Relationship between Serum Protein Electrophoresis, Endoscopic and Histopathological Scores in 99 Cats with Chronic Enteropathy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
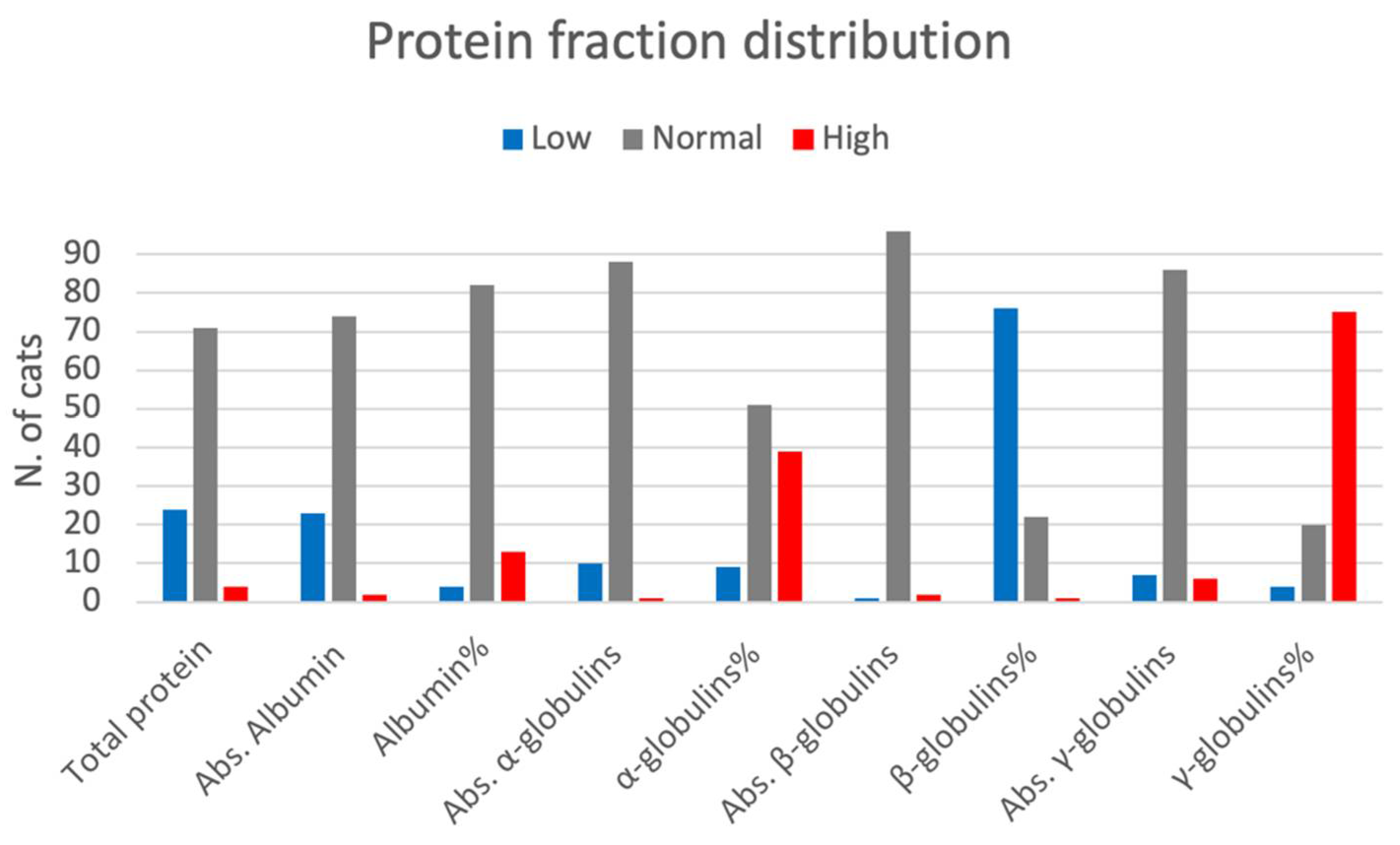
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richter, K.P. Feline Gastrointestinal Lymphoma. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 1083–1098. [Google Scholar] [CrossRef]
- Louwerens, M.; London, C.A.; Pedersen, N.C.; Lyons, L.A. Feline Lymphoma in the Post—Feline Leukemia Virus Era. J. Vet. Intern. Med. 2005, 19, 329–335. [Google Scholar] [CrossRef]
- Marsilio, S. Feline Chronic Enteropathy. J. Small Anim. Pract. 2021, 62, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Moore, F.M.; Haynes, J.S.; Miles, K.G. Idiopathic Inflammatory Bowel Disease in Dogs and Cats: 84 Cases (1987–1990). J. Am. Vet. Med. Assoc. 1992, 201, 1603–1608. [Google Scholar]
- Hart, J.R.; Shaker, E.; Patnaik, A.K.; Garvey, M.S. Lymphocytic-plasmacytic enterocolitis in cats: 60 cases (1988–1990). J. Am. An. Hosp. Assoc. 1994, 30, 505–514. [Google Scholar]
- Dennis, J.S.; Kruger, J.M.; Mullaney, T.P. Lymphocytic/plasmacytic gastroenteritis in cats: 14 cases (1985–1990). J. Am. Vet. Med. Assoc. 1992, 200, 1712–1718. [Google Scholar] [PubMed]
- Mahony, O.M.; Moore, A.S.; Cotter, S.M.; Engler, S.J.; Brown, D.; Penninck, D.G. Alimentary Lymphoma in Cats: 28 Cases (1988–1993). J. Am. Vet. Med. Assoc. 1995, 207, 1593–1598. [Google Scholar]
- Fondacaro, J.; Richter, K.; Carpenter, J.; Hart, J.R.; Hill, S.L.; Fettman, M.J. Feline gastrointestinal lymphoma: 67 cases (1988–1966). Eur. J. Comp. Gastr. 1999, 4, 5–11. [Google Scholar]
- Baez, J.L.; Hendrick, M.J.; Walker, L.M.; Washabau, R.J. Radiographic, Ultrasonographic, and Endoscopic Findings in Cats with Inflammatory Bowel Disease of the Stomach and Small Intestine: 33 Cases (1990–1997). J. Am. Vet. Med. Assoc. 1999, 215, 349–354. [Google Scholar]
- Burke, K.F.; Broussard, J.D.; Ruaux, C.G.; Suchodolski, J.S.; Williams, D.A.; Steiner, J.M. Evaluation of Fecal A1-Proteinase Inhibitor Concentrations in Cats with Idiopathic Inflammatory Bowel Disease and Cats with Gastrointestinal Neoplasia. Vet. J. Lond. Engl. 2012, 196, 189–196. [Google Scholar] [CrossRef]
- Jergens, A.E.; Crandell, J.M.; Evans, R.; Ackermann, M.; Miles, K.G.; Wang, C. A Clinical Index for Disease Activity in Cats with Chronic Enteropathy. J. Vet. Intern. Med. 2010, 24, 1027–1033. [Google Scholar] [CrossRef]
- McGrotty, Y.; Bell, R.; McLauchlan, G. Disorders of plasma protein. In BSAVA Manual of Canine and Feline Clinical Pathology, 3rd ed.; Villiers, E., Ristic, J.R., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2016; pp. 123–141. [Google Scholar]
- Vavricka, S.R.; Burri, E.; Beglinger, C.; Degen, L.; Manz, M. Serum Protein Electrophoresis: An Underused but Very Useful Test. Digestion 2009, 79, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Matar, M.; Rinawi, F.; Shamir, R.; Assa, A. Hypergammaglobulinemia Is a Marker of Extraintestinal Manifestations in Pediatric Inflammatory Bowel Disease. Turk. J. Gastroenterol. 2017, 28, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Pagnini, C.; Menasci, F.; Desideri, F.; Sanna, A.; Corleto, V.D.; Fave, G.D. Searching for Biomarkers in Clinical Practice: The Prevalence and Clinical Significance of Hypergammaglobulinemia in Inflammatory Bowel Disease Patients. J. Gastrointest. Liver Dis. JGLD 2016, 25, 565–566. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, K.W. Pancreatitis and Triaditis in Cats: Causes and Treatment. J. Small Anim. Pract. 2015, 56, 40–49. [Google Scholar] [CrossRef]
- Bailey, S.; Benigni, L.; Eastwood, J.; Garden, O.A.; McMahon, L.; Smith, K.; Steiner, J.M.; Suchodolski, J.S.; Allenspach, K. Comparisons between Cats with Normal and Increased FPLI Concentrations in Cats Diagnosed with Inflammatory Bowel Disease. J. Small Anim. Pract. 2010, 51, 484–489. [Google Scholar] [CrossRef]
- Steiner, J.M. Diagnosis of Pancreatitis. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 1181–1195. [Google Scholar] [CrossRef]
- Boland, L.; Beatty, J. Feline Cholangitis. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 703–724. [Google Scholar] [CrossRef]
- Group, T.W.I.G.S.; Washabau, R.J.; Day, M.J.; Willard, M.D.; Hall, E.J.; Jergens, A.E.; Mansell, J.; Minami, T.; Bilzer, T.W. Endoscopic, Biopsy, and Histopathologic Guidelines for the Evaluation of Gastrointestinal Inflammation in Companion Animals. J. Vet. Intern. Med. 2010, 24, 10–26. [Google Scholar] [CrossRef]
- Day, M.J.; Bilzer, T.; Mansell, J.; Wilcock, B.; Hall, E.J.; Jergens, A.; Minami, T.; Willard, M.; Washabau, R.; World Small Animal Veterinary Association Gastrointestinal Standardization Group. Histopathological Standards for the Diagnosis of Gastrointestinal Inflammation in Endoscopic Biopsy Samples from the Dog and Cat: A Report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 2008, 138, S1–S43. [Google Scholar] [CrossRef] [PubMed]
- Kiupel, M.; Smedley, R.C.; Pfent, C.; Xie, Y.; Xue, Y.; Wise, A.G.; DeVaul, J.M.; Maes, R.K. Diagnostic Algorithm to Differentiate Lymphoma from Inflammation in Feline Small Intestinal Biopsy Samples. Vet. Pathol. 2011, 48, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.; Hill, S.L.; Richter, K.P.; Marsilio, S.; Ackermann, M.R.; Lidbury, J.A.; Suchodolski, J.S.; Cocker, S.; Steiner, J.M. Comprehensive Comparison of Upper and Lower Endoscopic Small Intestinal Biopsy in Cats with Chronic Enteropathy. J. Vet. Intern. Med. 2021, 35, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Gaschen, L. Ultrasonography of Small Intestinal Inflammatory and Neoplastic Diseases in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S. Feline Abdominal Ultrasonography: What’s Normal? What’s Abnormal? the Liver. J. Feline Med. Surg. 2019, 21, 12–24. [Google Scholar] [CrossRef]
- Zini, E.; Hafner, M.; Kook, P.; Lutz, T.A.; Ohlerth, S.; Reusch, C.E. Longitudinal Evaluation of Serum Pancreatic Enzymes and Ultrasonographic Findings in Diabetic Cats Without Clinically Relevant Pancreatitis at Diagnosis. J. Vet. Intern. Med. 2015, 29, 589–596. [Google Scholar] [CrossRef]
- Gerou-Ferriani, M.; McBrearty, A.R.; Burchmore, R.J.; Jayawardena, K.G.I.; Eckersall, P.D.; Morris, J.S. Agarose Gel Serum Protein Electrophoresis in Cats with and without Lymphoma and Preliminary Results of Tandem Mass Fingerprinting Analysis. Vet. Clin. Pathol. 2011, 40, 159–173. [Google Scholar] [CrossRef]
- Moore, A.R.; Avery, P.R. Protein Characterization Using Electrophoresis and Immunofixation; a Case-based Review of Dogs and Cats. Vet. Clin. Pathol. 2019, 48, 29–44. [Google Scholar] [CrossRef]
- Tothova, C.; Nagy, O.; Kovac, G. Serum Proteins and Their Diagnostic Utility in Veterinary Medicine: A Review. Veterinární Med. 2016, 61, 475–496. [Google Scholar] [CrossRef]
- Taylor, S.S.; Tappin, S.W.; Dodkin, S.J.; Papasouliotis, K.; Casamian-Sorrosal, D.; Tasker, S. Serum Protein Electrophoresis in 155 Cats. J. Feline Med. Surg. 2010, 12, 643–653. [Google Scholar] [CrossRef]
- Kaneko, J.J. Serum Proteins and the Dysproteinemias. In Clinical Biochemistry of Domestic Animals, 5th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Allenspach, K. Diagnosis of Small Intestinal Disorders in Dogs and Cats. Clin. Lab. Med. 2015, 35, 521–534. [Google Scholar] [CrossRef]
- Terragni, R.; Morselli-Labate, A.M.; Vignoli, M.; Bottero, E.; Brunetti, B.; Saunders, J.H. Is Serum Total LDH Evaluation Able to Differentiate between Alimentary Lymphoma and Inflammatory Bowel Disease in a Real World Clinical Setting? PLoS ONE 2016, 11, e0151641. [Google Scholar] [CrossRef]
- Forman, M.A.; Marks, S.L.; Cock, H.E.V.; Hergesell, E.J.; Wisner, E.R.; Baker, T.W.; Kass, P.H.; Steiner, J.M.; Williams, D.A. Evaluation of Serum Feline Pancreatic Lipase Immunoreactivity and Helical Computed Tomography versus Conventional Testing for the Diagnosis of Feline Pancreatitis. J. Vet. Intern. Med. 2004, 18, 807–815. [Google Scholar] [CrossRef]
- Cerón, J.J.; Eckersall, P.D.; Martínez-Subiela, S. Acute Phase Proteins in Dogs and Cats: Current Knowledge and Future Perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef]
- Yuki, M.; Aoyama, R.; Nakagawa, M.; Hirano, T.; Naitoh, E.; Kainuma, D. A Clinical Investigation on Serum Amyloid a Concentration in Client-Owned Healthy and Diseased Cats in a Primary Care Animal Hospital. Vet. Sci. 2020, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, P.J. Metabolic Diseases of the Liver In Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat, 8th ed.; Ettinger, S.J., Feldman, E.C., Cote, E., Eds.; Elsevier: St. Louis, MO, USA, 2017; pp. 4040–4042. [Google Scholar]
- Clark, J.E.C.; Haddad, J.L.; Brown, D.C.; Morgan, M.J.; Winkle, T.J.V.; Rondeau, M.P. Feline Cholangitis: A Necropsy Study of 44 Cats (1986–2008). J. Feline Med. Surg. 2011, 13, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Freiche, V.; Faucher, M.R.; German, A.J. Can Clinical Signs, Clinicopathological Findings and Abdominal Ultrasonography Predict the Site of Histopathological Abnormalities of the Alimentary Tract in Cats? J. Feline Med. Surg. 2016, 18, 118–128. [Google Scholar] [CrossRef] [Green Version]
| Endoscopy | Histopathology | Ultrasonographic Findings | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Endo1 n = 46 | Endo2-3 n = 53 | p | HistoA n = 42 | HistoB n = 57 | p | UEI n = 45 | UI n = 30 | p | |
| Total protein | 6 (4–8.5) | 6 (3.4–10.1) | 0.378 | 6 (3.4–9.9) | 6.1 (4.2–10.1) | 0.202 | 7.1 (4.7–10.1) | 7.2 (5.1–10) | 0.897 |
| Absolute values (mg/dL) | |||||||||
| Albumin | 3 (2.1–3.6) | 3 (1.9–4.6) | 0.444 | 3 (1.9–3.9) | 3.1 (2.2–4.6) | 0.090 | 3.3 (1.6–4.6) | 3.5 (2.3–4.5) | 0.074 |
| α-globulins | 1.2 (0.5–2) | 1.3 (0.4–2.5) | 0.138 | 1.3 (0.5–2) | 1.3 (0.4–2.5) | 0.579 | 1.1 (0.6–2.5) | 1.6 (0.4–2) | 0.009 |
| β-globulins | 0.6 (0.2–2) | 0.6 (0.3–1.7) | 0.420 | 0.6 (0.2–1.7) | 0.7 (0.3–2) | 0.063 | 0.9 (0.3–2) | 0.8 (0.3–1.9) | 0.295 |
| γ-globulins | 1 (0.2–2.2) | 1 (0.3–5.4) | 0.872 | 0.9 (0.2–5.4) | 1 (0.2–2.9) | 0.294 | 1.5 (0.3–5.4) | 1.2 (0.4–2.9) | 0.034 |
| Percentage (%) | |||||||||
| Albumin | 50.5 (34.8–69.1) | 50.2 (26.8–63.1) | 0.861 | 50.3 (26.8–69.1) | 50.3 (34.8–61.6) | 0.599 | 45.9 (26.8–61.9) | 49.5 (37.9–62.1) | 0.047 |
| α-globulins | 20.6 (9.1–31.2) | 21.8 (6.9–33.8) | 0.260 | 21.5 (9.1–31.2) | 20.6 (6.9–33.8) | 0.496 | 17.5 (7.8–29.3) | 21 (6.9–33.8) | 0.02 |
| β-globulins | 10.4 (6–23.7) | 10.1 (4.6–30.6) | 0.154 | 9.4 (4.6–30.6) | 11.5 (5.3–23.7) | 0.182 | 14.2 (5.1–26.1) | 11.4 (4.6–27.4) | 0.100 |
| γ-globulins | 17.4 (5.2–30.1) | 16.4 (4.6–54.9) | 0.869 | 16.1 (5.2–54.9) | 17.4 (4.6–30.1) | 0.414 | 20.4 (6.1–54.9) | 16.8 (5.9-33.8) | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierini, A.; Gori, E.; Tulone, F.; Benvenuti, E.; Bottero, E.; Ruggiero, P.; Marchetti, V. Relationship between Serum Protein Electrophoresis, Endoscopic and Histopathological Scores in 99 Cats with Chronic Enteropathy. Vet. Sci. 2022, 9, 453. https://doi.org/10.3390/vetsci9090453
Pierini A, Gori E, Tulone F, Benvenuti E, Bottero E, Ruggiero P, Marchetti V. Relationship between Serum Protein Electrophoresis, Endoscopic and Histopathological Scores in 99 Cats with Chronic Enteropathy. Veterinary Sciences. 2022; 9(9):453. https://doi.org/10.3390/vetsci9090453
Chicago/Turabian StylePierini, Alessio, Eleonora Gori, Fiorenza Tulone, Elena Benvenuti, Enrico Bottero, Pietro Ruggiero, and Veronica Marchetti. 2022. "Relationship between Serum Protein Electrophoresis, Endoscopic and Histopathological Scores in 99 Cats with Chronic Enteropathy" Veterinary Sciences 9, no. 9: 453. https://doi.org/10.3390/vetsci9090453







