluoroscopy guidance. After removing the guidewire, an angiographic study using iohexol was performed showing the location and the morphology of the PDA. Following angiography, a Cook Rosen PTFE Curved Wire Guide 0.035″ × 145 cm was advanced into the descending aorta and the Berenstein guiding catheter or multipurpose catheter was removed. Based on the chosen AVP II size, a 5.5 Fr to 6 Fr × 40 cm guiding sheath (Flexor Balkin, Cook Medical Inc, Bloomington, IN, USA) was advanced over the guidewire into the descending aorta (
Figure 2) acting as delivering catheter, and the guidewire was subsequently removed.
The 180° curve of the Balkin guiding sheath enables it to easily pass through the right ventricle in order to reach the pulmonary artery, the PDA and the descending aorta.
The device was introduced into the Balkin guiding sheath through the Check-Flo Valve and then advanced carefully until the distal disc was expanded into the descending aorta near the ductus. The partially-deployed AVP II, the attached delivery cable and the guiding sheath were gently pulled back simultaneously, until the distal disc engaged the aortic ostium of the PDA. The guiding sheath then was retracted while slightly pushing the delivery wire, allowing the central component of the device to expand into the PDA ampulla (
Figure 3).
The proximal disk was then deployed in the main pulmonary artery with a firm but not excessive traction that was sufficient to create a slight distension of the proximal disc. This guarantees its position in the pulmonary artery, while the lack of waist in the device central component indicates that it is totally positioned in the ampulla and is not engaged in the ostium (
Figure 4).
Correct positioning of the device was also evaluated during the procedure using transthoracic echocardiography, which also enables the presence or absence of residual flow to be analyzed throughout the device as well as its stability within the duct (
Figure 5).
The AVP II device was then detached by counterclockwise rotation of the delivery wire. All catheters were then removed, and the right jugular vein was sutured with 4-0 monofilament polydioxanone absorbable suture (PDSII Ethicon) or ligated [
30]. Post-operative thoracic radiography confirmed the correct placement of the device in all patients (
Figure 6).
Clinical and echocardiographic check-ups were scheduled one day, one week, and three months after the procedure for all dogs. In all cases with the sutured jugular vein, the integrity of the vessel and flow normality were checked by vascular ultrasound.
3. Results
Seven dogs were included in this retrospective study. Three dogs were referred to the Anicura Istituto Veterinario Novara (Cases 1, 2 and 7), and four to the Pavia Veterinary Hospital (Cases 3, 4, 5 and 6) for the noninvasive closure of a previously diagnosed PDA. At clinical presentation, all dogs except Case 3 (which was dyspneic and had pulmonary edema managed with medical treatment before the procedure), were bright, alert, and responsive. All dogs presented a loud, continuous murmur at the left axillary area with a palpable precordial thrill and bounding femoral pulses. Color Doppler echocardiography confirmed the presence of the PDA with left-to-right flow in all the dogs, with a peak transductal flow velocity ≥ 5 m/s.
Case 3, on presentation, was dyspneic with a respiratory rate of 60 breaths per minute with cyanotic mucous membranes. Physical examination revealed pulmonary crackles. Lung ultrasound revealed numerous B lines in all lung fields. Focus cardiac ultrasonography showed severe left atrial and left ventricular enlargement and a restrictive transmitral flow pattern. The dog was managed with an IV constant rate infusion of furosemide and the pulmonary edema resolved in 10 h. When the patient was clinically stable, a complete echocardiogram was performed. There were no complications after the PDA closure and there was a complete resolution of the pulmonary edema. The dog was discharged with torasemide (0.1 mg/kg PO q24 h) and pimobendan (0.3 mg/Kg PO q12 h) for two months. The pharmacological treatment was interrupted one month before the last echocardiographic evaluation.
Table 1 reports clinical data, the devices, and all their technical characteristics (order numbers, diameters and lengths), selected to close the PDA based on transthoracic echocardiography.
The procedures were all performed as described in the Material and Methods with no complications. The total procedure time was between 20 and 45 min for all patients. After 24 h, the devices were visible within the ductus with no obvious extension into the pulmonary artery and no residual flow across the device in all dogs. The one-week and three-month echocardiographic examinations showed no residual flow across the device in any of the dogs. All patients showed a significant reduction in the left heart size after one week and three months (
Table 2). Cases 3, 4, 5 and 6 were subjected to jugular vein suture and flows were normal three months after the procedure (
Figure 7).
The thoracic radiographs performed before and just after the procedure showed a reduction in the cardiac silhouette as well as significant reduction of the over-circulation vascular pattern for all dogs (
Figure 8,
Figure 9,
Figure 10,
Figure 11 and
Figure 12).
4. Discussion
A high mortality rate is reported in dogs that do not undergo PDA closure within the first year after diagnosis [
15,
31,
32,
33,
34]. For interventional closure of PDA various types of devices have been used in dogs, mainly coils and ACDO using a transfemoral arterial approach [
19,
32,
33]. The main limitations of this transarterial approach are the risk of femoral artery hemorrhage after the procedure [
32,
33,
35], the risk of femoral nerve trauma, and the patient’s size [
26].
The present case series describes the use of the AVP II as a first-choice device for PDA occlusion using a transjugular technique in five puppies, of which 4 out of 5 had a body weight of less than 3 kg and 1 out of 5 was a large breed dog, and two adult small-sized dogs (respectively weighing more and less than 5 kg). The AVP II was originally developed for the occlusion of high-flow peripheral vessels such as arteriovenous malformations in human patients [
36,
37,
38,
39], but it has also been successfully used for interventional PDA closure in humans and dogs mainly using a transfemoral venous approach [
22,
23,
40,
41]. The transjugular approach is widely used in humans and dogs for minimally invasive procedures necessitating right heart catheterization, such as pulmonary balloon valvuloplasty, pacemaker implantation and radiofrequency ablations [
25,
27,
29], however, it has been rarely reported for ACDO release in dogs with PDA [
42,
43]. Among the venous approaches, the larger size of the vessel and the ease of isolation make the trans-jugular approach more accessible than the femoral one, and this reduces surgical or procedure times [
22,
26,
34,
44,
45]. Another advantage of the jugular access is that the vein can be sutured and not ligated, as was performed in 4 out of 7 dogs in the present case series. Considering the commonly young age of dogs undergoing PDA occlusion, it can be particularly useful to preserve the right jugular vein for potential future procedures (e.g., central venous access or pacemaker implantation). Lastly, we believe that the shorter distance between the jugular vein and the heart significantly reduces the length of the catheters, which are therefore shorter and more manageable for the surgeon during the procedure.
The AVP II device size selection was based on the type and size of the duct which was at least twice as large as the PDA minimal ductal diameter and at least 20% larger than the PDA maximal ampulla diameter as previously described [
22,
23]. The literature also reports an oversize factor of 130–150% of the PDA ampulla diameter, as per the manufacturer directions of use [
23]. Furthermore, it has been additionally described that in cases that have a PDA ampulla diameter smaller or equal to 10 mm (as in 6 of our 7 cases) lower oversizing factors (110–130%), were successfully used as the distensibility of smaller ampullas is suspected to be minimal [
23]. In our case series we used an oversizing factor of at least 120% of the PDA ampulla diameter in accordance with C, D the previous literature.
The present case series suggests that the use of the AVP II via a transjugular approach is a feasible, safe, and effective method for PDA closure in dogs, even in small patients. Embolization of the device did not occur in any case. Complete closure of the PDA and a significant hemodynamic reduction in pulmonary overcirculation and normalization of left heart size were demonstrated in all dogs. In our experience, the Flexor Balkin delivery catheter used in our study has an ideal curvature of the tip (180°), which allows for an easy approach and an adequate movement of the catheter into the right ventricle irrespective of the diameter of the catheter, even in small dogs. In addition, the Flexor Balkin guiding sheath is very handy due to its short length (40 cm), which is a key element for the accurate execution of the procedure.
The present study was not without limitations. Firstly, the retrospective nature of the study did not make it possible to delineate a study design comparing the outcomes between the dogs treated with the AVP II approach and a control group treated with ACDO. A second limit was the lack of homogeneity in the size, weight, and age of the included dogs. Third, the choice of the dimension of the occlusion device was based only on echocardiographic techniques. This may represent a limitation because a double check of the measurements with the angiography has not been performed. However, the device size selection appeared to be adequate in all cases, showing that the echocardiographic measurements obtained by trained operators together with the AVP II device adaptability properties may be the explanation for the high successful rate described in our case series. Finally, the low number of cases and the short period of follow up (3 months) make it impossible to compare the long-term outcomes with those reported in previous literature.
5. Conclusions
The present case series suggests that transjugular PDA occlusion using the AVP II is a safe and effective interventional technique in puppies and adult small breed dogs. It can be considered as a fast alternative to the arterial or venous femoral approach using both the ACDO and the AVP II, particularly in those subjects that have very small femoral vessels.
Author Contributions
Conceptualization, L.V. and O.D.; methodology, L.V., O.D., F.M., M.B. (Mara Bagardi), V.P., M.C. and V.V.; validation, L.V. and O.D.; formal analysis, L.V. and O.D.; investigation, M.B. (Mara Bagardi), F.M., M.B. (Martina Bini), V.P., M.C. and V.V.; resources, L.V. and O.D.; data curation, M.B (Mara Bagardi).; writing—original draft preparation, M.B. (Mara Bagardi); writing—review and editing, M.B. (Mara Bagardi), L.V., T.V. and O.D.; visualization, L.V.; supervision, O.D.; project administration, L.V. and O.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study have not been published elsewhere but are available on request from the corresponding author.
Acknowledgments
The authors thank their colleagues Alessia Orusa, Monica Cinti, Cristina Ubaldini and Annalisa Burlone for sending the cases to the two reference centers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Detweiler, D.K.; Patterson, D.F.; Hubben, K.; Botts, R.P. The prevalence of spontaneously occurring cardiovascular disease in dogs. Am. J. Public Health Nations Health 1961, 51, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Detweiler, D.K.; Patterson, D.F. The prevalence and types of cardiovascular disease in dogs. Ann. N. Y. Acad. Sci. 1965, 127, 481–516. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.; Glaus, T.M. Congenital cardiac diseases in dogs: A retrospective analysis. Schweiz. Arch. Tierheilkd. 2003, 145, 527–536. [Google Scholar] [CrossRef]
- Buchanan, J.W. Prevalence of cardiovascular disorders. In Textbook of Canine and Feline Cardiology, 2nd ed.; Fox, P.R., Sisson, D.D., Moise, N.S., Eds.; WB Saunders Company: Philadelphia, PA, USA, 1999; pp. 458–463. [Google Scholar]
- Hunt, G.B.; Church, D.B.; Malik, R.; Bellenger, C.R. A retrospective analysis of congenital cardiac anomalies (1977–1989). Aust. Vet. Pract. 1995, 20, 70–75. [Google Scholar]
- MacDonald, K.A. Congenital heart diseases of puppies and kittens. Vet. Clin. Small Anim. Pract. 2006, 36, 503–531. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Domenech, O.; Silva, J.; Vannini, S.; Bussadori, R.; Bussadori, C. Retrospective review of congenital heart disease in 976 Dogs. J. Vet. Intern. Med. 2011, 25, 477–483. [Google Scholar] [CrossRef]
- Tidholm, A. Retrospective study of congenital heart defects in 151 dogs. J. Small Anim. Pract. 1997, 38, 94–98. [Google Scholar] [CrossRef]
- Ghirlanda, S.; Acerbi, A.; Herzog, H.; Serpell, J.A. Fashion vs. function in cultural evolution: The case of dog breed popularity. PLoS ONE 2013, 9, e74770. [Google Scholar] [CrossRef]
- Ghirlanda, S.; Acerbi, A.; Herzog, H. Dog movie stars and dog breed popularity: A case study in media influence on choice. PLoS ONE 2014, 9, e106565. [Google Scholar] [CrossRef]
- Herzog, H. Forty-two thousand and one Dalmatians: Fads, social contagion, and dog breed popularity. Soc. Anim. 2006, 14, 383–397. [Google Scholar] [CrossRef]
- Bellumori, T.P.; Famula, T.R.; Bannasch, D.L.; Belanger, J.M.; Oberbauer, A.M. Prevalence of inherited disorders among mixed-breed and purebred dogs: 27,254 cases (1995–2010). J. Am. Vet. Med. Assoc. 2013, 242, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, P.G.; Polli, M.; Pradelli, D.; Papa, M.; Rizzi, R.; Bagardi, M.; Bussadori, C. Epidemiological study of congenital heart diseases in dogs: Prevalence, popularity, and volatility throughout twenty years of clinical practice. PLoS ONE 2020, 15, e0230160. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.W. Patent ductus arteriosus. Semin. Vet. Med. Surg. 1994, 9, 68–176. [Google Scholar]
- Eyster, G.E.; Eyster, J.T.; Cords, G.B.; Johnston, J. Patent ductus arteriosus in the dog: Characteristics of occurrence and results of surgery in one hundred consecutive cases. J. Am. Vet. Med. Assoc. 1976, 168, 435–438. [Google Scholar] [PubMed]
- Galie, N.; Manes, A.; Palazzini, M.; Negro, L.; Marinelli, A.; Gambetti, S.; Mariucci, E.; Donti, A.; Branzi, A.; Picchio, F.M. Management of Pulmonary Arterial Hypertension Associated with Congenital Systemic-to-Pulmonary Shunts and Eisenmengers Syndrome. Drugs 2008, 68, 1049–1066. [Google Scholar] [CrossRef] [PubMed]
- Achen, S.E.; Miller, M.W.; Gordon, S.G.; Saunders, A.B.; Roland, R.M.; Drourr, L.T. Transarterial ductal occlusion with the Amplatzer vascular plug in 31 dogs. J. Vet. Intern. Med. 2008, 22, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.F.; Green, F.W.; Sanders, R.A. Transcatheter closure of patent ductus arteriosus in a dog with a peripheral vascular occlusion device. J. Vet. Cardiol. 2006, 8, 139–143. [Google Scholar] [CrossRef]
- Nguyenba, T.P.; Tobias, A.H. The Amplatz canine duct occluder: A novel device for patent ductus arteriosus occlusion. J. Vet. Cardiol. 2007, 9, 109–117. [Google Scholar] [CrossRef]
- Smith, P.J.; Martin, M.W.S. Transcatheter embolisation of patent ductus arteriosus using an Amplatzer vascular plug in six dogs. Small Anim. Pract. 2007, 48, 80–86. [Google Scholar] [CrossRef]
- Singh, M.K.; Kittleson, M.D.; Kass, P.H.; Griffiths, L.G. Occlusion devices and approaches in canine patent ductus arteriosus: Comparison of outcomes. J. Vet. Intern. Med. 2012, 26, 85–92. [Google Scholar] [CrossRef]
- Hildebrandt, N.; Stosic, A.; Henrich, E.; Wiedemann, N.; Wurtinger, G.; Schneider, M. Transvenous embolization of moderate to large patent ductus arteriosus in dogs using the Amplatzer vascular plug II. J. Vet. Intern. Med. 2022, 36, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Belachsen, O.; Sargent, J.; Koffas, H.; Schneider, M.; Wagner, T. The use of Amplatzer vascular plug II in 32 consecutive dogs for transvenous occlusion of patent ductus arteriosus. J. Vet. Card. 2022, 41, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Seldinger, S.I. Catheter replacement of the needle in percutaneous arteriography: A new technique. Acta Radiol. 1953, 39, 368e76. [Google Scholar] [CrossRef] [PubMed]
- Lilbert, J.; Mowat, V. Common Vascular Changes in the Jugular Vein of Saline Controls in Continuous Infusion Studies in the Beagle Dog. Toxicol. Pathol. 2004, 32, 694–700. [Google Scholar] [CrossRef]
- Schrope, D.P. Balloon valvuloplasty of valvular pulmonic stenosis in the dog. Clin. Tech. Small Anim. Pract. 2005, 20, 182–195. [Google Scholar] [CrossRef]
- Hoetama, E.; Prakoso, R.; Roebiono, P.S.; Sakidjan, I.; Kurniawati, Y.; Siagian, S.N.; Lelya, O.; Rahajoe, A.U.; Harimurti, G.M.; Lilyasari, O. Balloon pulmonary valvuloplasty in neonates with critical pulmonary stenosis: Jugular or femoral. Ann. Pediatr. Cardiol. 2020, 13, 11–15. [Google Scholar] [CrossRef]
- Medical Practice Committee and Council of the British Cardiac Society. Choice of route for insertion of temporary pacing wires: Recommendations of the Medical Practice Committee and Council of the British Cardiac Society. Br. Heart J. 1993, 70, 592. [Google Scholar]
- Ergül, Y.; Özgür, S.; Şahin, G.T.; Kafalı, H.; Çelebi, S.B.; Bay, B.; Güzeltaş, A. The Transjugular Approach: An Alternative Route to Improve Ablation Success in Right Anteriorly and Anterolaterally-Located Supraventricular Tachycardia Substrates in Children. Pediatric Cardiol. 2019, 40, 477–482. [Google Scholar] [CrossRef]
- Gillinov, A.M.; Lee, A.W.; Redmond, J.M.; Zehr, K.J.; Jackson, L.; Davis, E.A.; Hruban, R.H.; Williams, G.M.; Cameron, D.E. Absorbable suture improves growth of venous anastomoses. J. Vas. Surg. 1992, 16, 769–773. [Google Scholar] [CrossRef]
- Saunders, A.B.; Gordon, S.G.; Boggess, M.M.; Miller, M.W. Long-Term Outcome in Dogs with Patent Ductus Arteriosus: 520 Cases (1994–2009). J. Vet. Intern. Med. 2014, 28, 401–410. [Google Scholar] [CrossRef]
- Campbell, F.E.; Thomas, W.P.; Miller, S.J.; Berger, D.; Kittleson, M.D. Immediate and late outcomes of transarterial coil occlusion of patent ductus arteriosus in dogs. J. Vet. Intern. Med. 2006, 20, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.G.; Saunders, A.B.; Achen, S.E.; Roland, R.M.; Drourr, L.T.; Hariu, C.; Miller, M.W. Transarterial ductal occlusion using the Amplatz canine duct occluder in 40 dogs. J. Vet. Cardiol. 2010, 12, 85–92. [Google Scholar] [CrossRef]
- Schneider, M.; Hildebrandt, N.; Schweigl, T.; Schneider, I.; Hagel, K.H.; Neu, H. Transvenous embolization of small patent ductus arteriosus with single detachable coils in dogs. J. Vet. Intern. Med. 2001, 15, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Szatmári, V. Chitosan hemostatic dressing for control of hemorrhage from femoral arterial puncture site in dogs. J. Vet. Sci. 2015, 16, 517–523. [Google Scholar] [CrossRef]
- Hijazi, Z.M. New device for percutaneous closure of aortopulmonary collaterals. Catheter. Cardiovasc. Interv. 2004, 63, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Lopera, J.E. The Amplatzer vascular plug: Review of evolution and current applications. Semin. Interv. Radiol. 2015, 32, 356–369. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Tam, M.D.; Zhou, D.; Wang, D.X.; Spain, J. The Amplatzer vascular plug: A review of the device and its clinical applications. Cardiovasc. Interv. Radiol. 2012, 35, 725–740. [Google Scholar] [CrossRef]
- Delaney, J.W.; Fletcher, S.E. Patent ductus arteriosus closure using the amplatzer (R) vascular plug II for all anatomic variants. Catheter. Cardiovasc. Interv. 2013, 81, 820–824. [Google Scholar] [CrossRef]
- Garay, F.J.; Aguirre, D.; Cardenas, L.; Springmuller, D.; Heusser, F. Use of the Amplatzer vascular plug II device to occlude different types of patent ductus arteriosus in pediatric patients. J. Interv. Cardiol. 2015, 28, 198–204. [Google Scholar] [CrossRef]
- Schwartz, M.; Glatz, A.C.; Rome, J.J.; Gillespie, M.J. The amplatzer vascular plug and amplatzer vascular plug II for vascular occlusion procedures in 50 patients with congenital cardiovascular disease. Catheter. Cardiovasc. Interv. 2010, 76, 411–417. [Google Scholar] [CrossRef]
- Choi, R.; Hyun, C. Transjugular occlusion of patent ductus arteriosus using an Amplatz canine ductal occluder in a Cocker spaniel dog. Korean J. Vet. Res. 2010, 50, 49–53. [Google Scholar]
- Lavennes, M.; Chetboul, V.; Passavin, P.; Gouni, V.; Damoiseaux, C.; Poissonnier, C.; Carazo Arias, L.E.; Alvarado, M.P.; Morlet, A.; Chevènement, O.; et al. Successful transcatheter retrieval of embolized Amplatz Canine Duct Occluders in two dogs. J. Vet. Card. 2018, 20, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Blossom, J.E.; Bright, J.M.; Griffiths, L.G. Transvenous occlusion of patent ductus arteriosus in 56 consecutive dogs. J. Vet. Cardiol. 2010, 12, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Henrich, E.; Hildebrandt, N.; Schneider, C.; Hassdenteufel, E.; Schneider, M. Transvenous coil embolization of patent ductus arteriosus in small (≤3.0 kg) dogs. J. Vet. Intern. Med. 2011, 25, 65e70. [Google Scholar] [CrossRef]
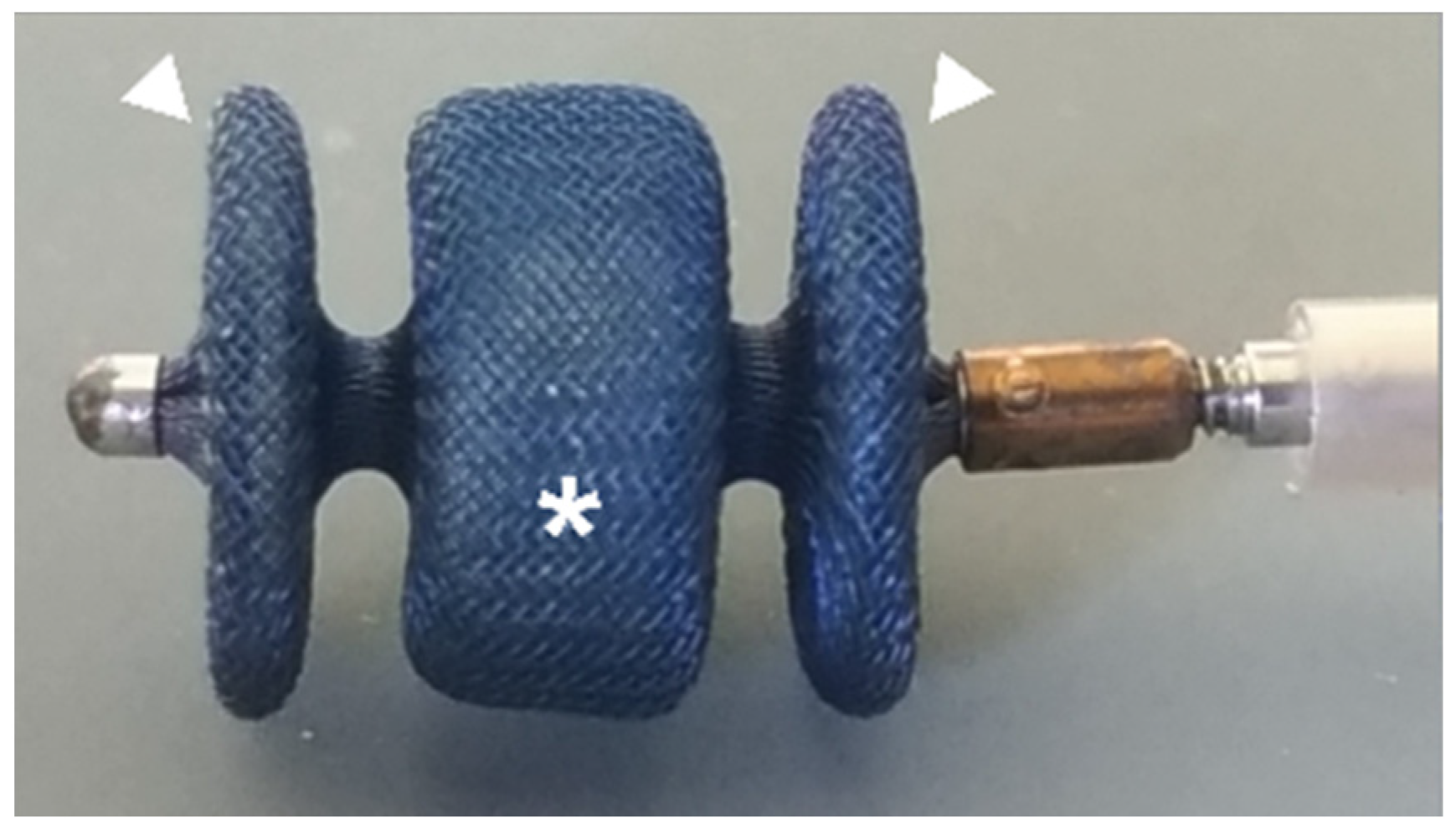
Figure 1.
Amplatzer vascular plug II with two lateral retention discs (arrowhead) and central component (asterisk): self-expanding device made from a nitinol wire mesh. The device is attached by a microscrew to a 135 cm nitinol delivery wire in a hoop dispenser and preloaded in a loader.
Figure 1.
Amplatzer vascular plug II with two lateral retention discs (arrowhead) and central component (asterisk): self-expanding device made from a nitinol wire mesh. The device is attached by a microscrew to a 135 cm nitinol delivery wire in a hoop dispenser and preloaded in a loader.
Figure 2.
Cook Medical Flexor Balkin Guiding Sheath (5.5 Fr) with Check-Flo Valve and hydrophilic coating. On the right, magnification of the 180° curve guiding sheath.
Figure 2.
Cook Medical Flexor Balkin Guiding Sheath (5.5 Fr) with Check-Flo Valve and hydrophilic coating. On the right, magnification of the 180° curve guiding sheath.
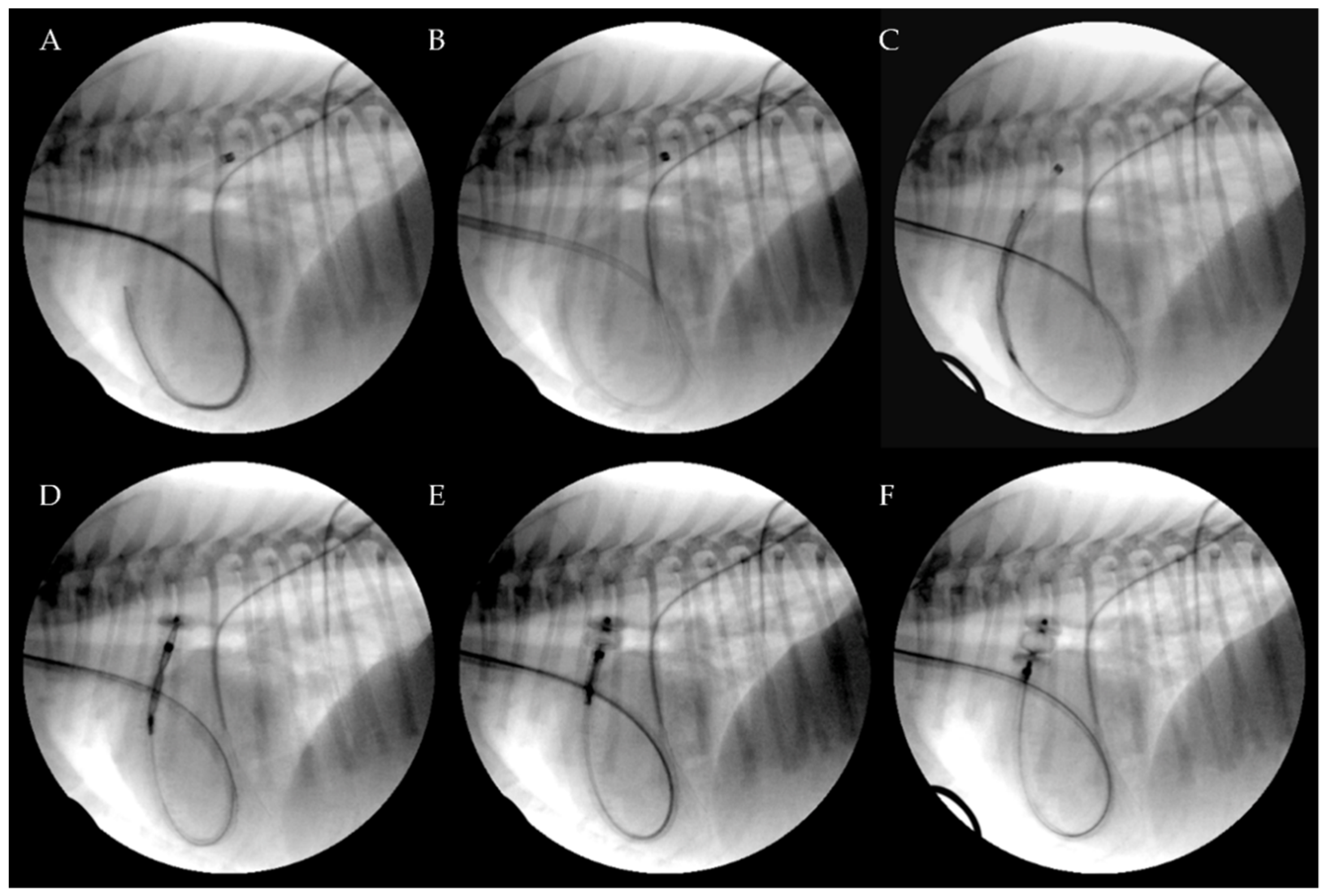
Figure 3.
Transjugular patent ductus arteriosus occlusion using the Amplatzer Vascular Plug II. The PDA was crossed retrograde using a straight soft guidewire advanced into the descending aorta through the right ventricle and the pulmonary artery on a 5 Fr multipurpose catheter. The soft guidewire was replaced by a standard Cook Rosen PTFE curved guidewire used to position the tip of the Flexor Balkin guiding sheath into the aorta and was then removed (A). The Flexor Balkin guiding sheath is in correct position (B). The device was introduced into the Balkin guiding sheath (C). The Amplatzer Vascular Plug II was advanced (C), partially (D) and fully extruded (E) and occluding the PDA (F).
Figure 3.
Transjugular patent ductus arteriosus occlusion using the Amplatzer Vascular Plug II. The PDA was crossed retrograde using a straight soft guidewire advanced into the descending aorta through the right ventricle and the pulmonary artery on a 5 Fr multipurpose catheter. The soft guidewire was replaced by a standard Cook Rosen PTFE curved guidewire used to position the tip of the Flexor Balkin guiding sheath into the aorta and was then removed (A). The Flexor Balkin guiding sheath is in correct position (B). The device was introduced into the Balkin guiding sheath (C). The Amplatzer Vascular Plug II was advanced (C), partially (D) and fully extruded (E) and occluding the PDA (F).
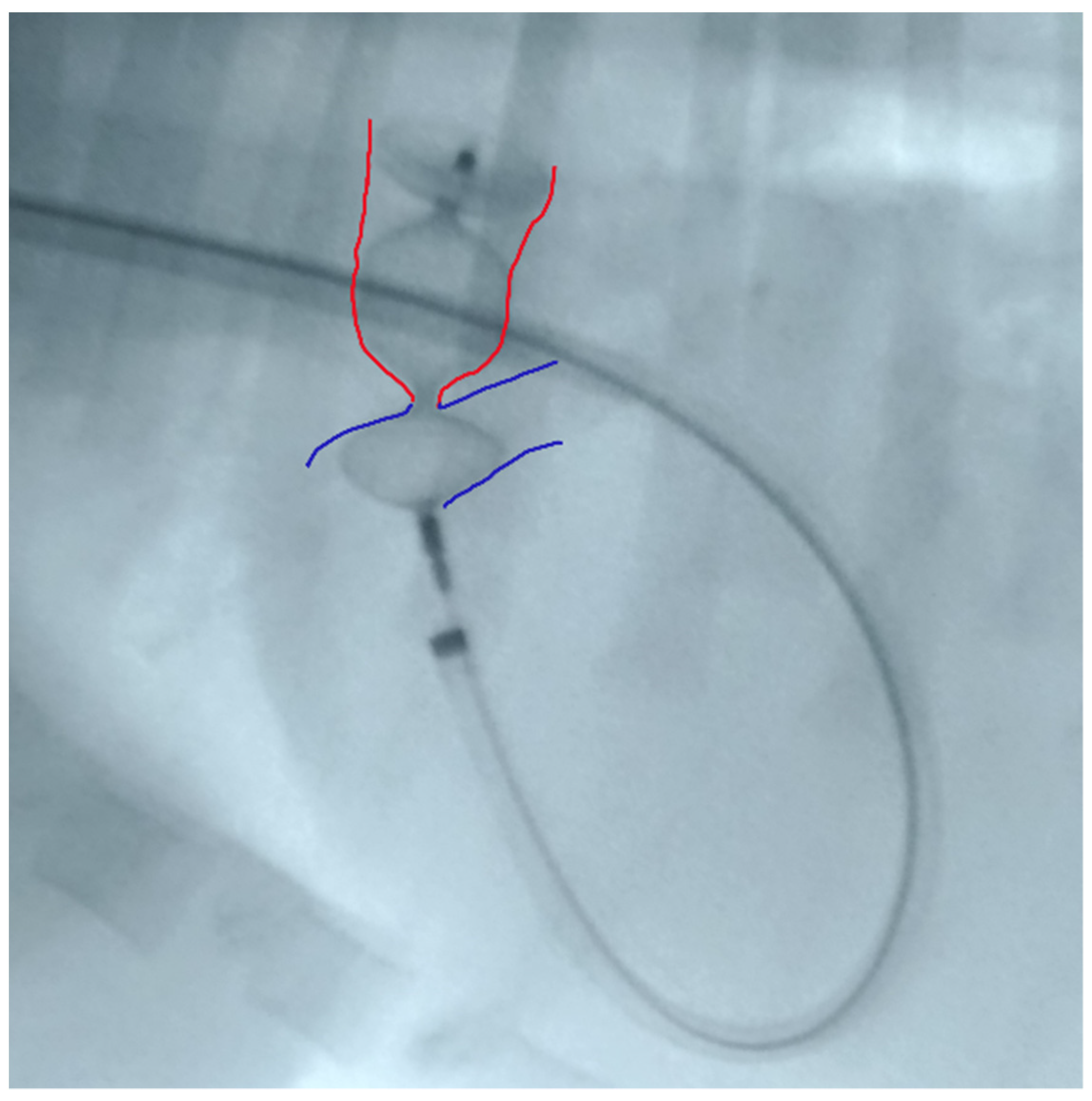
Figure 4.
Device deployed in the main pulmonary artery with slight distension of the proximal disc. Red: ductal ampulla; Blue: pulmonary artery.
Figure 4.
Device deployed in the main pulmonary artery with slight distension of the proximal disc. Red: ductal ampulla; Blue: pulmonary artery.
Figure 5.
Transthoracic echocardiography from left parasternal cranial view during the procedure. Balkin catheter through the PDA (A). Distal disc and central component within the PDA ampulla (B). Distal disc and central component within the PDA ampulla and proximal disc within the PA just before the device deployment (C). Device released within the PDA structure (D). Device within the PDA with no residual flow (E). Ao: aorta; PA: pulmonary artery; MPA: main pulmonary artery; RPA: right pulmonary artery; V: probe marker.
Figure 5.
Transthoracic echocardiography from left parasternal cranial view during the procedure. Balkin catheter through the PDA (A). Distal disc and central component within the PDA ampulla (B). Distal disc and central component within the PDA ampulla and proximal disc within the PA just before the device deployment (C). Device released within the PDA structure (D). Device within the PDA with no residual flow (E). Ao: aorta; PA: pulmonary artery; MPA: main pulmonary artery; RPA: right pulmonary artery; V: probe marker.
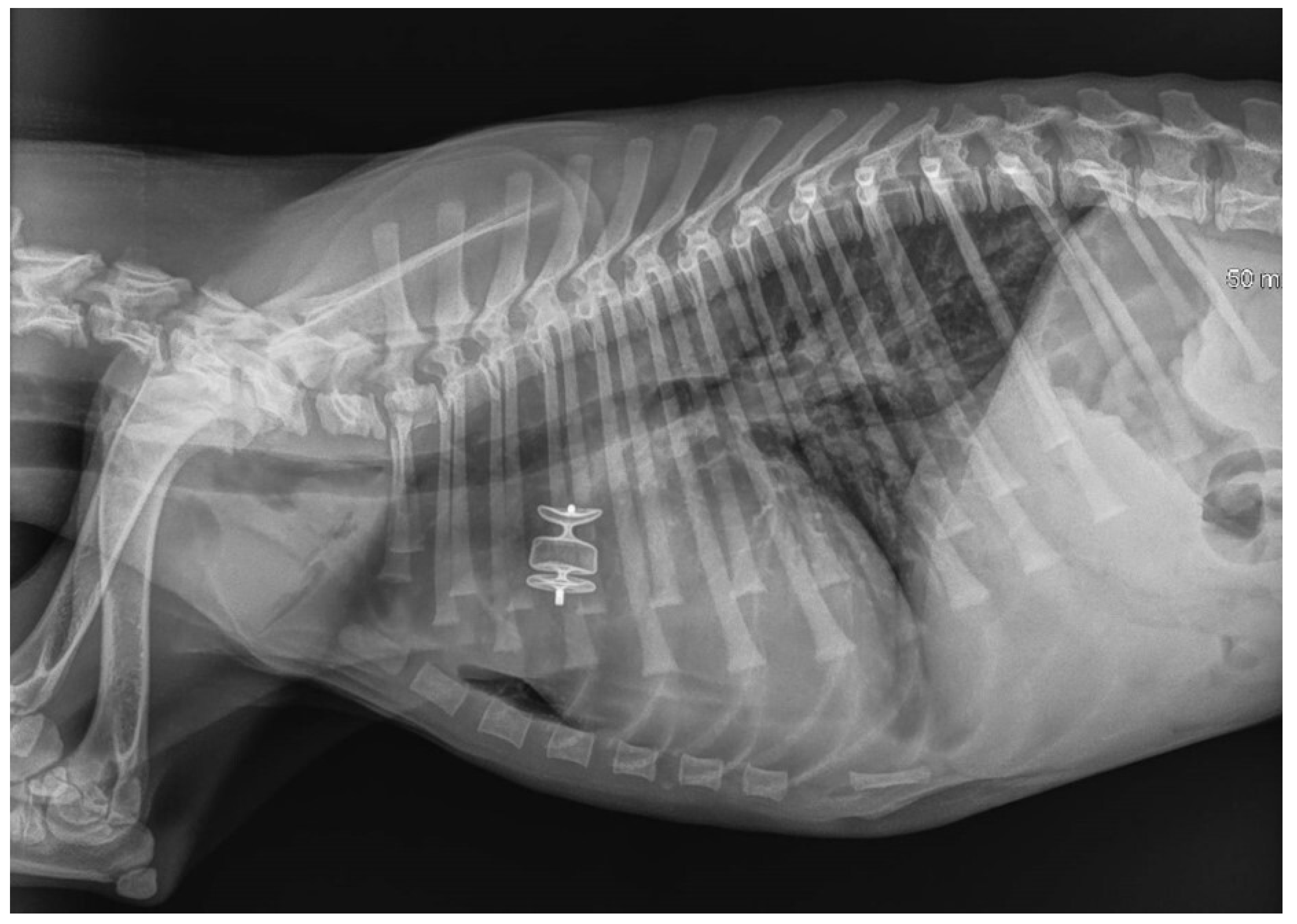
Figure 6.
Immediate post-procedure right lateral thoracic radiograph (Case 3). The dog had pulmonary edema before the procedure, which was managed with medical treatment. The device is readily visible in situ. Mild pulmonary infiltrate was still observed in the lung field, especially the caudal lung lobes, after the procedure.
Figure 6.
Immediate post-procedure right lateral thoracic radiograph (Case 3). The dog had pulmonary edema before the procedure, which was managed with medical treatment. The device is readily visible in situ. Mild pulmonary infiltrate was still observed in the lung field, especially the caudal lung lobes, after the procedure.
Figure 7.
Doppler flow evaluation with linear probe (12 Hz) of the up (A) and down (B) jugular veins three months after the procedure (Case 5) demonstrating normal size of the right jugular vein compared to the left one, and normal flow within the vessel.
Figure 7.
Doppler flow evaluation with linear probe (12 Hz) of the up (A) and down (B) jugular veins three months after the procedure (Case 5) demonstrating normal size of the right jugular vein compared to the left one, and normal flow within the vessel.
Figure 8.
Pre-procedure (A,B) and post-procedure (C,D) left lateral and dorso-ventral thoracic radiographs of Case 1. L: left; R: right.
Figure 8.
Pre-procedure (A,B) and post-procedure (C,D) left lateral and dorso-ventral thoracic radiographs of Case 1. L: left; R: right.
Figure 9.
Pre-procedure (A,B) and post-procedure (C,D) left lateral and dorso-ventral thoracic radiographs of Case 2. L: left; R: right.
Figure 9.
Pre-procedure (A,B) and post-procedure (C,D) left lateral and dorso-ventral thoracic radiographs of Case 2. L: left; R: right.
Figure 10.
Pre- (A) and post-procedure (B) right lateral thoracic radiographs of Case 4.
Figure 10.
Pre- (A) and post-procedure (B) right lateral thoracic radiographs of Case 4.
Figure 11.
Pre-procedure (A,B) and post-procedure (C,D) right lateral and dorso-ventral thoracic radiographs of Case 6.
Figure 11.
Pre-procedure (A,B) and post-procedure (C,D) right lateral and dorso-ventral thoracic radiographs of Case 6.
Figure 12.
Pre-procedure (A,B) and post-procedure (C,D) right and left lateral and dorso-ventral thoracic radiographs of Case 7. L: left; R: right.
Figure 12.
Pre-procedure (A,B) and post-procedure (C,D) right and left lateral and dorso-ventral thoracic radiographs of Case 7. L: left; R: right.
Table 1.
Clinical data, minimum ductal ostium and ampulla diameters, dimension of Cook Flexor Balkin Guiding Sheath catheters at delivery, and Amplatzer Vascular Plug II order numbers and sizes (pre-implanted device length, minimum internal diameter required and maximum length) for each patient.
Table 1.
Clinical data, minimum ductal ostium and ampulla diameters, dimension of Cook Flexor Balkin Guiding Sheath catheters at delivery, and Amplatzer Vascular Plug II order numbers and sizes (pre-implanted device length, minimum internal diameter required and maximum length) for each patient.
| Case | Breed | Sex | Age | Weight (kg) | PDA Ostium (mm) | PDA
Ampulla (mm) | Cook Flexor Balkin Guiding Sheath (Fr) | AMPLATZER Vascular Plug II Order Number | AMPLATZER Vascular Plug II Diameter (mm) | Pre-Implanted Device Length (mm) | Minimum Internal Diameter Required
(inches) | Maximum Length (cm) |
|---|
| Case 1 | Poodle | M | 4 months | 2.8 | 2 | 6 | 6 | 9-AVP2-010 | 10 | 7 | 0.070 | 100 |
| Case 2 | Poodle | F | 4 months | 2.9 | 3 | 7 | 6 | 9-AVP2-010 | 10 | 7 | 0.070 | 100 |
| Case 3 | Border collie | F | 3 months | 2.9 | 2.7 | 10 | 6 | 9-AVP2-012 | 12 | 9 | 0.070 | 100 |
| Case 4 | Maltese | M | 6 months | 2.9 | 2.4 | 6.6 | 5.5 | 9-AVP2-010 | 10 | 7 | 0.070 | 100 |
| Case 5 | American Staffordshire Terrier | F | 3 months | 8.5 | 2.8 | 8.6 | 6 | 9-AVP2-012 | 12 | 9 | 0.070 | 100 |
| Case 6 | Poodle | F | 5 years | 7.3 | 2.9 | 9.1 | 6 | 9-AVP2-012 | 12 | 9 | 0.070 | 100 |
| Case 7 | Poodle | F | 1 year | 4.6 | 3.4 | 11.2 | 7 | 9-AVP2-014 | 14 | 10 | 0.086 | 100 |
Table 2.
Echocardiographic measurements before transvenous Amplatzer Vascular Plug II occlusion (T0) and at 24 h (T1), one week (T2), and three-month check-ups (T3).
Table 2.
Echocardiographic measurements before transvenous Amplatzer Vascular Plug II occlusion (T0) and at 24 h (T1), one week (T2), and three-month check-ups (T3).
| Case | Timing | LA/Ao | LVIDDn |
|---|
| Case 1 | T0 | 1.55 | 2.02 |
| T1 | 1.41 | 1.80 |
| T2 | 1.30 | 1.60 |
| T3 | 1.20 | 1.47 |
| Case 2 | T0 | 1.68 | 2.16 |
| T1 | 1.32 | 1.48 |
| T2 | 1.32 | 1.37 |
| T3 | 1.30 | 1.30 |
| Case 3 | T0 | 1.85 | 2.96 |
| T1 | 1.41 | 2.16 |
| T2 | 1.10 | 1.56 |
| T3 | 1.10 | 1.64 |
| Case 4 | T0 | 1.39 | 2.52 |
| T1 | 1.26 | 1.84 |
| T2 | 1.24 | 1.75 |
| T3 | 1.21 | 1.60 |
| Case 5 | T0 | 1.22 | 1.84 |
| T1 | 1.23 | 1.68 |
| T2 | 1.19 | 1.69 |
| T3 | 1.20 | 1.69 |
| Case 6 | T0 | 1.33 | 2.46 |
| T1 | 1.18 | 1.73 |
| T2 | 1.10 | 1.65 |
| T3 | 1.10 | 1.52 |
| Case 7 | T0 | 1.90 | 2.20 |
| T1 | 1.55 | 1.90 |
| T2 | 1.39 | 1.50 |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).