Regulatory Role of Vacuolar Calcium Transport Proteins in Growth, Calcium Signaling, and Cellulase Production in Trichoderma reesei
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Vector Construction for Gene Deletion
2.3. Transformation of T. reesei
2.4. Enzyme Activity
2.5. RNA Extraction and Transcript Analysis Using Quantitative PCR (RT-qPCR)
2.6. Confocal Microscopy
2.7. Bioinformatics and Statistical Analysis
3. Results
3.1. Identification of Putative Homologues in T. reesei: Three for PMC1, Five for YVC1, and Seven for VCX1 of S. cerevisiae
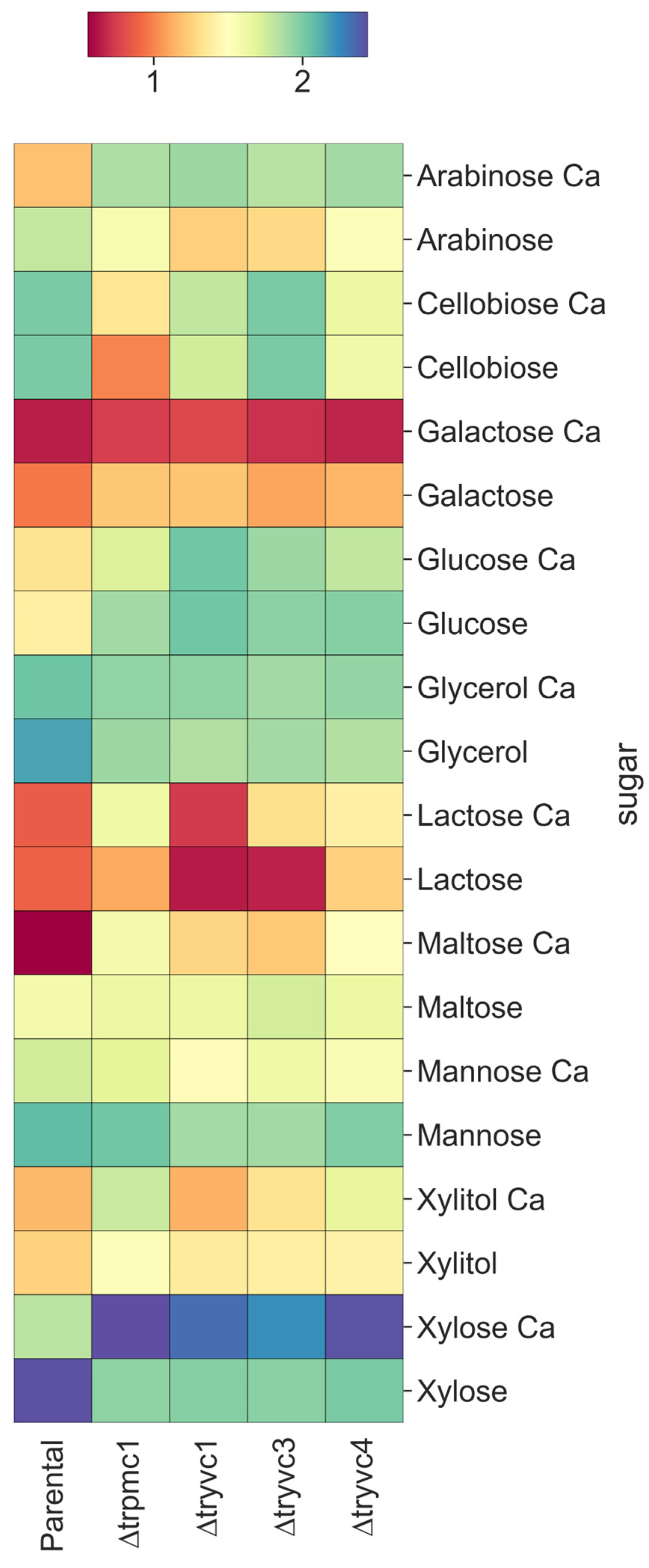
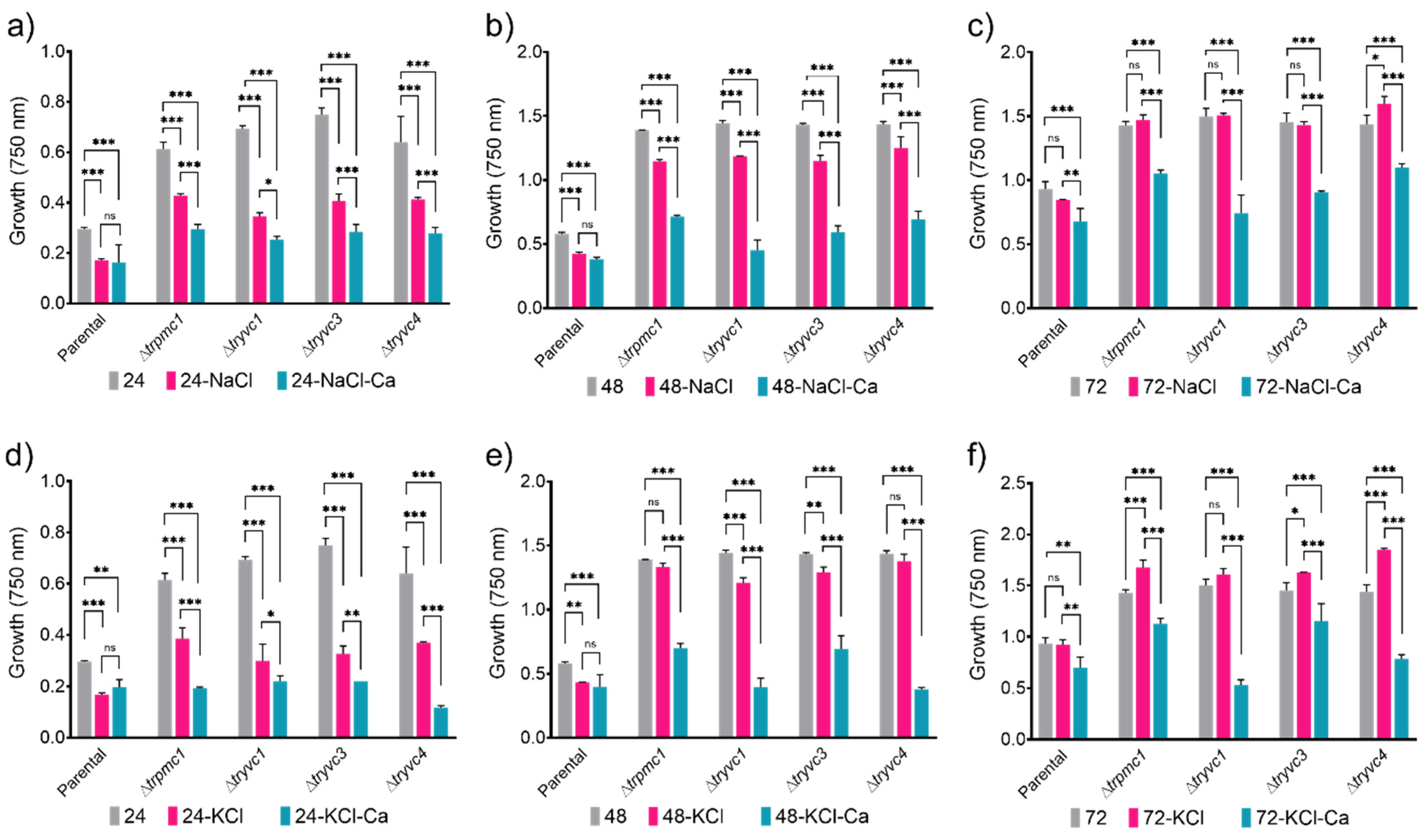
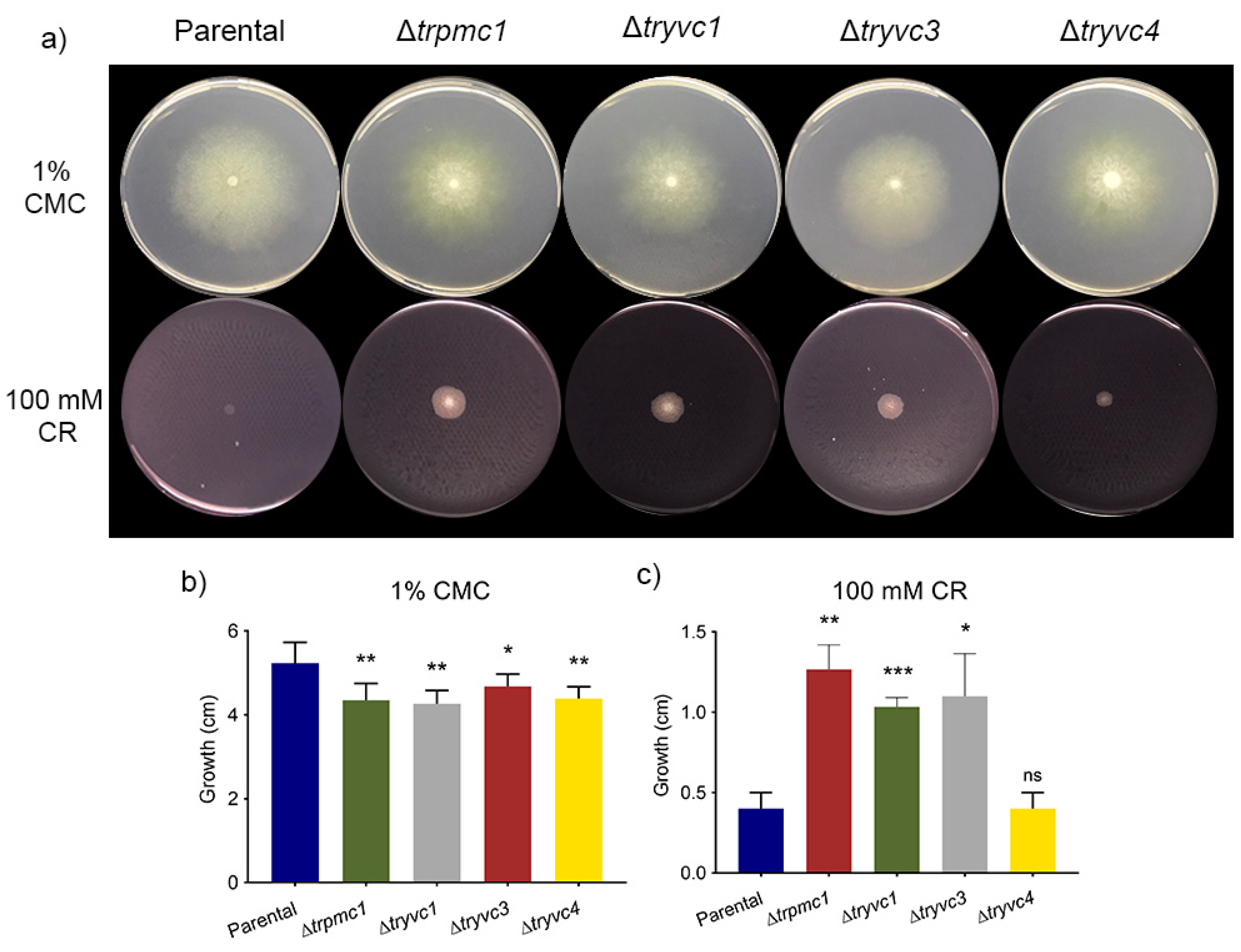
3.2. The Vacuolar Calcium Transport Proteins Are Involved in Sugars Assimilation, Manganese and Osmotic Stress Responses, Cellulose Deconstruction, and Cell Wall Stress Resistance
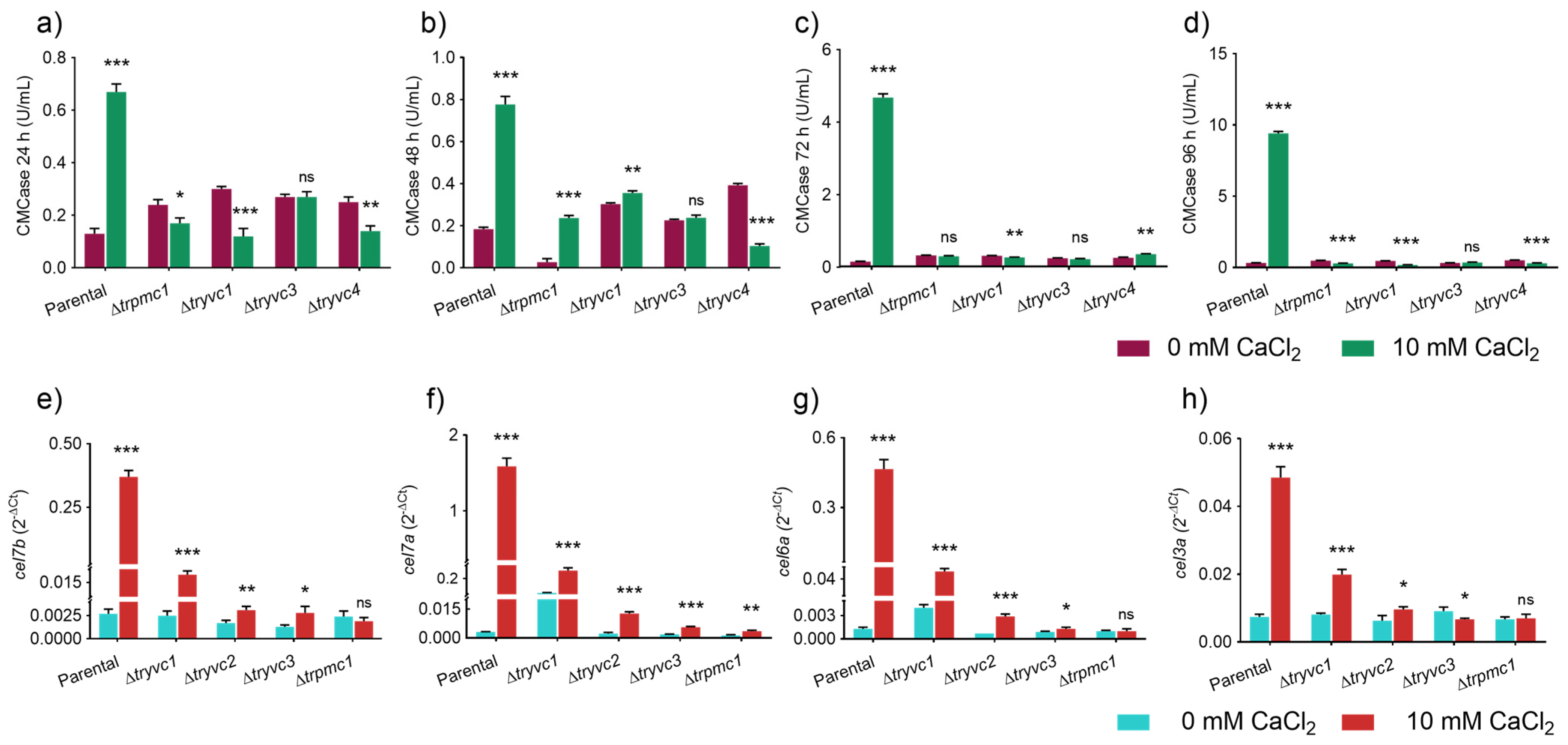
3.3. TrPMC1, TrYVC1, TrYVC3, and TrYVC4 Are Key Factors in Cellulases Production Under Calcium Supplementation
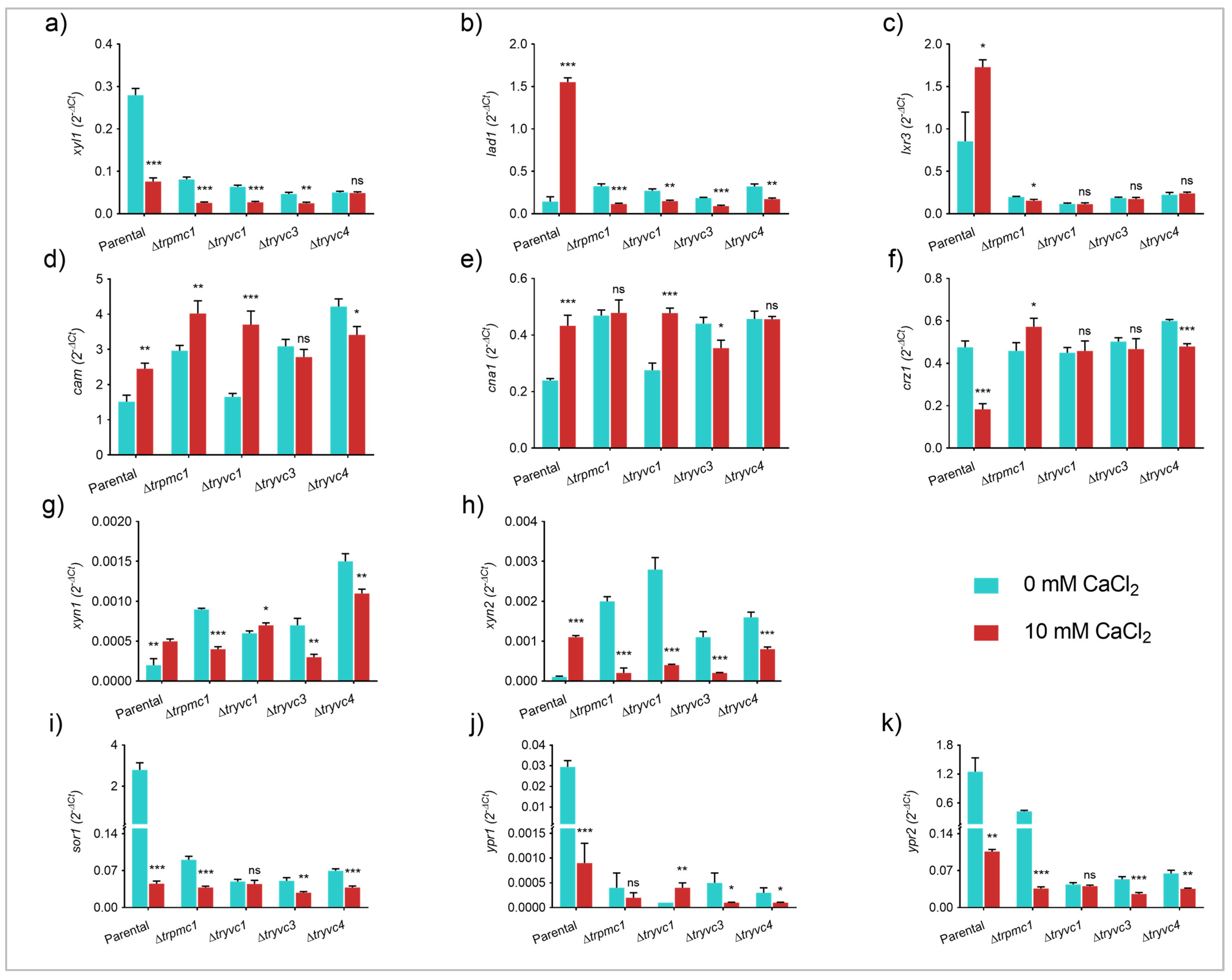
3.4. The Production of Xylanases and Secondary Metabolites Are Impacted by the Vacuolar Calcium Transport Proteins in T. reesei in the Presence of Xylose
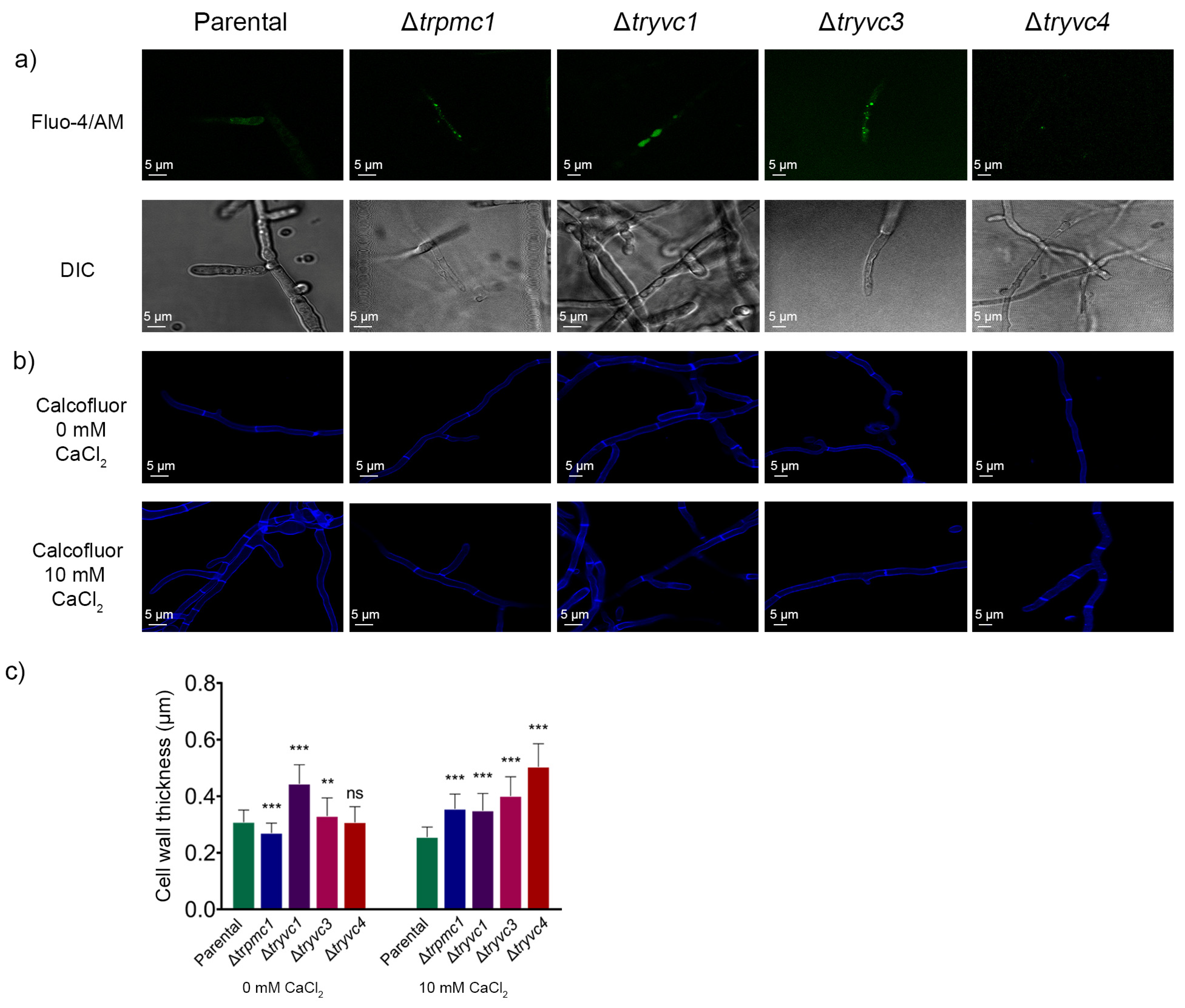
3.5. Microscopy Analysis Reveals the Role of TrPMC1, TrYVC1, TrYVC3, and TrYVC4 in Cell Wall Thickness and Calcium Dynamics in T. reesei
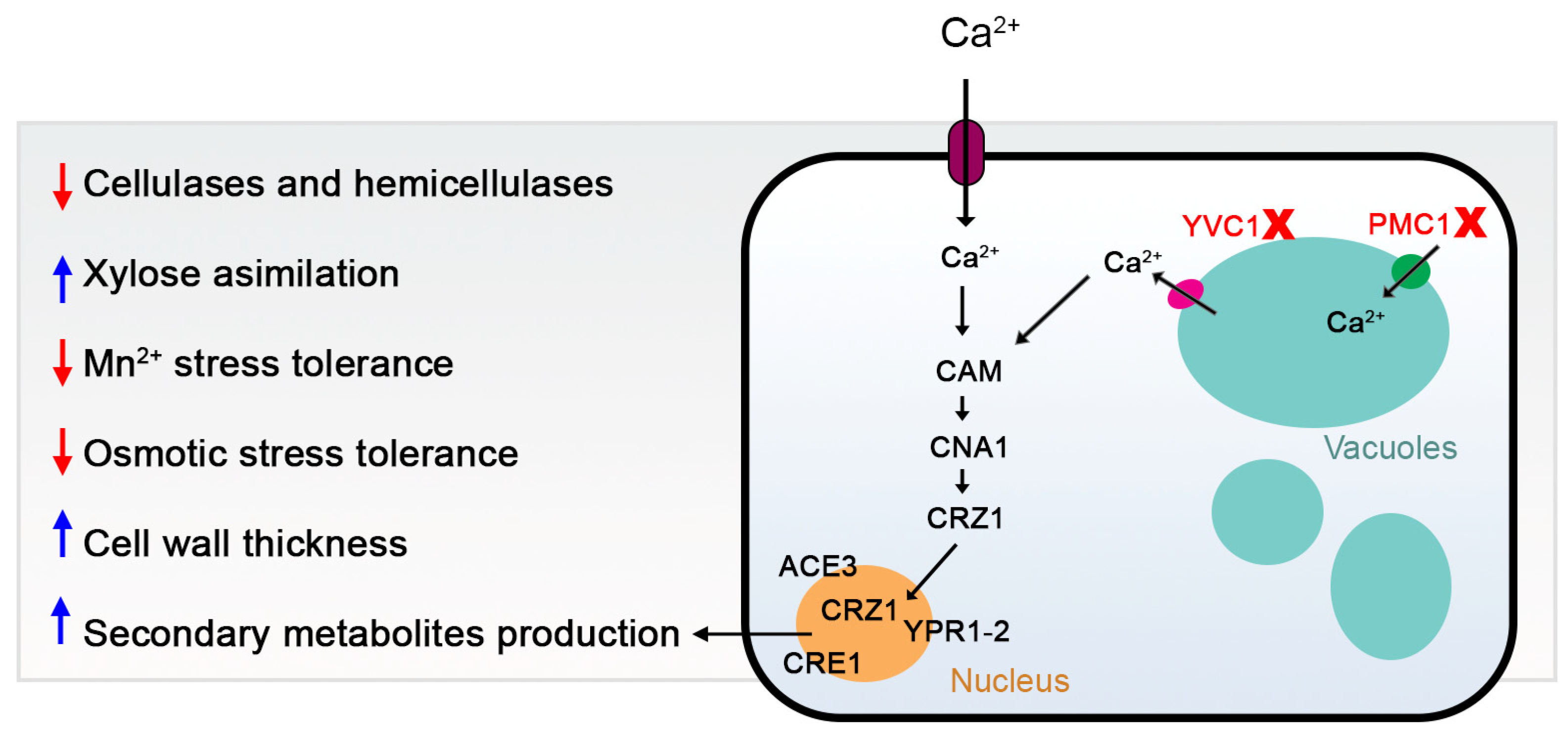
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; O’hara, I.M.; Mundree, S.; Gao, B.; Ball, A.S.; Zhu, N.; Bai, Z.; Jin, B. Biofuels from food processing wastes. Curr. Opin. Biotechnol. 2016, 38, 97–105. [Google Scholar] [CrossRef] [PubMed]
- de Paula, R.G.; Antoniêto, A.C.C.; Ribeiro, L.F.C.; Carraro, C.B.; Nogueira, K.M.V.; Lopes, D.C.B.; Silva, A.C.; Zerbini, M.T.; Pedersoli, W.R.; do Nascimento Costa, M.; et al. New Genomic Approaches to Enhance Biomass Degradation by the Industrial Fungus Trichoderma reesei. Int. J. Genom. 2018, 2018, 1974151. [Google Scholar] [CrossRef]
- Jansen, M.L.A.; Bracher, J.M.; Papapetridis, I.; Verhoeven, M.D.; de Bruijn, H.; de Waal, P.P.; van Maris, A.J.A.; Klaassen, P.; Pronk, J.T. Saccharomyces cerevisiae strains for second-generation ethanol production: From academic exploration to industrial implementation. FEMS Yeast Res. 2017, 17, fox044. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Bao, J. Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling. Bioprocess Biosyst. Eng. 2016, 39, 133–140. [Google Scholar] [CrossRef]
- dos Santos Castro, L.; Pedersoli, W.; Antoniêto, A.C.; Steindorff, A.; Silva-Rocha, R.; Martinez-Rossi, N.M.; Rossi, A.; Brown, N.A.; Goldman, G.H.; Faça, V.M.; et al. Comparative metabolism of cellulose, sophorose and glucose in Trichoderma reesei using high-throughput genomic and proteomic analyses. Biotechnol. Biofuels 2014, 7, 41. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Y.; Wang, W.; Wei, D. Mn2+ modulates the expression of cellulase genes in Trichoderma reesei Rut-C30 via calcium signaling. Biotechnol. Biofuels 2018, 11, 54. [Google Scholar] [CrossRef]
- Li, N.; Li, J.; Chen, Y.; Shen, Y.; Wei, D.; Wang, W. Mechanism of Zn2+ regulation of cellulase production in Trichoderma reesei Rut-C30. Biotechnol. Biofuels Bioprod. 2023, 16, 73. [Google Scholar] [CrossRef]
- Li, N.; Zeng, Y.; Chen, Y.; Shen, Y.; Wang, W. Induction of cellulase production by Sr2+ in Trichoderma reesei via calcium signaling transduction. Bioresour. Bioprocess. 2022, 9, 96. [Google Scholar] [CrossRef]
- Chen, L.; Zou, G.; Wang, J.; Wang, J.; Liu, R.; Jiang, Y.; Zhao, G.; Zhou, Z. Characterization of the Ca2+-responsive signaling pathway in regulating the expression and secretion of cellulases in Trichoderma reesei Rut-C30. Mol. Microbiol. 2016, 100, 560–575. [Google Scholar] [CrossRef]
- Martins-Santana, L.; de Paula, R.G.; Silva, A.G.; Lopes, D.C.B.; Silva, R.D.N.; Silva-Rocha, R. CRZ1 regulator and calcium cooperatively modulate holocellulases gene expression in Trichoderma reesei QM6a. Genet. Mol. Biol. 2020, 43, e20190244. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Peiter, E. Calcium Transport Proteins in Fungi: The Phylogenetic Diversity of Their Relevance for Growth, Virulence, and Stress Resistance. Front. Microbiol. 2020, 10, 3100. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot. Cell 2015, 14, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, P.; Li, Y.; Bi, Y.; Prusky, D.B. Updating Insights into the Regulatory Mechanisms of Calcineurin-Activated Transcription Factor Crz1 in Pathogenic Fungi. J. Fungi 2022, 8, 1082. [Google Scholar] [CrossRef]
- Tisi, R.; Rigamonti, M.; Groppi, S.; Belotti, F. Calcium homeostasis and signaling in fungi and their relevance forpathogenicity of yeasts and filamentous fungi. AIMS Mol. Sci. 2016, 3, 505–549. [Google Scholar] [CrossRef]
- Schmoll, M.; Dattenböck, C.; Carreras-Villaseñor, N.; Mendoza-Mendoza, A.; Tisch, D.; Alemán, M.I.; Baker, S.E.; Brown, C.; Cervantes-Badillo, M.G.; Cetz-Chel, J.; et al. The Genomes of Three Uneven Siblings: Footprints of the Lifestyles of Three Trichoderma Species. Microbiol. Mol. Biol. Rev. 2016, 80, 205–327. [Google Scholar] [CrossRef]
- De Paula, R.G.; Antoniêto, A.C.C.; Carraro, C.B.; Lopes, D.C.B.; Persinoti, G.F.; Peres, N.T.A.; Martinez-Rossi, N.M.; Silva-Rocha, R.; Silva, R.N. The Duality of the MAPK Signaling Pathway in the Control of Metabolic Processes and Cellulase Production in Trichoderma reesei. Sci. Rep. 2018, 8, 14931. [Google Scholar] [CrossRef]
- Silva, A.C.; Oshiquiri, L.H.; de Morais Costa de Jesus, L.F.; Maués, D.B.; do Nascimento Silva, R. The Cerato-Platanin EPL2 from Trichoderma reesei Is Not Directly Involved in Cellulase Formation but in Cell Wall Remodeling. Microorganisms 2023, 11, 1965. [Google Scholar] [CrossRef]
- Campos Antoniêto, A.C.; Graciano de Paula, R.; Santos Castro L dos Silva-Rocha, R.; Felix Persinoti, G.; Nascimento Silva, R. Trichoderma reesei CRE1-mediated Carbon Catabolite Repression in Response to Sophorose Through RNA Sequencing Analysis. Curr. Genom. 2015, 17, 119–131. [Google Scholar] [CrossRef]
- Dos Santos Castro, L.; de Paula, R.G.; Antoniêto, A.C.C.; Persinoti, G.F.; Silva-Rocha, R.; Silva, R.N. Understanding the Role of the Master Regulator XYR1 in Trichoderma reesei by Global Transcriptional Analysis. Front. Microbiol. 2016, 7, 175. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.; Shen, Y.; Ma, Y.; Wei, D.; Wang, W. N,N-dimethylformamide induces cellulase production in the filamentous fungus Trichoderma reesei. Biotechnol. Biofuels 2019, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, X.; Zhao, X.; Shen, Y.; Xu, X.; Wei, L.; Wang, W.; Wei, D. cAMP activates calcium signalling via phospholipase C to regulate cellulase production in the filamentous fungus Trichoderma reesei. Biotechnol. Biofuels 2021, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Quan, L.; Yang, M.; Wang, D.; Wang, Y.Z. Regulation of cellulase production via calcium signaling in Trichoderma reesei under PEG8000 stress. Appl. Microbiol. Biotechnol. 2024, 108, 178. [Google Scholar] [CrossRef] [PubMed]
- Derntl, C.; Kiesenhofer, D.P.; Mach, R.L.; Mach-Aigner, A.R. Novel Strategies for Genomic Manipulation of Trichoderma reesei with the Purpose of Strain Engineering. Appl. Environ. Microbiol. 2015, 81, 6314–6323. [Google Scholar] [CrossRef]
- Nogueira, K.M.V.; de Paula, R.G.; Antoniêto, A.C.C.; dos Reis, T.F.; Carraro, C.B.; Silva, A.C.; Almeida, F.; Rechia, C.G.V.; Goldman, G.H.; Silva, R.N. Characterization of a novel sugar transporter involved in sugarcane bagasse degradation in Trichoderma reesei. Biotechnol. Biofuels 2018, 11, 84. [Google Scholar] [CrossRef]
- Przylucka, A.; Akcapinar, G.B.; Chenthamara, K.; Cai, F.; Grujic, M.; Karpenko, J.; Livoi, M.; Shen, Q.; Kubicek, C.P.; Druzhinina, I.S. HFB7—A novel orphan hydrophobin of the Harzianum and Virens clades of Trichoderma, is involved in response to biotic and abiotic stresses. Fungal Genet. Biol. 2017, 102, 63–76. [Google Scholar] [CrossRef]
- Schmoll, M.; Schuster, A.; Silva, R.D.N.; Kubicek, C.P. The G-alpha protein GNA3 of Hypocrea jecorina (anamorph Trichoderma reesei) regulates cellulase gene expression in the presence of light. Eukaryot. Cell 2009, 8, 410–420. [Google Scholar] [CrossRef]
- Derntl, C.; Rassinger, A.; Srebotnik, E.; Mach, R.L.; Mach-Aigner, A.R. Identification of the main regulator responsible for synthesis of the typical yellow pigment produced by Trichoderma reesei. Appl. Environ. Microbiol. 2016, 82, 6247–6257. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Preparation and Transformation of Competent E. coli Using Calcium Chloride. Cold Spring Harb. Protoc. 2006, 2006, pdb.prot3932. [Google Scholar] [CrossRef]
- Bimboim, H.C.; Doly, J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979, 7, 1513–1523. [Google Scholar] [CrossRef]
- Teepe, A.G.; Loprete, D.M.; He, Z.; Hoggard, T.A.; Hill, T.W. The protein kinase C orthologue PkcA plays a role in cell wall integrity and polarized growth in Aspergillus nidulans. Fungal Genet. Biol. 2007, 44, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Schiestl, R.H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Collopy, P.D.; Colot, H.V.; Park, G.; Ringelberg, C.; Crew, C.M.; Borkovich, K.A.; Dunlap, J.C. High-throughput construction of gene deletion cassettes for generation of Neurospora crassa knockout strains. Methods Mol. Biol. 2010, 638, 33–40. [Google Scholar] [PubMed]
- Colot, H.V.; Park, G.; Turner, G.E.; Ringelberg, C.; Crew, C.M.; Litvinkova, L.; Weiss, R.L.; Borkovich, K.A.; Dunlap, J.C. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 2006, 103, 10352–10357. [Google Scholar] [CrossRef]
- Goldman, G.H.; Dos Reis Marques, E.; Duarte Ribeiro, D.C.; De Souza Bernardes, L.Â.; Quiapin, A.C.; Vitorelli, P.M.; Savoldi, M.; Semighini, C.P.; de Oliveira, R.C.; Nunes, L.R.; et al. Expressed sequence tag analysis of the human pathogen Paracoccidioides brasiliensis yeast phase: Identification of putative homologues of Candida albicans virulence and pathogenicity genes. Eukaryot. Cell 2003, 2, 34–48. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Vitikainen, M.; Arvas, M.; Pakula, T.; Oja, M.; Penttilä, M.; Saloheimo, M. Array comparative genomic hybridization analysis of Trichoderma reesei strains with enhanced cellulase production properties. BMC Genom. 2010, 11, 441. [Google Scholar] [CrossRef]
- Huang, S.; He, Z.; Zhang, S.; Keyhani, N.O.; Song, Y.; Yang, Z.; Jiang, Y.; Zhang, W.; Pei, Y.; Zhang, Y. Interplay between calcineurin and the Slt2 MAP-kinase in mediating cell wall integrity, conidiation and virulence in the insect fungal pathogen Beauveria bassiana. Fungal Genet. Biol. 2015, 83, 78–91. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Xiao, Z.; Storms, R.; Tsang, A. Microplate-based carboxymethylcellulose assay for endoglucanase activity. Anal. Biochem. 2005, 342, 176–178. [Google Scholar] [CrossRef]
- Castro, L.D.S.; Antoniêto, A.C.C.; Pedersoli, W.R.; Silva-Rocha, R.; Persinoti, G.F.; Silva, R.N. Expression pattern of cellulolytic and xylanolytic genes regulated by transcriptional factors XYR1 and CRE1 are affected by carbon source in Trichoderma reesei. Gene Expr. Patterns 2014, 14, 88–95. [Google Scholar] [CrossRef]
- Verbeke, J.; Coutinho, P.; Mathis, H.; Quenot, A.; Record, E.; Asther, M.; Heiss-Blanquet, S. Transcriptional profiling of cellulase and expansin-related genes in a hypercellulolytic Trichoderma reesei. Biotechnol. Lett. 2009, 31, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Berka, R.M.; Henrissat, B.; Saloheimo, M.; Arvas, M.; Baker, S.E.; Chapman, J.; Chertkov, O.; Coutinho, P.M.; Cullen, D.; et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat. Biotechnol. 2008, 26, 553–560. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. Poon AFY, editor. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Antonieto, A.C.C.; Nogueira, K.M.V.; de Paula, R.G.; Nora, L.C.; Cassiano, M.H.A.; Guazzaroni, M.-E.; Almeida, F.; da Silva, T.A.; Ries, L.N.A.; de Assis, L.J.; et al. A Novel Cys2His2 Zinc Finger Homolog of AZF1 Modulates Holocellulase Expression in Trichoderma reesei. mSystems 2019, 4, 10.1128. [Google Scholar] [CrossRef]
- Waskom, M. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Herold, S.; Bischof, R.; Metz, B.; Seiboth, B.; Kubicek, C.P. Xylanase gene transcription in Trichoderma reesei is triggered by different inducers representing different hemicellulosic pentose polymers. Eukaryot. Cell 2013, 12, 390–398. [Google Scholar] [CrossRef]
- Bowman, B.J.; Abreu, S.; Margolles-Clark, E.; Draskovic, M.; Bowman, E.J. Role of four calcium transport proteins, encoded by nca-1, nca-2, nca-3, and cax, in maintaining intracellular calcium levels in Neurospora crassa. Eukaryot. Cell 2011, 10, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Weihmann, F.; Schliebner, I.; Horbach, R.; Deising, H.B.; Wirsel, S.G.R.; Peiter, E. The transient receptor potential (TRP) channel family in colletotrichum graminicola: A molecular and physiological analysis. PLoS ONE 2016, 11, e0158561. [Google Scholar] [CrossRef] [PubMed]
- Dinamarco, T.M.; Freitas, F.Z.; Almeida, R.S.; Brown, N.A.; dos Reis, T.F.; Ramalho, L.N.Z.; Savoldi, M.; Goldman, M.H.S.; Bertolini, M.C.; Goldman, G.H. Functional Characterization of an Aspergillus fumigatus Calcium Transporter (PmcA) that Is Essential for Fungal Infection. PLoS ONE 2012, 7, e37591. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.G.; Ying, S.H.; Feng, M.G. Differential Roles for Six P-Type Calcium ATPases in Sustaining Intracellular Ca2+ Homeostasis, Asexual Cycle and Environmental Fitness of Beauveria bassiana. Sci. Rep. 2017, 7, 1040. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N.O. Molecular Genetics of Beauveria bassiana Infection of Insects. Adv. Genet. 2016, 94, 165–249. [Google Scholar]
- Antoniêto, A.C.C.; Castro, L.S.; Silva-Rocha, R.; Persinoti, G.F.; Silva, R.N. Defining the genome-wide role of CRE1 during carbon catabolite repression in Trichoderma reesei using RNA-Seq analysis. Fungal Genet. Biol. 2014, 73, 93–103. [Google Scholar] [CrossRef]
- Peterson, R.; Nevalainen, H. Trichoderma reesei RUT-C30—Thirty years of strain improvement. Microbiology 2012, 158, 58–68. [Google Scholar] [CrossRef]
- Davis, T.R.; Zucchi, P.C.; Kumamoto, C.A. Calmodulin Binding to Dfi1p Promotes Invasiveness of Candida albicans. PLoS ONE 2013, 8, e76239. [Google Scholar] [CrossRef]
- Groppi, S.; Belotti, F.; Brandão, R.L.; Martegani, E.; Tisi, R. Glucose-induced calcium influx in budding yeast involves a novel calcium transport system and can activate calcineurin. Cell Calcium 2011, 49, 376–386. [Google Scholar] [CrossRef]
- Fonseca-Peralta, H.M.; Pineda-Hidalgo, K.V.; Castro-Martínez, C.; Contreras-Andrade, I. Effect of Zinc-Calcium on Xylose Consumption by Mucor circinelloides (MN128960): Xylitol and Ethanol Yield Optimization. Energies 2022, 15, 906. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, Y.J.; Kwon, B.; Choo, Y.M.; Yu, K.Y.; Kim, J. Regulation of secondary metabolism by calmodulin signaling in filamentous fungi. Rev. Iberoam. Micol. 2019, 36, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liu, Z.; Li, M.; Hu, Y.; Yang, L.; Song, X.; Qin, Y. Drafting Penicillium oxalicum calcineurin-CrzA pathway by combining the analysis of phenotype, transcriptome, and endogenous protein–protein interactions. Fungal Genet. Biol. 2022, 158, 103652. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F. Vacuolal and Peroxisomal Calcium Ion Transporters in Yeasts and Fungi: Key Role in the Translocation of Intermediates in the Biosynthesis of Fungal Metabolites. Genes 2022, 13, 1450. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Liras, P. The PenV vacuolar membrane protein that controls penicillin biosynthesis is a putative member of a subfamily of stress-gated transient receptor calcium channels. Curr. Res. Biotechnol. 2021, 3, 317–322. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.L.; Zhang LBin Ying, S.H.; Feng, M.G. The role of three calcineurin subunits and a related transcription factor (Crz1) in conidiation, multistress tolerance and virulence in Beauveria bassiana. Appl. Microbiol. Biotechnol. 2015, 99, 827–840. [Google Scholar] [CrossRef]
- Kumar, A.; Roy, A.; Deshmukh, M.V.; Tamuli, R. Dominant mutants of the calcineurin catalytic subunit (CNA-1) showed developmental defects, increased sensitivity to stress conditions, and CNA-1 interacts with CaM and CRZ-1 in Neurospora crassa. Arch. Microbiol. 2020, 202, 921–934. [Google Scholar] [CrossRef]
- Wang, S.; Cao, J.; Liu, X.; Hu, H.; Shi, J.; Zhang, S.; Keller, N.P.; Lu, L. Putative Calcium Channels CchA and MidA Play the Important Roles in Conidiation, Hyphal Polarity and Cell Wall Components in Aspergillus nidulans. PLoS ONE 2012, 7, e46564. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Saltsman, K.; Gasch, A.P.; Li, H.X.; Ogawa, N.; Botstein, D.; Brown, P.O.; Cyert, M.S. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2002, 277, 31079–31088. [Google Scholar] [CrossRef]
- Annick Ries, L.N.; Rocha, M.C.; De Castro, P.A.; Silva-Rocha, R.; Silva, R.N.; Freitas, F.Z.; de Assis, L.J.; Bertolini, M.C.; Malavazi, I.; Goldman, G.H. The Aspergillus fumigatus CrzA transcription factor activates chitin synthase gene expression during the caspofungin paradoxical effect. mBio 2017, 8, e00705-17. [Google Scholar]
- Lev, S.; Desmarini, D.; Chayakulkeeree, M.; Sorrell, T.C.; Djordjevic, J.T. The Crz1/Sp1 Transcription Factor of Cryptococcus neoformans Is Activated by Calcineurin and Regulates Cell Wall Integrity. PLoS ONE 2012, 7, e51403. [Google Scholar] [CrossRef]
- Palmer, C.P.; Zhou, X.L.; Lin, J.; Loukin, S.H.; Kung, C.; Saimi, Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA 2001, 98, 7801–7805. [Google Scholar] [CrossRef] [PubMed]
- Pozos, T.C.; Sekler, I.; Cyert, M.S. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol. Cell Biol. 1996, 16, 3730–3741. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.W.; Fink, G.R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 1994, 124, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, F.; Zhao, Q.; Chen, J.; Zhang, B.; Ding, X.; Wang, H.; Yang, B.; Lu, G.; Zhang, B.; et al. A novel role of the vacuolar calcium channel Yvc1 in stress response, morphogenesis and pathogenicity of Candida albicans. Int. J. Med. Microbiol. 2014, 304, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhang, K.; Zhang, D.; Yu, Q.; Xiao, C.; Dong, Y.; Chu, M.; Zou, S.; Li, M. Effects of Disruption of PMC1 in the tfp1∆/∆ Mutant on Calcium Homeostasis, Oxidative and Osmotic Stress Resistance in Candida albicans. Mycopathologia 2018, 183, 315–327. [Google Scholar] [CrossRef] [PubMed]
- De Castro, P.A.; Chiaratto, J.; Winkelströter, L.K.; Bom, V.L.P.; Ramalho, L.N.Z.; Goldman, M.H.S.; Brown, N.A.; Goldman, G.H. The involvement of the Mid1/Cch1/Yvc1 calcium channels in Aspergillus fumigatus virulence. PLoS ONE 2014, 9, e103957. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, J.-E.; Frailey, D.; Nohe, A.; Duncan, R.; Czymmek, K.J.; Kang, S. Roles of three Fusarium oxysporum calcium ion (Ca2+) channels in generating Ca2+ signatures and controlling growth. Fungal Genet. Biol. 2015, 82, 145–157. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Kadotani, N.; Kasahara, S.; Tosa, Y.; Mayama, S.; Nakayashiki, H. Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Mol. Microbiol. 2008, 68, 1348–1365. [Google Scholar] [CrossRef]
- Kmetzsch, L.; Staats, C.C.; Simon, E.; Fonseca, F.L.; de Oliveira, D.L.; Sobrino, L.; Rodrigues, J.; Leal, A.L.; Nimrichter, L.; Rodrigues, M.L.; et al. The vacuolar Ca2+ exchanger vcx1 is involved in calcineurin-dependent Ca2+ tolerance and virulence in Cryptococcus neoformans. Eukaryot. Cell 2010, 9, 1798–1805. [Google Scholar] [CrossRef]
- Kmetzsch, L.; Staats, C.C.; Cupertino, J.B.; Fonseca, F.L.; Rodrigues, M.L.; Schrank, A.; Vainstein, M.H. The calcium transporter Pmc1 provides Ca2+ tolerance and influences the progression of murine cryptococcal infection. FEBS J. 2013, 280, 4853–4864. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Ying, S.H.; Feng, M.G. Five vacuolar Ca2+ exchangers play different roles in calcineurin-dependent Ca2+/Mn2+ tolerance, multistress responses and virulence of a filamentous entomopathogen. Fungal Genet. Biol. 2014, 73, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Fokina, A.V.; Sokolov, S.S.; Kang, H.A.; Kalebina, T.S.; Ter-Avanesyan, M.D.; Agaphonov, M.O. Inactivation of Pmc1 vacuolar Ca2+ ATPase causes G2 cell cycle delay in Hansenula polymorpha. Cell Cycle 2012, 11, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.C.G.; Katoh-Fukui, R.; Moto, K.; Ribas, J.C.; Ishiguro, J. Schizosaccharomyces pombe Pmr1p is essential for cell wall integrity and is required for polarized cell growth and cytokinesis. Eukaryot. Cell 2004, 3, 1124–1135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, H.; Liu, F.; Zhang, S.; Lu, L. Putative PmrA and PmcA are important for normal growth, Morphogenesis and cell wall integrity, But not for viability in Aspergillus nidulans. Microbiology 2014, 160, 2387–2395. [Google Scholar] [CrossRef]
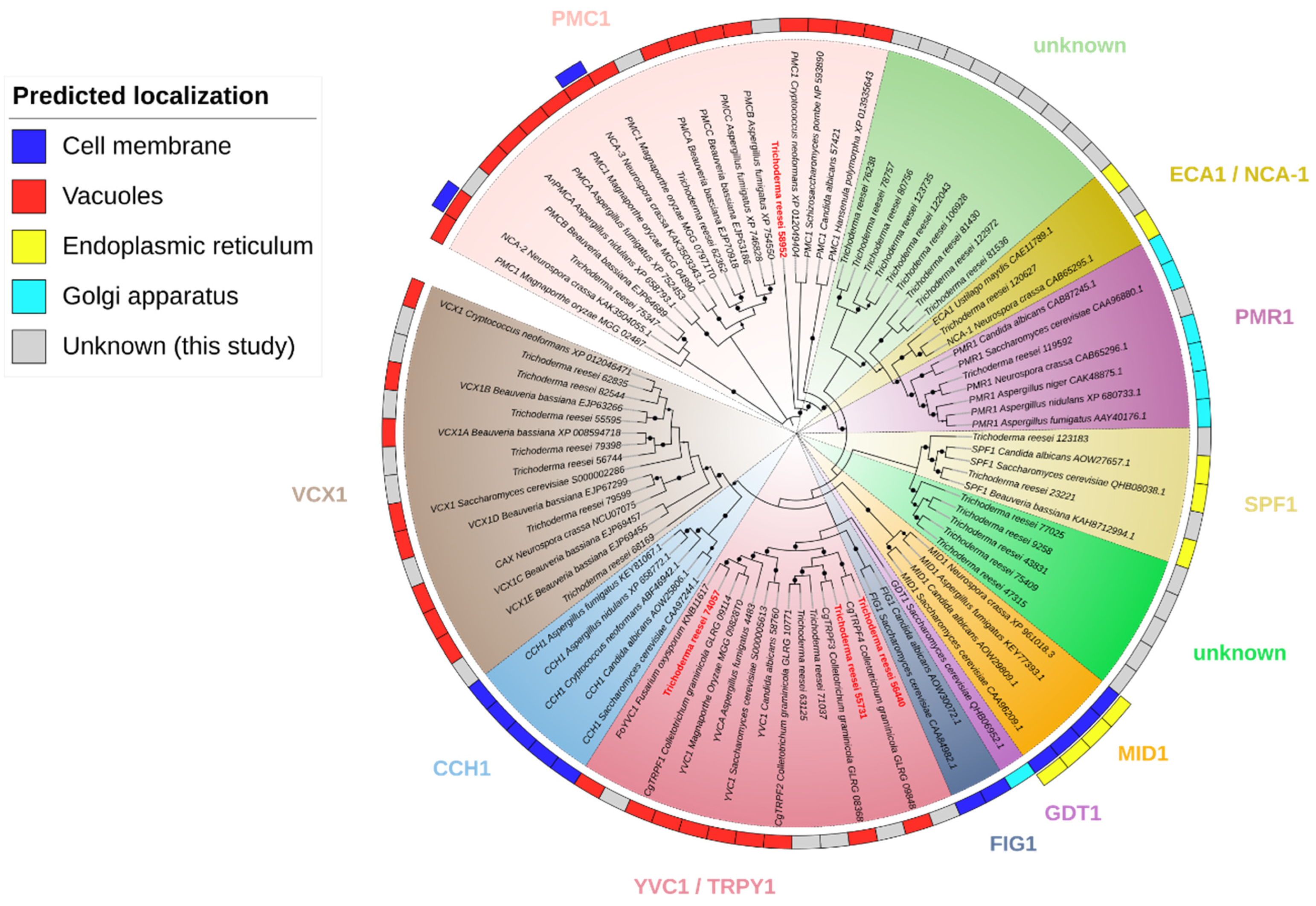



| JGI ID | Possible Homologue in S. cerevisiae | Size (aa) | Annotation | Full e-Value (Hmmsearch) |
|---|---|---|---|---|
| 74057 | YVC1 | 625 | Nonselective cation channel protein | 1.8 × 10−256 |
| 55731 | YVC1 | 1167 | Nonselective cation channel protein | 7 × 10−164 |
| 56440 | YVC1 | 701 | Nonselective cation channel protein | 5 × 10−145 |
| 63125 | YVC1 | 631 | Nonselective cation channel protein | 3.7 × 10−121 |
| 71037 | YVC1 | 1104 | Nonselective cation channel protein | 3.4 × 10−117 |
| 79599 | VCX1 | 462 | Vacuolar calcium ion transporter | 1.4 × 10−178 |
| 55595 | VCX1 | 421 | Vacuolar calcium ion transporter | 2 × 10−166 |
| 79398 | VCX1 | 339 | Vacuolar calcium ion transporter | 2.1 × 10−151 |
| 56744 | VCX1 | 381 | Vacuolar calcium ion transporter | 8.5 × 10−100 |
| 82544 | VCX1 | 433 | Vacuolar calcium ion transporter | 4.3 × 10−87 |
| 68169 | VCX1 | 394 | Vacuolar calcium ion transporter | 1 × 10−81 |
| 62835 | VCX1 | 1115 | Vacuolar calcium ion transporter | 7.4 × 10−48 |
| 75347 | PMC1 | 1379 | Calcium-translocating P-type ATPase, PMCA-type | 0 |
| 58952 | PMC1 | 1204 | Calcium-translocating P-type ATPase, PMCA-type | 0 |
| 62362 | PMC1 | 1281 | Calcium-translocating P-type ATPase, PMCA-type | 0 |
| 120627 | PMC1 | 998 | Calcium-translocating P-type ATPase, SERCA-type | 2.4 × 10−142 |
| 119592 | PMC1 | 1062 | Calcium-transporting ATPase | 1.5 × 10−137 |
| 81536 | PMC1 | 1101 | Calcium-transporting ATPase | 4.3 × 10−111 |
| 122972 | PMC1 | 1071 | Calcium-transporting ATPase | 8.4 × 10−109 |
| 81430 | PMC1 | 1023 | Calcium-transporting ATPase | 1.3 × 10−106 |
| 106928 | PMC1 | 1049 | Sodium/potassium-transporting ATPase subunit alpha | 6.3 × 10−94 |
| 78757 | PMC1 | 923 | Calcium-transporting ATPase | 1.9 × 10−47 |
| 76238 | PMC1 | 982 | Calcium-transporting ATPase | 1.4 × 10−45 |
| 123183 | PMC1 | 1309 | Cation-transporting ATPase-related | 1.1 × 10−38 |
| 122043 | PMC1 | 1171 | Heavy metal translocating P-type ATPase | 2.2 × 10−36 |
| 43831 | PMC1 | 1354 | Probable phospholipid-transporting ATPase | 1.2 × 10−32 |
| 23221 | PMC1 | 1318 | Cation-transporting ATPase-related | 3.6 × 10−31 |
| 123735 | PMC1 | 1105 | Heavy metal translocating P-type ATPase | 5 × 10−30 |
| 77025 | PMC1 | 1300 | Probable phospholipid-transporting ATPase | 7.4 × 10−30 |
| 80756 | PMC1 | 1133 | Heavy metal translocating P-type ATPase | 1 × 10−27 |
| 79258 | PMC1 | 1534 | Probable phospholipid-transporting ATPase | 3.2 × 10−27 |
| 47315 | PMC1 | 1392 | Probable phospholipid-transporting ATPase | 2.7 × 10−23 |
| 75409 | PMC1 | 1368 | Probable phospholipid-transporting ATPase | 2.4 × 10−20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshiquiri, L.H.; Pereira, L.M.S.; Maués, D.B.; Milani, E.R.; Silva, A.C.; Jesus, L.F.d.M.C.d.; Silva-Neto, J.A.; Veras, F.P.; de Paula, R.G.; Silva, R.N. Regulatory Role of Vacuolar Calcium Transport Proteins in Growth, Calcium Signaling, and Cellulase Production in Trichoderma reesei. J. Fungi 2024, 10, 853. https://doi.org/10.3390/jof10120853
Oshiquiri LH, Pereira LMS, Maués DB, Milani ER, Silva AC, Jesus LFdMCd, Silva-Neto JA, Veras FP, de Paula RG, Silva RN. Regulatory Role of Vacuolar Calcium Transport Proteins in Growth, Calcium Signaling, and Cellulase Production in Trichoderma reesei. Journal of Fungi. 2024; 10(12):853. https://doi.org/10.3390/jof10120853
Chicago/Turabian StyleOshiquiri, Letícia Harumi, Lucas Matheus Soares Pereira, David Batista Maués, Elizabete Rosa Milani, Alinne Costa Silva, Luiz Felipe de Morais Costa de Jesus, Julio Alves Silva-Neto, Flávio Protásio Veras, Renato Graciano de Paula, and Roberto Nascimento Silva. 2024. "Regulatory Role of Vacuolar Calcium Transport Proteins in Growth, Calcium Signaling, and Cellulase Production in Trichoderma reesei" Journal of Fungi 10, no. 12: 853. https://doi.org/10.3390/jof10120853
APA StyleOshiquiri, L. H., Pereira, L. M. S., Maués, D. B., Milani, E. R., Silva, A. C., Jesus, L. F. d. M. C. d., Silva-Neto, J. A., Veras, F. P., de Paula, R. G., & Silva, R. N. (2024). Regulatory Role of Vacuolar Calcium Transport Proteins in Growth, Calcium Signaling, and Cellulase Production in Trichoderma reesei. Journal of Fungi, 10(12), 853. https://doi.org/10.3390/jof10120853









