Unlocking the Power: New Insights into the Anti-Aging Properties of Mushrooms
Abstract
:1. Introduction
2. Aging
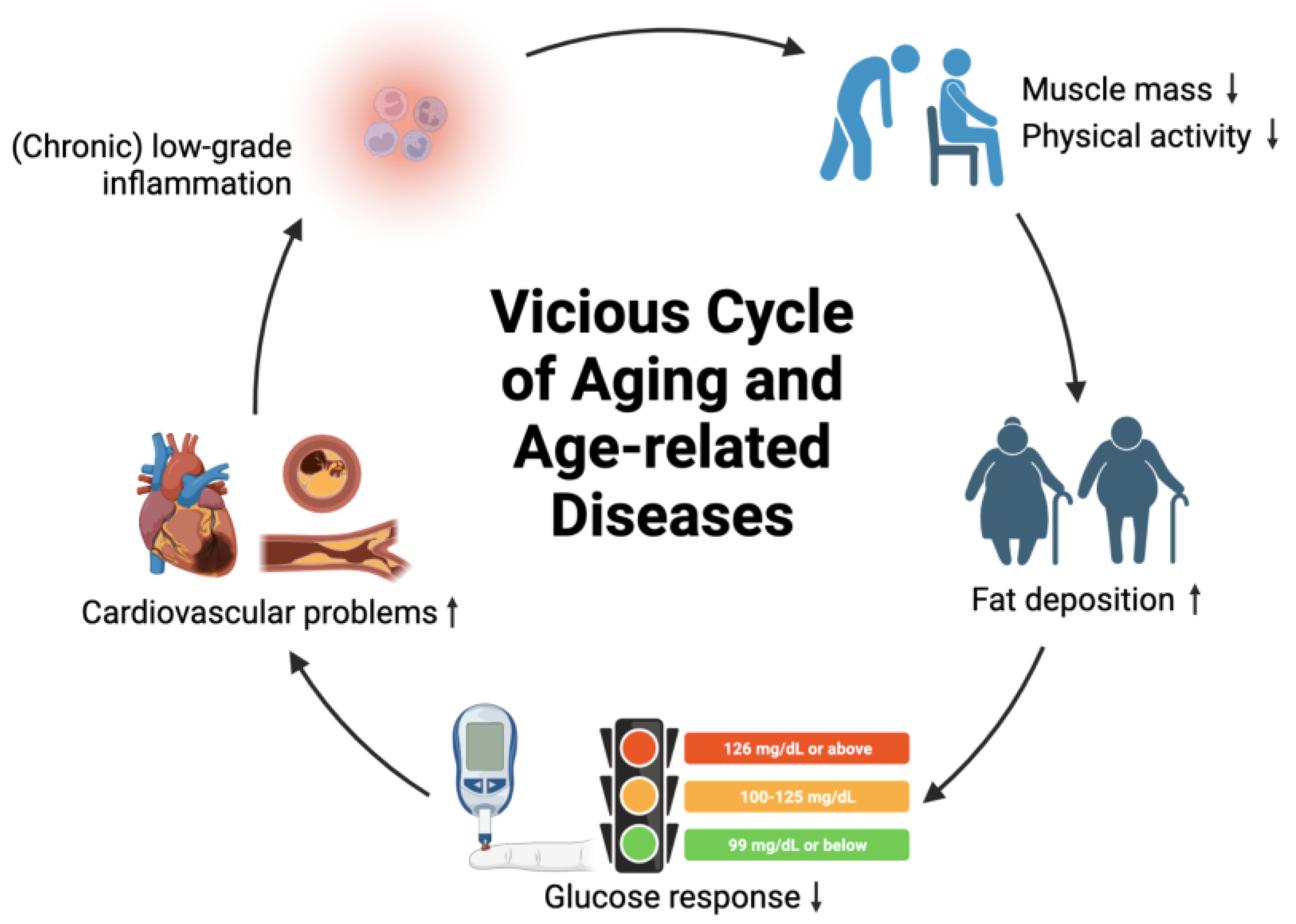
2.1. Aging and Age-Related Diseases
2.2. Aging and Dietary Intervention
2.3. Ageing, Mental Health and Gender
3. Components of Mushrooms and Their Anti-Aging Effects
3.1. Bioactive Compounds in Mushrooms
3.1.1. Carbohydrates
3.1.2. Proteins
3.1.3. Lipids
3.1.4. Phenolic Compounds
4. Effects of Mushrooms and Their Anti-Aging Properties
4.1. Anti-Oxidant Activity
4.2. Anti-Wrinkle Effects
4.3. Immunomodulatory Effects
4.4. Cardioprotective Effects
4.5. Neuroprotective Effects
4.6. Anti-Diabetic Effects
4.7. Beneficial for Age-Related Diseases
4.8. Structure–Activity Relationship
5. Molecular and Cellular Mechanisms Underlying Aging Processes
5.1. Cell Senescence
5.2. Telomere Maintenance
5.3. Mitochondrial Dysfunction
5.4. DNA Damage
5.5. Epigenetic Changes
5.6. Chronic Low-Grade Inflammation
6. Concluding Remarks and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.; Barber, R.M.; Foreman, K.J.; Ozgoren, A.A.; Abdallah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; Aburaddad, L.J. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- World Health Organization. Preventing chronic diseases: A vital investment. Prev. Chronic Dis. A Vital Invest. 2008, 126, 95. [Google Scholar]
- de Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Baiamonte, E.; Guarrera, M.; Parisi, A.; Ruffolo, C.; Tagliaferri, F.; Barbagallo, M. Healthy aging and dietary patterns. Nutrients 2022, 14, 889. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, H.; Mohammed Ali, A. Royal jelly and its components promote healthy aging and longevity: From animal models to humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Johnson, E.; Lyberg, T.; Bernardshaw, S.; Tryggestad, A.M.A.; Grinde, B. Effects of the medicinal mushroom Agaricus blazei Murill on immunity, infection and cancer. Scand. J. Immunol. 2008, 68, 157015. [Google Scholar] [CrossRef] [PubMed]
- Im, K.H.; Nguyen, T.K.; Choi, J.; Lee, T.S. In vitro antioxidant, anti-diabetes, anti-dementia, and inflammation inhibitory effect of Trametes pubescens fruiting body extracts. Molecules 2016, 21, 639. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, I.S.; Kim, K.C.; Yoo, I.D.; Yang, H.M. ROS scavenging and anti-wrinkle effects of clitocybin A isolated from the mycelium of the mushroom Clitocybe aurantiaca. J. Microbiol. Biotechnol. 2017, 27, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Gao, Z.; Liu, W.; Li, H.; Zhang, Y.; Feng, Y.; Song, X.; Wang, W.; Zhang, J.; Huang, C.; et al. Characterization, antioxidant, anti-aging and organ protective effects of sulfated polysaccharides from Flammulina velutipes. Molecules 2019, 24, 3517. [Google Scholar] [CrossRef]
- Jo Feeney, M.; Miller, A.M.; Roupas, P. Mushrooms-biologically distinct and nutritionally unique: Exploring a “Third Food Kingdom”. Nutr. Today 2014, 49, 301–307. [Google Scholar] [CrossRef]
- Elkhateeb, W.A. What medicinal mushroom can do? J. Chem. Res. 2020, 5, 106–118. [Google Scholar]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of aging: Dietary implications. Am. J. Clin. Nutr. 1972, 25, 839–843. [Google Scholar] [CrossRef]
- Van Remmen, H.; Ikeno, Y.; Hamilton, M.; Pahlavani, M.; Wolf, N.; Thorpe, S.R.; Alderson, N.L.; Baynes, J.W.; Epstein, C.J.; Huang, T.T.; et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genom. 2003, 16, 29–37. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, B.J.; Huang, W.S.; Amrouche, A.T.; Maurizio, B.; Simal-Gandara, J.; Tundis, R.; Xiao, J.B.; Zou, L.; Lu, B.Y. Edible flowers as functional raw materials: A review on anti-aging properties. Trends Food Sci. Technol. 2020, 106, 30–47. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Belikov, A.V. Age-related diseases as vicious cycles. Ageing Res. Rev. 2019, 49, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Galluzzi, L.; Freije, J.M.P.; Madeo, F.; Kroemer, G. Metabolic control of longevity. Cell 2016, 166, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Lee, S.H.; Min, K.J. Caloric restriction and its mimetics. BMB Rep. 2013, 46, 181–187. [Google Scholar] [CrossRef]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition 1989, 5, 155–171, discussion 172. [Google Scholar]
- Martins, I.; Galluzzi, L.; Kroemer, G. Hormesis, cell death and aging. Aging 2011, 3, 821–828. [Google Scholar] [CrossRef]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Miller, R.A. Cell stress and aging: New emphasis on multiplex resistance mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 179–182. [Google Scholar] [CrossRef]
- Cava, E.; Fontana, L. Will calorie restriction work in humans? Aging 2013, 5, 507–514. [Google Scholar] [CrossRef]
- de Magalhaes, J.P. The scientific quest for lasting youth: Prospects for curing aging. Rejuvenation Res. 2014, 17, 458–467. [Google Scholar] [CrossRef]
- Dirks, A.J.; Leeuwenburgh, C. Caloric restriction in humans: Potential pitfalls and health concerns. Mech. Ageing Dev. 2006, 127, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A time to fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xie, F.; Xiao, Y.; Lu, C.; Zhong, J.; Huang, D.; Chen, J.; Wei, J.; Jiang, Y.; Zhong, T. Metformin: A potential candidate for targeting aging mechanisms. Aging Dis. 2021, 12, 480–493. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wang, S. The role of rapamycin in healthspan extension via the delay of organ aging. Ageing Res. Rev. 2021, 70, 101376. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as anti-aging therapy: Is it for everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef]
- Martel, J.; Ko, Y.F.; Liau, J.C.; Lee, C.S.; Ojcius, D.M.; Lai, H.C.; Young, J.D. Myths and realities surrounding the mysterious caterpillar fungus. Trends Biotechnol. 2017, 35, 1017–1021. [Google Scholar] [CrossRef]
- Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Chang, C.J.; Lin, C.S.; Lai, H.C.; Young, J.D. Immunomodulatory properties of plants and mushrooms. Trends Pharmacol. Sci. 2017, 38, 967–981. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Kiely, K.M.; Brady, B.; Byles, J. Gender, mental health and ageing. Maturitas 2019, 129, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Cedrone, F.; Catalini, A.; Stacchini, L.; Berselli, N.; Caminiti, M.; Mazza, C.; Cosma, C.; Minutolo, G.; Di Martino, G. The role of gender in the association between mental health and potentially preventable hospitalizations: A single-center retrospective observational study. Int. J. Environ. Res. Public Health 2022, 19, 14691. [Google Scholar] [CrossRef] [PubMed]
- Rinsky-Halivni, L.; Brammli-Greenberg, S.; Christiani, D.C. Ageing workers’ mental health during COVID-19: A multilevel observational study on the association with the work environment, perceived workplace safety and individual factors. BMJ Open 2022, 12, e064590. [Google Scholar] [CrossRef] [PubMed]
- Bockting, W.; Coleman, E.; Deutsch, M.B.; Guillamon, A.; Meyer, I.; Meyer, W., 3rd; Reisner, S.; Sevelius, J.; Ettner, R. Adult development and quality of life of transgender and gender nonconforming people. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 188–197. [Google Scholar] [CrossRef]
- Thomas Tobin, C.S.; Erving, C.L.; Hargrove, T.W.; Satcher, L.A. Is the Black-White mental health paradox consistent across age, gender, and psychiatric disorders? Aging Ment. Health 2022, 26, 196–204. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Y.; Ye, Z.; Xie, J. Descriptive analysis of depression among adolescents in Huangshi, China. BMC Psychiatry 2023, 23, 176. [Google Scholar] [CrossRef]
- Ulep, V.G.T.; Uy, J.; Casas, L.D. What explains the large disparity in child stunting in the Philippines? A decomposition analysis. Public Health Nutr. 2022, 25, 2995–3007. [Google Scholar] [CrossRef]
- Xu, C.; Ganesan, K.; Liu, X.; Ye, Q.; Cheung, Y.; Liu, D.; Zhong, S.; Chen, J. Prognostic value of negative emotions on the incidence of breast cancer: A systematic review and meta-analysis of 129,621 patients with breast cancer. Cancers 2022, 14, 475. [Google Scholar] [CrossRef]
- Lorenzo, E.C.; Kuchel, G.A.; Kuo, C.L.; Moffitt, T.E.; Diniz, B.S. Major depression and the biological hallmarks of aging. Ageing Res. Rev. 2023, 83, 101805. [Google Scholar] [CrossRef]
- Yeap, B.B. Hormonal changes and their impact on cognition and mental health of ageing men. Maturitas 2014, 79, 227–235. [Google Scholar] [CrossRef]
- Barros, L.; Correia, D.M.; Ferreira, I.C.; Baptista, P.; Santos-Buelga, C. Optimization of the determination of tocopherols in Agaricus sp. edible mushrooms by a normal phase liquid chromatographic method. Food Chem. 2008, 110, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Konko, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Kalac, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.; Barros, L.; Abreu, R.M. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.E.; Hernandez-Perez, T.; Paredes-Lopez, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Falch, B.H.; Espevik, T.; Ryan, L.; Stokke, B.T. The cytokine stimulating activity of (1→3)-beta-D-glucans is dependent on the triple helix conformation. Carbohydr. Res. 2000, 329, 587–596. [Google Scholar] [CrossRef]
- Kataoka, K.; Muta, T.; Yamazaki, S.; Takeshige, K. Activation of macrophages by linear (1→3)-beta-D-glucans. Impliations for the recognition of fungi by innate immunity. J. Biol. Chem. 2002, 277, 36825–36831. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M.; Liu, R.; Rahman, M.M. Hericium erinaceus: An edible mushroom with medicinal values. J. Complement. Integr. Med. 2013, 10, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Yvin, J.C. Effects of marine beta-1,3 glucan on immune reactions. Int. Immunopharmacol. 2004, 4, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, B.Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.; Santos-Buelga, C.; Ferreira, I.C. Phenolic, polysaccharidic, and lipidic fractions of mushrooms from northeastern Portugal: Chemical compounds with antioxidant properties. J. Agric. Food Chem. 2012, 60, 4634–4640. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ou, Y.; Yew, T.W.; Liu, J.; Leng, B.; Lin, Z.; Su, Y.; Zhuang, Y.; Lin, J.; Li, X.; et al. Hepatoprotective effects of polysaccharide isolated from Agaricus bisporus industrial wastewater against CCl₄-induced hepatic injury in mice. Int. J. Biol. Macromol. 2016, 82, 678–686. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Wang, W.; Wang, X.; Zhang, C.; Zhang, J.; Jing, H.; Ren, Z.; Gao, Z.; Song, X.; et al. Antioxidant and anti-aging effects of acidic-extractable polysaccharides by Agaricus bisporus. Int. J. Biol. Macromol. 2018, 106, 1297–1306. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, G.; Fang, L.; Wang, L.; Xie, J. The immunostimulatory and anti-tumor activities of polysaccharide from Agaricus bisporus (brown). J. Food Nutr. Res. 2014, 2, 122–126. [Google Scholar] [CrossRef]
- Pires, A.; Ruthes, A.C.; Cadena, S.; Iacomini, M. Cytotoxic effect of a mannogalactoglucan extracted from Agaricus bisporus on HepG2 cells. Carbohydr. Polym. 2017, 170, 33–42. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Alquini, G.; Tadra-Sfeir, M.Z.; Iacomini, M.; Wichers, H.J.; Van Griensven, L.J. Agaricus bisporus and Agaricus brasiliensis (1→6)-β-D-glucans show immunostimulatory activity on human THP-1 derived macrophages. Carbohydr. Polym. 2013, 94, 91–99. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef]
- Govindan, S.; Johnson, E.E.; Christopher, J.; Shanmugam, J.; Thirumalairaj, V.; Gopalan, J. Antioxidant and anti-aging activities of polysaccharides from Calocybe indica var. APK2. Exp. Toxicol. Pathol. 2016, 68, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.T.; Fu, Y.X.; Yang, W.J.; Hu, Q.H.; Zhao, L.Y. Effects of polysaccharides from the base of Flammulina velutipes stipe on growth of murine RAW264.7, B16F10 and L929 cells. Int. J. Biol. Macromol. 2018, 107, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, J.; Zhao, L.; Ma, N.; Fang, Y.; Pei, F.; Mariga, A.M.; Hu, Q. Polysaccharides from Flammulina velutipes improve scopolamine-induced impairment of learning and memory of rats. J. Funct. Foods 2015, 18, 411–422. [Google Scholar] [CrossRef]
- Xu, S.; Dou, Y.; Ye, B.; Wu, Q.; Wang, Y.; Hu, M.; Ma, F.; Rong, X.; Guo, J. Ganoderma lucidum polysaccharides improve insulin sensitivity by regulating inflammatory cytokines and gut microbiota composition in mice. J. Funct. Foods 2017, 38, 545–552. [Google Scholar] [CrossRef]
- Jin, M.; Zhu, Y.; Shao, D.; Zhao, K.; Xu, C.; Li, Q.; Yang, H.; Huang, Q.; Shi, J. Effects of polysaccharide from mycelia of Ganoderma lucidum on intestinal barrier functions of rats. Int. J. Biol. Macromol. 2017, 94, 1–9. [Google Scholar] [CrossRef]
- Yang, G.; Yang, L.; Zhuang, Y.; Qian, X.; Shen, Y. Ganoderma lucidum polysaccharide exerts anti-tumor activity via MAPK pathways in HL-60 acute leukemia cells. J. Recept. Signal Transduct. Res. 2016, 36, 6–13. [Google Scholar] [CrossRef]
- Huang, S.; Mao, J.; Ding, K.; Zhou, Y.; Zeng, X.; Yang, W.; Wang, P.; Zhao, C.; Yao, J.; Xia, P.; et al. Polysaccharides from Ganoderma lucidum promote cognitive function and neural progenitor proliferation in mouse model of Alzheimer’s disease. Stem Cell Rep. 2017, 8, 84–94. [Google Scholar] [CrossRef]
- Ya, G. A Lentinus edodes polysaccharide induces mitochondrial-mediated apoptosis in human cervical carcinoma HeLa cells. Int. J. Biol. Macromol. 2017, 103, 676–682. [Google Scholar] [CrossRef]
- Ren, Z.; Li, J.; Song, X.; Zhang, J.; Wang, W.; Wang, X.; Gao, Z.; Jing, H.; Li, S.; Jia, L. The regulation of inflammation and oxidative status against lung injury of residue polysaccharides by Lentinula edodes. Int. J. Biol. Macromol. 2018, 106, 185–192. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Huang, X.; Liu, Y.; Li, Q.; Zheng, Z.; Wang, K. A polysaccharide from Lentinus edodes inhibits human colon cancer cell proliferation and suppresses tumor growth in athymic nude mice. Oncotarget 2017, 8, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Jeff, I.B.; Fan, E.; Tian, M.; Song, C.; Yan, J.; Zhou, Y. In vivo anticancer and immunomodulating activities of mannogalactoglucan-type polysaccharides from Lentinus edodes (Berkeley) Singer. Cent. Eur. J. Immunol. 2016, 41, 47–53. [Google Scholar] [CrossRef]
- Xu, H.; Zou, S.; Xu, X.; Zhang, L. Anti-tumor effect of β-glucan from Lentinus edodes and the underlying mechanism. Sci. Rep. 2016, 6, 288–302. [Google Scholar] [CrossRef]
- Carneiro, A.A.; Ferreira, I.C.; Dueñas, M.; Barros, L.; da Silva, R.; Gomes, E.; Santos-Buelga, C. Chemical composition and antioxidant activity of dried powder formulations of Agaricus blazei and Lentinus edodes. Food Chem. 2013, 138, 2168–2173. [Google Scholar] [CrossRef]
- Ren, D.; Wang, N.; Guo, J.; Yuan, L.; Yang, X. Chemical characterization of Pleurotus eryngii polysaccharide and its tumor-inhibitory effects against human hepatoblastoma HepG-2 cells. Carbohydr. Polym. 2016, 138, 123–133. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Mariga, A.M.; Fang, Y.; Ma, N.; Pei, F.; Hu, Q. Purification, characterization and antitumor activity of polysaccharides from Pleurotus eryngii residue. Carbohydr. Polym. 2014, 114, 297–305. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Zheng, W.; Gao, Y.; Wang, M.; Zhang, Y.; Gao, Q. Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 2016, 92, 30–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Jin, G.; Yang, X.; Zhang, Y. Polysaccharides from Pleurotus ostreatus alleviate cognitive impairment in a rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 92, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Jin, G.; Yang, X.; Zhou, H. Regulating dyslipidemia effect of polysaccharides from Pleurotus ostreatus on fat-emulsion-induced hyperlipidemia rats. Int. J. Biol. Macromol. 2017, 101, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Liu, J.L.; Yang, W.; Hou, X.; Li, Q.J. Antitumor activity of polysaccharide extracted from Pleurotus ostreatus mycelia against gastric cancer in vitro and in vivo. Mol. Med. Rep. 2015, 12, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar] [CrossRef]
- Chang, H.H.; Sheu, F. Anti-tumor mechanisms of orally administered a fungal immunomodulatory protein from Flammulina velutipes in mice. FASEB J. 2006, 20, 297–306. [Google Scholar] [CrossRef]
- Lin, C.H.; Sheu, G.T.; Lin, Y.W.; Yeh, C.S.; Huang, Y.H.; Lai, Y.C.; Chang, J.G.; Ko, J.L. A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochem. 2010, 45, 1537–1542. [Google Scholar] [CrossRef]
- Peek, H.W.; Halkes, S.B.A.; Tomassen, M.M.M.; Mes, J.J.; Landman, W.J.M. In vivo screening of five phytochemicals/extracts and a fungal immunomodulatory protein against colibacillosis in broilers. Avian Pathol. 2013, 42, 235–247. [Google Scholar] [CrossRef]
- Lin, W.H.; Hung, C.H.; Hsu, C.-N.; Lin, J.Y. Dimerization of the N-terminal amphipathic helix domain of the fungal immunomodulatory protein from Ganoderma tsugae defined by a yeast two-hybrid system and site-directed mutagenesis. J. Biol. Chem. 1997, 272, 20044–20048. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Yeh, C.H.; Sheu, F. A novel immunomodulatory protein from Poria cocos induces toll-like receptor 4-dependent activation within mouse peritoneal macrophages. J. Agric. Food Chem. 2009, 57, 6129–6139. [Google Scholar] [CrossRef]
- Ditamo, Y.; Rupil, L.L.; Sendra, V.G.; Nores, G.A.; Roth, G.A.; Irazoqui, F.J. In vivo immunomodulatory effect of the lectin from edible mushroom Agaricus bisporus. Food Funct. 2016, 7, 262–279. [Google Scholar] [CrossRef]
- Matuszewska, A.; Karp, M.; Jaszek, M.; Janusz, G.; Osińska-Jaroszuk, M.; Sulej, J.; Stefaniuk, D.; Tomczak, W.; Giannopoulos, K. Laccase purified from Cerrena unicolor exerts antitumor activity against leukemic cells. Oncol. Lett. 2016, 11, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Rong, C.B.; Kong, C.; Liu, Y.; Xu, F.; Miao, Q.J.; Wang, S.X.; Wang, H.X.; Zhang, G.Q. A novel laccase with potent antiproliferative and HIV-1 reverse transcriptase inhibitory activities from mycelia of mushroom Coprinus comatus. Biomed. Res. Int. 2014, 2014, 417461. [Google Scholar] [CrossRef]
- Chu, P.Y.; Sun, H.L.; Ko, J.L.; Ku, M.S.; Lin, L.J.; Lee, Y.T.; Liao, P.F.; Pan, H.H.; Lu, H.L.; Lue, K.H. Oral fungal immunomodulatory protein-Flammulina velutipes has influence on pulmonary inflammatory process and potential treatment for allergic airway disease: A mouse model. J. Microbiol. Immunol. Infect. 2017, 50, 297–306. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 2001, 68, 2151–2168. [Google Scholar] [CrossRef]
- Kumaran, S.; Pandurangan, A.K.; Shenbhagaraman, R.; Esa, N.M. Isolation and characterization of lectin from the Artist’s Conk medicinal mushroom, Ganoderma applanatum (Agaricomycetes), and evaluation of its antiproliferative activity in HT-29 colon cancer cells. Int. J. Med. Mushrooms 2017, 19, 675–684. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. A laccase from the medicinal mushroom Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2006, 72, 508–513. [Google Scholar] [CrossRef]
- Hsin, I.L.; Wang, S.C.; Li, J.R.; Ciou, T.C.; Wu, C.H.; Wu, H.M.; Ko, J.L. Immunomodulatory proteins FIP-gts and chloroquine induce caspase-independent cell death via autophagy for resensitizing cisplatin-resistant urothelial cancer cells. Phytomedicine 2016, 23, 1566–1573. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Hypsin, a novel thermostable ribosome-inactivating protein with antifungal and antiproliferative activities from fruiting bodies of the edible mushroom Hypsizigus marmoreus. Biochem. Biophys. Res. Commun. 2001, 285, 1071–1085. [Google Scholar] [CrossRef]
- Wong, J.H.; Wang, H.X.; Ng, T.B. Marmorin, a new ribosome inactivating protein with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from the mushroom Hypsizigus marmoreus. Appl. Microbiol. Biotechnol. 2008, 81, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, Q.J.; Zhu, M.-U.; Wang, H.X.; Zhang, G.Q. An extracellular laccase with antiproliferative activity from the sanghuang mushroom Inonotus baumii. J. Mol. Catal. B Enzym. 2014, 99, 20–25. [Google Scholar] [CrossRef]
- Žurga, S.; Nanut, M.P.; Kos, J.; Sabotič, J. Fungal lectin MpL enables entry of protein drugs into cancer cells and their subcellular targeting. Oncotarget 2017, 8, 26896–26910. [Google Scholar] [CrossRef]
- Wu, X.; Huang, C.; Chen, Q.; Wang, H.; Zhang, J. A novel laccase with inhibitory activity towards HIV-I reverse transcriptase and antiproliferative effects on tumor cells from the fermentation broth of mushroom Pleurotus cornucopiae. Biomed. Chromatogr. 2014, 28, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Ng, T.B. Purification of a laccase from fruiting bodies of the mushroom Pleurotus eryngii. Appl. Microbiol. Biotechnol. 2006, 69, 521–535. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Su, D.; Liu, Q.; Gao, W.; Kang, Y. Mushroom lectin overcomes hepatitis B virus tolerance via TLR6 signaling. Sci. Rep. 2017, 7, 5814. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, G.; Lee, Y.C.; Park, H.; Wu, Y.; Shin, H.J. Antibacterial and antifungal activities of lectin extracted from fruiting bodies of the Korean cauliflower medicinal mushroom, Sparassis latifolia (Agaricomycetes). Int. J. Med. Mushrooms 2016, 18, 291–309. [Google Scholar] [CrossRef]
- May, M.J.; Hartley, M.R.; Roberts, L.M.; Krieg, P.A.; Osborn, R.W.; Lord, J.M. Ribosome inactivation by ricin A chain: A sensitive method to assess the activity of wild-type and mutant polypeptides. EMBO J. 1989, 8, 301–308. [Google Scholar] [CrossRef]
- Zhou, K.; Fu, Z.; Chen, M.; Lin, Y.; Pan, K. Structure of trichosanthin at 1.88 Å resolution. Proteins Struct. Funct. Bioinform. 1994, 19, 4–13. [Google Scholar] [CrossRef]
- Zhu, F.; Zhou, Y.K.; Ji, Z.L.; Chen, X.R. The plant ribosome-inactivating proteins play important roles in defense against pathogens and insect pest attacks. Front. Plant Sci. 2018, 9, 146. [Google Scholar] [CrossRef]
- Domashevskiy, A.V.; Goss, D.J. Pokeweed antiviral protein, a ribosome inactivating protein: Activity, inhibition and prospects. Toxins 2015, 7, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Lam, J.S.; Wong, J.H.; Lam, S.K.; Ngai, P.H.; Wang, H.X.; Chu, K.T.; Chan, W.Y. Differential abilities of the mushroom ribosome-inactivating proteins hypsin and velutin to perturb normal development of cultured mouse embryos. Toxicol. In Vitro 2010, 24, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Sande, D.; Oliveira, G.P.d.; Moura, M.A.F.E.; Martins, B.d.A.; Lima, M.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Günç Ergönül, P.; Akata, I.; Kalyoncu, F.; Ergönül, B. Fatty acid compositions of six wild edible mushroom species. Sci. World J. 2013, 2013, 163964. [Google Scholar] [CrossRef] [PubMed]
- Sinanoglou, V.J.; Zoumpoulakis, P.; Heropoulos, G.; Proestos, C.; Ćirić, A.; Petrovic, J.; Glamoclija, J.; Sokovic, M. Lipid and fatty acid profile of the edible fungus Laetiporus sulphurous. Antifungal and antibacterial properties. J. Food Sci. Technol. 2015, 52, 3264–3272. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Nimrichter, L.; Oliveira, D.L.; Frases, S.; Miranda, K.; Zaragoza, O.; Alvarez, M.; Nakouzi, A.; Feldmesser, M.; Casadevall, A. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell 2007, 6, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D’Arrigo, M.; Martínez, J. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef]
- Weng, Y.; Xiang, L.; Matsuura, A.; Zhang, Y.; Huang, Q.; Qi, J. Ganodermasides A and B, two novel anti-aging ergosterols from spores of a medicinal mushroom Ganoderma lucidum on yeast via UTH1 gene. Bioorg. Med. Chem. 2010, 18, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Saiki, P.; Kawano, Y.; Griensven, L.; Miyazaki, K. The anti-inflammatory effect of Agaricus brasiliensis is partly due to its linoleic acid content. Food Funct. 2017, 8, 4150–4158. [Google Scholar] [CrossRef] [PubMed]
- Ergon, P.; Ergonul, B.; Kalyoncu, F.; Akata, I. Fatty acid compositions of five wild edible mushroom species collected from Turkey. Int. J. Pharmacol. 2012, 8, 463–466. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Critic. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.; Zielinski, A.A.F.; Helm, C.V.; Maciel, G.M.; Pedro, A.C.; Stafussa, A.P.; Ávila, S.; Haminiuk, C.W.I. Bio compounds of edible mushrooms: In vitro antioxidant and antimicrobial activities. LWT 2019, 107, 214–220. [Google Scholar] [CrossRef]
- Contato, A.G.; Inácio, F.D.; de Araújo, C.A.V.; Brugnari, T.; Maciel, G.M.; Haminiuk, C.W.I.; Bracht, A.; Peralta, R.M.; de Souza, C.G.M. Comparison between the aqueous extracts of mycelium and basidioma of the edible mushroom Pleurotus pulmonarius: Chemical composition and antioxidant analysis. J. Food Meas. Charact. 2020, 14, 830–837. [Google Scholar] [CrossRef]
- Mutukwa, I.B.; Hall, C.A., III; Cihacek, L.; Lee, C.W. Evaluation of drying method and pretreatment effects on the nutritional and antioxidant properties of oyster mushroom (Pleurotus ostreatus). J. Food Process. Preserv. 2019, 43, e13910. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Sarikurkcu, C.; Yalcin, O.U.; Cengiz, M.; Gungor, H. Metal concentration, phenolics profiling, and antioxidant activity of two wild edible Melanoleuca mushrooms (M. cognata and M. stridula). Microchem. J. 2019, 150, 104172. [Google Scholar] [CrossRef]
- Hwang, A.Y.; Yang, S.C.; Kim, J.; Lim, T.; Cho, H.; Hwang, K.T. Effects of non-traditional extraction methods on extracting bioactive compounds from chaga mushroom (Inonotus obliquus) compared with hot water extraction. LWT 2019, 110, 80–84. [Google Scholar] [CrossRef]
- Stojkovic, D.; Smiljkovic, M.; Ciric, A.; Glamoclija, J.; Griensven, L.V.; Ferreira, I.; Sokovic, M. An insight into antidiabetic properties of six medicinal and edible mushrooms: Inhibition of α-amylase and α-glucosidase linked to type-2 diabetes. S. Afr. J. Bot. 2019, 120, 100–103. [Google Scholar] [CrossRef]
- Lin, S.; Ching, L.T.; Lam, K.; Cheung, P.C.K. Anti-angiogenic effect of water extract from the fruiting body of Agrocybe aegerita. LWT 2017, 75, 155–163. [Google Scholar] [CrossRef]
- Alam, N.; Sikder, M.M.; Karim, M.A.; Amin, S. Antioxidant and antityrosinase activities of milky white mushroom. Bangladesh J. Bot. 2019, 48, 1065–1073. [Google Scholar] [CrossRef]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Veljović, S.; Veljović, M.; Nikićević, N.; Despotović, S.; Radulović, S.; Nikšić, M.; Filipović, L. Chemical composition, antiproliferative and antioxidant activity of differently processed Ganoderma lucidum ethanol extracts. J. Food Sci. Technol. 2017, 54, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, M.; Siwulski, M.; Mleczek, M. Evaluation of bioactive compounds content and antioxidant properties of soil-growing and wood-growing edible mushrooms. J. Food Process. Preserv. 2018, 42, e13386. [Google Scholar] [CrossRef]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Lumyong, S. Phenolic profile of various wild edible mushroom extracts from Thailand and their antioxidant properties, anti-tyrosinase and hyperglycaemic inhibitory activities. J. Funct. Foods 2016, 27, 352–364. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Xu, B. Phenolic profiles, antioxidant capacities and metal chelating ability of edible mushrooms commonly consumed in China. LWT 2016, 72, 423–431. [Google Scholar] [CrossRef]
- Alkan, S.; Uysal, A.; Kasik, G.; Vlaisavljevic, S.; Berežni, S.; Zengin, G. Chemical characterization, antioxidant, enzyme inhibition and antimutagenic properties of eight mushroom species: A comparative study. J. Fungi 2020, 6, 166. [Google Scholar] [CrossRef]
- de Souza Campos Junior, F.A.; Petrarca, M.H.; Meinhart, A.D.; de Jesus Filho, M.; Godoy, H.T. Multivariate optimization of extraction and validation of phenolic acids in edible mushrooms by capillary electrophoresis. Food Res. Int. 2019, 126, 108685. [Google Scholar] [CrossRef]
- Souilem, F.; Fernandes, Â.; Calhelha, R.C.; Barreira, J.C.M.; Barros, L.; Skhiri, F.; Martins, A.; Ferreira, I.C.F.R. Wild mushrooms and their mycelia as sources of bioactive compounds: Antioxidant, anti-inflammatory and cytotoxic properties. Food Chem. 2017, 230, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Nowacka-Jechalke, N.; Olech, M.; Nowak, R. Chapter 11—Mushroom polyphenols as chemopreventive agents. In Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 137–150. [Google Scholar]
- Gogoi, P.; Chutia, P.; Singh, P.; Mahanta, C.L. Effect of optimized ultrasound-assisted aqueous and ethanolic extraction of Pleurotus citrinopileatus mushroom on total phenol, flavonoids and antioxidant properties. J. Food Proc. Engin. 2019, 42, e13172. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sun, J.; Luo, Z.Y.; Rao, S.Q.; Su, Y.J.; Xu, R.R.; Yang, Y.J. Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef]
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M.E. Identification and quantification of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J. Food Measu. Charact. 2020, 14, 1690–1698. [Google Scholar] [CrossRef]
- Yahia, E.M.; Gutiérrez-Orozco, F.; Moreno-Pérez, M.A. Identification of phenolic compounds by liquid chromatography-mass spectrometry in seventeen species of wild mushrooms in Central Mexico and determination of their antioxidant activity and bioactive compounds. Food Chem. 2017, 226, 14–22. [Google Scholar] [CrossRef]
- Akindahunsi, A.A.; Oyetayo, F.L. Nutrient and antinutrient distribution of edible mushroom, Pleurotus tuber-regium (fries) singer. LWT 2006, 39, 548–553. [Google Scholar] [CrossRef]
- Garrab, M.; Edziri, H.; El Mokni, R.; Mastouri, M.; Mabrouk, H.; Douki, W. Phenolic composition, antioxidant and anticholinesterase properties of the three mushrooms Agaricus silvaticus Schaeff., Hydnum rufescens Pers. and Meripilus giganteus (Pers.) Karst. in Tunisia. S. Afr. J. Bot. 2019, 124, 359–363. [Google Scholar] [CrossRef]
- Pavithra, M.; Sridhar, K.R.; Greeshma, A.A.; Tomita-Yokotani, K. Bioactive potential of the wild mushroom Astraeus hygrometricus in South-west India. Mycology 2016, 7, 191–202. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef]
- Jayakumar, T.; Thomas, P.A.; Geraldine, P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innov. Food Sci. Emerg. Technol. 2009, 10, 228–234. [Google Scholar] [CrossRef]
- Wu, Y.; Moon-Hee, C.; Li, J.; Yang, H.; Hyun-Jae, S. Mushroom cosmetics: The present and future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Hyde, K.D.; Bahkali, A.H.; Moslem, M.A. Fungi—An unusual source for cosmetics. Fungal Divers. 2010, 43, 1–9. [Google Scholar] [CrossRef]
- Cheung, L.M.; Cheung, P.; Ooi, V. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Dubost, N.J.; Beelman, R.B.; Peterson, D.; Royse, D.J. Identification and quantification of ergothioneine in cultivated mushrooms by liquid chromatography-mass spectroscopy. Int. J. Med. Mush. 2006, 8, 215–222. [Google Scholar] [CrossRef]
- Maity, K.; Kar Mandal, E.; Maity, S.; Gantait, S.K.; Das, D.; Maiti, S.; Maiti, T.K.; Sikdar, S.R.; Islam, S.S. Structural characterization and study of immunoenhancing and antioxidant property of a novel polysaccharide isolated from the aqueous extract of a somatic hybrid mushroom of Pleurotus florida and Calocybe indica variety APK2. Int. J. Biol. Macromol. 2011, 48, 304–310. [Google Scholar] [CrossRef]
- Yuan, C.; Huang, X.; Cheng, L.; Bu, Y.; Liu, G.; Yi, F.; Yang, Z.; Song, F. Evaluation of antioxidant and immune activity of Phellinus ribis glucan in mice. Food Chem. 2009, 115, 581–584. [Google Scholar] [CrossRef]
- Sun, J.; He, H.; Xie, B.J. Novel antioxidant peptides from fermented mushroom Ganoderma lucidum. J. Agric. Food Chem. 2004, 52, 6646–6652. [Google Scholar] [CrossRef]
- Lupo, M.P.; Cole, A.L. Cosmeceutical peptides. Dermatol. Ther. 2007, 20, 343–359. [Google Scholar] [CrossRef]
- Kim, S.Y.; Go, K.C.; Song, Y.S.; Jeong, Y.S.; Kim, E.J.; Kim, B.J. Extract of the mycelium of T. matsutake inhibits elastase activity and TPA-induced MMP-1 expression in human fibroblasts. Int. J. Mol. Med. 2014, 34, 1613–1621. [Google Scholar] [CrossRef]
- Stanikunaite, R.; Khan, S.I.; Trappe, J.M.; Ross, S.A. Cyclooxygenase-2 inhibitory and antioxidant compounds from the truffle Elaphomyces granulatus. Phytother. Res. 2009, 23, 575–588. [Google Scholar] [CrossRef]
- Shahid, A.; Huang, M.; Liu, M.; Shamim, M.A.; Parsa, C.; Orlando, R.; Huang, Y. The medicinal mushroom Ganoderma lucidum attenuates UV-induced skin carcinogenesis and immunosuppression. PLoS ONE 2022, 17, e0265615. [Google Scholar] [CrossRef]
- Thangthaeng, N.; Miller, M.G.; Gomes, S.M.; Shukitt-Hale, B. Daily supplementation with mushroom (Agaricus bisporus) improves balance and working memory in aged rats. Nutr. Res. 2015, 35, 1079–1084. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Lin, J.; Lim, S.; Cao, Y.; Chen, S.; Xu, P.; Xu, C.; Zheng, H.; Fu, K.C.; et al. Structure and anti-inflammatory activity relationship of ergostanes and lanostanes in Antrodia cinnamomea. Foods 2022, 11, 1831. [Google Scholar] [CrossRef]
- Calvo, M.S.; Mehrotra, A.; Beelman, R.B.; Nadkarni, G.; Wang, L.; Cai, W.; Goh, B.C.; Kalaras, M.D.; Uribarri, J. A retrospective study in adults with metabolic syndrome: Diabetic risk factor response to daily consumption of Agaricus bisporus (white button mushrooms). Plant Foods Hum. Nutr. 2016, 71, 245–251. [Google Scholar] [CrossRef]
- Song, T.Y.; Yang, N.C.; Chen, C.L.; Thi, T.L.V. Protective effects and possible mechanisms of ergothioneine and hispidin against methylglyoxal-induced injuries in rat pheochromocytoma cells. Oxid. Med. Cell. Longev. 2017, 2017, 4824371. [Google Scholar] [CrossRef]
- Hagiwara, S.-Y.; Takahashi, M.; Shen, Y.; Kaihou, S.; Tomiyama, T.; Yazawa, M.; Tamai, Y.; Sin, Y.; Kazusaka, A.; Terazawa, M. A phytochemical in the edible tamogitake mushroom (Pleurotus cornucopiae), D-mannitol, inhibits ACE activity and lowers the blood pressure of spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2005, 69, 1603–1605. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal mushrooms: Their bioactive components, nutritional value and application in functional food production—A review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Seweryn, E.; Ziała, A.; Gamian, A. Health-promoting of polysaccharides extracted from Ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Togashi, Y. Aging, cancer, and antitumor immunity. Int. J. Clin. Oncol. 2022, 27, 316–322. [Google Scholar] [CrossRef]
- Johnson, E.; Førland, D.T.; Sætre, L.; Bernardshaw, S.V.; Lyberg, T.; Hetland, G. Effect of an extract based on the medicinal mushroom Agaricus blazei Murill on release of cytokines, chemokines and leukocyte growth factors in human blood ex vivo and in vivo. Scand. J. Immunol. 2009, 69, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Roupas, P.; Keogh, J.; Noakes, M.; Margetts, C.; Taylor, P. The role of edible mushrooms in health: Evaluation of the evidence. J. Funct. Foods 2012, 4, 687–709. [Google Scholar] [CrossRef]
- Pandimeena, M.; Prabu, M.; Sumathy, R.; Kumuthakalavalli, R. Evaluation of phytochemicals and in vitro anti-inflammatiry, anti-diabetic activity of the white oyster mushroom, Pleurotus florida. Int. Res. J. Pharm. Appl. Sci. 2015, 5, 16–21. [Google Scholar]
- Tanaka, A.; Nishimura, M.; Sato, Y.; Sato, H.; Nishihira, J. Enhancement of the Th1-phenotype immune system by the intake of Oyster mushroom (Tamogitake) extract in a double-blind, placebo-controlled study. J. Tradit. Complement. Med. 2016, 6, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Shao, J.; Wu, B.; Li, B. Potential immunomodulatory activities of a lectin from the mushroom Latiporus sulphureus. Int. J. Biol. Macromol. 2019, 130, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wan, Y.Z.; Qiao, C.X.; Xu, X.F.; Shen, Y. Immunoregenerative effects of the bionically cultured Sanghuang mushrooms (Inonotus sanghuagn) on the immunodeficient mice. J. Ethnopharmacol. 2019, 245, 112047. [Google Scholar] [CrossRef] [PubMed]
- Vlassopoulou, M.; Paschalidis, N.; Savvides, A.L.; Saxami, G.; Mitsou, E.K.; Kerezoudi, E.N.; Koutrotsios, G.; Zervakis, G.I.; Georgiadis, P.; Kyriacou, A.; et al. Immunomodulating activity of Pleurotus eryngii mushrooms following their in vitro fermentation by human fecal microbiota. J. Fungi 2022, 8, 329. [Google Scholar] [CrossRef]
- Boulaka, A.; Mantellou, P.; Stanc, G.-M.; Souka, E.; Valavanis, C.; Saxami, G.; Mitsou, E.; Koutrotsios, G.; Zervakis, G.I.; Kyriacou, A.; et al. Genoprotective activity of the Pleurotus eryngii mushrooms following their in vitro and in vivo fermentation by fecal microbiota. Front. Nutr. 2022, 9, 988517. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A "set up" for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef]
- Adachi, K.; Nanba, H.; Otsuka, M.; Kuroda, H. Blood pressure-lowering activity present in the fruit body of Grifola frondosa (maitake). I. Chem. Pharm. Bull. 1988, 36, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Tian, G.; Zhang, W.; Zhao, Y.; Zhao, L.; Wang, H.; Ng, T.B. A Tricholoma matsutake peptide with angiotensin converting enzyme inhibitory and antioxidative activities and antihypertensive effects in spontaneously hypertensive rats. Sci. Rep. 2016, 6, 24130. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich lentils and their health promoting effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-obesity effects of medicinal and edible mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef] [PubMed]
- Fombang, E.; Ee, L. Pleurotus florida aqueous extracts and powder influence lipid profile and suppress weight gain in rats fed high cholesterol diet. J. Nutr. Food Sci. 2016, 6, 2. [Google Scholar] [CrossRef]
- Mori, K.; Kobayashi, C.; Tomita, T.; Inatomi, S.; Ikeda, M. Antiatherosclerotic effect of the edible mushrooms Pleurotus eryngii (Eringi), Grifola frondosa (Maitake), and Hypsizygus marmoreus (Bunashimeji) in apolipoprotein E-deficient mice. Nutr. Res. 2008, 28, 335–342. [Google Scholar] [CrossRef]
- Koh, J.H.; Kim, J.M.; Chang, U.J.; Suh, H.J. Hypocholesterolemic effect of hot-water extract from mycelia of Cordyceps sinensis. Biol. Pharm. Bull. 2003, 26, 84–87. [Google Scholar] [CrossRef]
- de Sá-Nakanishi, A.B.; Soares, A.A.; Natali, M.R.; Comar, J.F.; Peralta, R.M.; Bracht, A. Effects of the continuous administration of an Agaricus blazei extract to rats on oxidative parameters of the brain and liver during aging. Molecules 2014, 19, 18590–18603. [Google Scholar] [CrossRef]
- Sillapachaiyaporn, C.; Rangsinth, P.; Nilkhet, S.; Ung, A.T.; Chuchawankul, S.; Tencomnao, T. Neuroprotective effects against glutamate-induced HT-22 hippocampal cell damage and Caenorhabditis elegans lifespan/healthspan enhancing activity of Auricularia polytricha mushroom extracts. Pharmaceuticals 2021, 14, 1001. [Google Scholar] [CrossRef]
- Kushairi, N.; Phan, C.W.; Sabaratnam, V.; David, P.; Naidu, M. Lion’s mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. suppresses H2O2-induced oxidative damage and LPS-induced inflammation in HT22 hippocampal neurons and BV2 microglia. Antioxidants 2019, 8, 261. [Google Scholar] [CrossRef]
- Tripodi, F.; Falletta, E.; Leri, M.; Angeloni, C.; Beghelli, D.; Giusti, L.; Milanesi, R.; Sampaio-Marques, B.; Ludovico, P.; Goppa, L.; et al. Anti-aging and neuroprotective properties of Grifola frondosa and Hericium erinaceus extracts. Nutrients 2022, 14, 4368. [Google Scholar] [CrossRef] [PubMed]
- Yanshree; Yu, W.S.; Fung, M.L.; Lee, C.W.; Lim, L.W.; Wong, K.H. The monkey head mushroom and memory enhancement in Alzheimer’s disease. Cells 2022, 11, 2284. [Google Scholar] [CrossRef]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium erinaceus improves recognition memory and induces hippocampal and cerebellar neurogenesis in frail mice during aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, X.; Liu, W.; Lu, Y.; Cheng, J.; Chen, Y. Beta cell aging and age-related diabetes. Aging 2021, 13, 7691–7706. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Hwang, H.J.; Kim, S.W.; Oh, J.Y.; Baek, Y.M.; Choi, J.W.; Bae, S.H.; Yun, J.W. Hypoglycemic effects of exopolysaccharides produced by mycelial cultures of two different mushrooms Tremella fuciformis and Phellinus baumii in ob/ob mice. Appl. Microbiol. Biotechnol. 2007, 75, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.C.; Jeong, Y.T.; Yang, B.K.; Islam, R.; Koyyalamudi, S.R.; Pang, G.; Cho, K.Y.; Song, C.H. White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr. Res. 2010, 30, 49–56. [Google Scholar] [CrossRef]
- Kim, H.M.; Kang, J.S.; Kim, J.Y.; Park, S.K.; Kim, H.S.; Lee, Y.J.; Yun, J.; Hong, J.T.; Kim, Y.; Han, S.B. Evaluation of antidiabetic activity of polysaccharide isolated from Phellinus linteus in non-obese diabetic mouse. Int. Immunopharmacol. 2010, 10, 72–78. [Google Scholar] [CrossRef]
- Oh, T.W.; Kim, Y.A.; Jang, W.J.; Byeon, J.I.; Ryu, C.H.; Kim, J.O.; Ha, Y.L. Semipurified fractions from the submerged-culture broth of Agaricus blazei Murill reduce blood glucose levels in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2010, 58, 4113–4129. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Li, C.; Qi, P.; Bao, J. Agaricus bisporus lectins mediates islet β-cell proliferation through regulation of cell cycle proteins. Exp. Biol. Med. 2012, 237, 287–296. [Google Scholar] [CrossRef]
- Guggenheim, A.G.; Wright, K.M.; Zwickey, H.L. Immune modulation from five major mushrooms: Application to integrative oncology. Integr. Med. 2014, 13, 32–44. [Google Scholar]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Jeitler, M.; Michalsen, A.; Frings, D.; Hübner, M.; Kessler, C.S. Significance of medicinal mushrooms in integrative oncology: A narrative review. Front. Pharmacol. 2020, 11, 580656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Chen, A.F.; Lin, Z.B. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J. Ethnopharmacol. 2007, 111, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hsiao, Y.M.; Ou, C.C.; Lin, Y.W.; Chiu, Y.L.; Lue, K.H.; Chang, J.G.; Ko, J.L. GMI, a Ganoderma immunomodulatory protein, down-regulates tumor necrosis factor α-induced expression of matrix metalloproteinase 9 via NF-κB pathway in human alveolar epithelial A549 cells. J. Agric. Food Chem. 2010, 58, 12014–12021. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Jayachandran, M.; Xu, B. A critical review on hepatoprotective effects of bioactive food components. Crit. Rev. Food Sci. Nutr. 2018, 58, 1165–1229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jayachandran, M.; Ganesan, K.; Xu, B. The black truffle, Tuber melanosporum (Ascomycetes), ameliorates hyperglycemia and regulates insulin signaling pathway in STZ-induced diabetic rats. Int. J. Med. Mushrooms 2020, 22, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017, 97, 228–237. [Google Scholar] [CrossRef]
- Jin, X.; Ruiz Beguerie, J.; Sze, D.M.; Chan, G.C. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst. Rev. 2016, 4, Cd007731. [Google Scholar] [CrossRef]
- Shah, S.K.; Walker, P.A.; Moore-Olufemi, S.D.; Sundaresan, A.; Kulkarni, A.D.; Andrassy, R.J. An evidence-based review of a Lentinula edodes mushroom extract as complementary therapy in the surgical oncology patient. JPEN J. Parenter. Enteral Nutr. 2011, 35, 449–458. [Google Scholar] [CrossRef]
- Gong, P.; Wang, X.; Liu, M.; Wang, M.; Wang, S.; Guo, Y.; Chang, X.; Yang, W.; Chen, X.; Chen, F. Hypoglycemic effect of a novel polysaccharide from Lentinus edodes on STZ-induced diabetic mice via metabolomics study and Nrf2/HO-1 pathway. Food Funct. 2022, 13, 3036–3049. [Google Scholar] [CrossRef]
- Saretzki, G. Telomeres, telomerase and ageing. Subcell. Biochem. 2018, 90, 221–308. [Google Scholar] [CrossRef] [PubMed]
- Bernardes de Jesus, B.; Blasco, M.A. Telomerase at the intersection of cancer and aging. Trends Genet. 2013, 29, 513–520. [Google Scholar] [CrossRef]
- Yuen, J.W.; Gohel, M.D.; Au, D.W. Telomerase-associated apoptotic events by mushroom Ganoderma lucidum on premalignant human urothelial cells. Nutr. Cancer 2008, 60, 109–119. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase inhibitors from natural products and their anticancer potential. Int. J. Mol. Sci. 2017, 19, 13. [Google Scholar] [CrossRef]
- Xu, B.; Li, C.; Sung, C. Telomerase inhibitory effects of medicinal mushrooms and lichens, and their anticancer activity. Int. J. Med. Mushrooms 2014, 16, 17–28. [Google Scholar] [CrossRef]
- Sukalingam, K.; Ganesan, K.; Xu, B. Trianthema portulacastrum L. (giant pigweed): Phytochemistry and pharmacological properties. Phytochem. Rev. 2017, 16, 461–478. [Google Scholar] [CrossRef]
- Jayasuriya, R.; Dhamodharan, U.; Ali, D.; Ganesan, K.; Xu, B.; Ramkumar, K.M. Targeting Nrf2/Keap1 signaling pathway by bioactive natural agents: Possible therapeutic strategy to combat liver disease. Phytomedicine 2021, 92, 153755. [Google Scholar] [CrossRef]
- Sakshi, S.; Jayasuriya, R.; Ganesan, K.; Xu, B.; Ramkumar, K.M. Role of circRNA-miRNA-mRNA interaction network in diabetes and its associated complications. Mol. Ther. Nucleic Acids 2021, 26, 1291–1302. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.A.; Coppotelli, G.; Rolo, A.P.; Palmeira, C.M.; Ross, J.M.; Sinclair, D.A. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial dysfunction in aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Wanasundara, P.K. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, A.E.S.S.; Koehnlein, E.A.; Soares, A.A.; Eler, G.J.; Nakashima, A.T.A.; Bracht, A.; Peralta, R.M. Bioactives of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. LWT 2012, 46, 493–499. [Google Scholar] [CrossRef]
- Ajith, T.A.; Sudheesh, N.P.; Roshny, D.; Abishek, G.; Janardhanan, K.K. Effect of Ganoderma lucidum on the activities of mitochondrial dehydrogenases and complex I and II of electron transport chain in the brain of aged rats. Exp. Gerontol. 2009, 44, 219–223. [Google Scholar] [CrossRef]
- de Sá-Nakanishi, A.B.; Soares, A.A.; de Oliveira, A.L.; Comar, J.F.; Peralta, R.M.; Bracht, A. Effects of treating old rats with an aqueous Agaricus blazei extract on oxidative and functional parameters of the brain tissue and brain mitochondria. Oxid. Med. Cell. Longev. 2014, 2014, 563179. [Google Scholar] [CrossRef]
- Ganesan, K.; Wang, Y.; Gao, F.; Liu, Q.; Zhang, C.; Li, P.; Zhang, J.; Chen, J. Targeting engineered nanoparticles for breast cancer therapy. Pharmaceutics 2021, 13, 1829. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Nair, S.P.; Azalewor, H.G.; Letha, N.; Gani, S.B. Preliminary phytochemical screening and in vitro antioxidant activity of Datura stramonium L. collected from Jimma, South West Ethiopia. Int. J. Pharma Bio Sci. 2016, 7, P261–P266. [Google Scholar]
- Ganesh, G.V.; Ganesan, K.; Xu, B.; Ramkumar, K.M. Nrf2 driven macrophage responses in diverse pathophysiological contexts: Disparate pieces from a shared molecular puzzle. BioFactors 2022, 48, 795–812. [Google Scholar] [CrossRef]
- Nagarajan, S.; Mohandas, S.; Ganesan, K.; Xu, B.; Ramkumar, K.M. New insights into dietary pterostilbene: Sources, metabolism, and health promotion effects. Molecules 2022, 27, 6316. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Ganesan, K.; Xu, B. New insight into mycochemical profiles and antioxidant potential of edible and medicinal mushrooms: A review. Int. J. Med. Mushrooms 2019, 21, 237–251. [Google Scholar] [CrossRef]
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Chung, S.S.M. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. Euro. J. Pharmacol. 2018, 829, 112–120. [Google Scholar] [CrossRef]
- Islam, T.; Ganesan, K.; Xu, B. Insights into health-promoting effects of Jew’s ear (Auricularia auricula-judae). Trends Food Sci. Technol. 2021, 114, 552–569. [Google Scholar] [CrossRef]
- Sukalingam, K.; Ganesan, K.; Das, S.; Thent, Z.C. An insight into the harmful effects of soy protein: A review. Clin. Ter. 2015, 166, 131–139. [Google Scholar] [CrossRef]
- Gurovic, M.S.V.; Viceconte, F.R.; Pereyra, M.T.; Bidegain, M.A.; Cubitto, M.A. DNA damaging potential of Ganoderma lucidum extracts. J. Ethnopharmacol. 2018, 217, 83–88. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.H.; Song, C.H.; Jang, K.J.; Kim, C.H.; Kang, J.S.; Choi, Y.H.; Yoon, H.M. Ethanol extract of Ganoderma lucidum augments ellular anti-oxidant defense through activation of Nrf2/HO-1. J. Pharmacopunct. 2016, 19, 59–69. [Google Scholar] [CrossRef]
- Lin, J.; Lu, Y.Y.; Shi, H.Y.; Lin, P. Chaga medicinal mushroom, Inonotus obliquus (Agaricomycetes), polysaccharides alleviate photoaging by regulating Nrf2 pathway and autophagy. Int. J. Med. Mushrooms 2023, 25, 49–64. [Google Scholar] [CrossRef]
- Sevindik, M.; Akgul, H.; Selamoglu, Z.; Braidy, N. Antioxidant and antigenotoxic potential of Infundibulicybe geotropa mushroom collected from northwestern Turkey. Oxid. Med. Cell. Longev. 2020, 2020, 5620484. [Google Scholar] [CrossRef]
- Lillycrop, K.A.; Hoile, S.P.; Grenfell, L.; Burdge, G.C. DNA methylation, ageing and the influence of early life nutrition. Proc. Nutr. Soc. 2014, 73, 413–421. [Google Scholar] [CrossRef]
- Lau, C.E.; Robinson, O. DNA methylation age as a biomarker for cancer. Int. J. Cancer 2021, 148, 2652–2663. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.L.; Meng, X.; Wang, C.; Dai, W.; Luo, Z.; Yin, Z.; Ju, Z.; Fu, X.; Yang, J.; Ye, Q.; et al. Histone H3K4me3 modification is a transgenerational epigenetic signal for lipid metabolism in Caenorhabditis elegans. Nat. Commun. 2022, 13, 768. [Google Scholar] [CrossRef] [PubMed]
- Ilango, S.; Paital, B.; Jayachandran, P.; Padma, P.R.; Nirmaladevi, R. Epigenetic alterations in cancer. Front. Biosci. 2020, 25, 1058–1109. [Google Scholar] [CrossRef]
- Saul, D.; Kosinsky, R.L. Epigenetics of aging and aging-associated diseases. Int. J. Mol. Sci. 2021, 22, 401. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Guo, Y.; Chen, D.; Tang, X.; Shuai, O.; Yong, T.; Wang, D.; Xiao, C.; Zhou, G.; Xie, Y.; et al. Alcohol extracts from Ganoderma lucidum delay the progress of Alzheimer’s disease by regulating DNA methylation in rodents. Front. Pharmacol. 2019, 10, 272. [Google Scholar] [CrossRef]
- Yi, S.J.; Kim, K. New insights into the role of histone changes in aging. Int. J. Mol. Sci. 2020, 21, 8241. [Google Scholar] [CrossRef] [PubMed]
- Bhukel, A.; Beuschel, C.B.; Maglione, M.; Lehmann, M.; Juhász, G.; Madeo, F.; Sigrist, S.J. Autophagy within the mushroom body protects from synapse aging in a non-cell autonomous manner. Nat. Commun. 2019, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Glück, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Simon, M.; Seluanov, A.; Gorbunova, V. DNA damage and repair in age-related inflammation. Nat. Rev. Immunol. 2023, 23, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Pietrocola, F.; Roiz-Valle, D.; Galluzzi, L.; Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 2023, 35, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Sharmila Banu, G.; Murugesan, A.G.; Rajasekara Pandian, M. Preliminary toxicity and phytochemical studies of aqueous bark extract of Helicteres isora L. Int. J. Pharmacol. 2007, 3, 96–100. [Google Scholar] [CrossRef]
- Sharmila Banu, G.; Kumar, G.; Murugesan, A.G. Ethanolic leaves extract of Trianthema portulacastrum L. ameliorates aflatoxin B1 induced hepatic damage in rats. Indian J. Clin. Biochem. 2009, 24, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Sharmila Banu, G.; Maheswaran, R.; Rema, S.; Rajasekara Pandian, M.; Murugesan, A.G. Effect of Plumbago zeylanica L. on blood glucose and plasma antioxidant status in STZ diabetic rats. J. Nat. Rem. 2007, 7, 66–71. [Google Scholar]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in health, disease, and aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sim, H.A.; Jung, D.Y.; Lim, E.Y.; Kim, Y.T.; Kim, B.J.; Jung, M.H. Poria cocus wolf extract ameliorates hepatic steatosis through regulation of lipid metabolism, inhibition of ER stress, and activation of autophagy via AMPK ctivation. Int. J. Mol. Sci. 2019, 20, 4801. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem. 2021, 358, 129883. [Google Scholar] [CrossRef]
- Yang, T.K.; Lee, Y.H.; Paudel, U.; Bhattarai, G.; Yun, B.S.; Hwang, P.H.; Yi, H.K. Davallialactone from mushroom reduced premature senescence and inflammation on glucose oxidative stress in human diploid fibroblast cells. J. Agric. Food Chem. 2013, 61, 7089–7095. [Google Scholar] [CrossRef]
- Sillapachaiyaporn, C.; Chuchawankul, S.; Nilkhet, S.; Moungkote, N.; Sarachana, T.; Ung, A.T.; Baek, S.J.; Tencomnao, T. Ergosterol isolated from cloud ear mushroom (Auricularia polytricha) attenuates bisphenol A-induced BV2 microglial cell inflammation. Food Res. Int. 2022, 157, 111433. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumor drug in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 263–296. [Google Scholar] [CrossRef] [PubMed]
- Sousa, P.; Tavares-Valente, D.; Amorim, M.; Azevedo-Silva, J.; Pintado, M.; Fernandes, J. β-Glucan extracts as high-value multifunctional ingredients for skin health: A review. Carbohydr. Polym. 2023, 322, 121329. [Google Scholar] [CrossRef] [PubMed]




| Mushrooms | Common Names | Bioactive Compounds | Source and Yield | Bioactivities | References |
|---|---|---|---|---|---|
| Agaricus bisporus | Button mushroom | Heteropolysaccharide Abnp1001, Abnp1002, Abap1001, Abap1002 | Concentrated industrial wastewater of A. bisporus; 0.989 mg/g, 1.849 mg/g, 0.128 mg/g, and 0.68 mg/g (Abnp1001, Abnp1002, Abap1001, Abap1002) | Hepatoprotective | [65] |
| Heteropolysaccharide AcAPS, AcAPS-1, AcAPS-2, AcAPS-3, with rhamnose and glucose as major monosaccharide | Dried fruiting body; yield n.s. | Hepatoprotective, nephroprotective, antioxidative | [66] | ||
| Polysaccharide extracts, main components n.s. | Whole mushroom; yield n.s. | Anti-tumor, immunostimulatory | [67] | ||
| Heteropolysaccharide/Mannogalacoglucan mannose, galactose, glucose | Freeze-dried fresh fruiting body; 41.4% yield (w/w dry weight) | Anti-tumor | [68] | ||
| β-glucan | Dried fresh fruiting body; yield n.s. | Immunostimulatory | [69] | ||
| Fructose, mannitol, trehalose | Fresh fruiting body; 5.79% (white mushroom) & 4.27% (brown mushroom) (w/w fresh weight) | n.s. | [70] | ||
| Calocybe indica | Milky mushroom | Polysaccharide extracts, main components n.s. | Fresh fruiting body; 3.27% (w/w dry weight) | Anti-oxidant, neuroprotective | [71] |
| Flammulina velutipes | Enoki/Golden needle mushroom | Polysaccharide extracts, main components n.s. | Base of stipe; yield n.s. | Anti-tumor | [72] |
| Polysaccharide extracts, main components n.s. | Fresh whole-mushroom; yield n.s. | Neuroprotective | [73] | ||
| Fructose, mannitol, sucrose, trehalose | Fresh fruiting body; 8.29% (w/w fresh weight) | n.s. | [70] | ||
| Ganoderma lucidum | Ling Zhi | Polysaccharide extracts, main components n.s. | Mycelia; 71.99% (w/w dry weight) | Anti-inflammation, ameliorating insulin resistance, suppressing lipid accumulation, regulation of gut microbiota | [74] |
| Polysaccharide extracts, main components n.s. | Commercialized spray dried mycelia; 91.48% (w/w dry weight) | Improving intestinal barrier functions | [75] | ||
| Arabinose, galactose, glucose, xylose | Whole mushroom; yield n.s. | Anti-tumor | [76] | ||
| Polysaccharide extracts, main components n.s. | Dried conidial powder; 2% (w/w dry weight, crude extracts) | Promote cognitive function and neural progenitor proliferation | [77] | ||
| Lentinula edodes | Shiitake mushroom | Glucose, galactose, mannose, arabinose | Fruiting body; 1.3% (w/w dry weight, purified polysaccharide cLEP1) | Therapeutic to cervical carcinoma | [78] |
| Rhamnose | Residue/byproduct; yield n.s. | Anti-inflammatory, anti-oxidant | [79] | ||
| Pyranose, β-d-glucans (β-(1→3)-D-glucose as backbone & β-(1→6)-D-glucose as side chains) | Dried fruiting body; 0.76% (w/w dry weight) | Anti-tumor | [80] | ||
| Mannogalactoglucan-type polysaccharides WPLE-N-2, WPLE-A0.5-2 | Fruiting body; yield n.s. | Anti-cancer, immunomodulatory | [81] | ||
| Lentinan (β-(1,3)-glucan with β-(1,6) branches) | Dried fruiting body (commercial product); 2.6% (w/w dry weight) | Anti-tumor | [82] | ||
| Mannitol, trehalose, arabinose | Dried powder; 23.3% (mannitol), 13.2% (trehalose), 1.79% (arabinose) (w/w dry weight) | n.s. | [83] | ||
| Pleurotus eryngii | King oyster mushroom | Mannose, glucose, galactose | Fresh whole-mushroom; 5.4% (w/w dry weight) | Anti-tumor | [84] |
| Heteropolysaccharides, novel fractions PEPE-1, PEPE-2, PEPE-3 (mannose, glucose, galactose, xylose) | Fresh mushroom residue; yield n.s. | Anti-tumor | [85] | ||
| Mannose, glucose, galactose | Fresh whole-mushroom; 28.3% (w/w dry weight) | Immunomodulatory | [86] | ||
| Pleurotus ostreatus | Oyster mushroom | Crude polysaccharide extracts | Fresh whole-mushroom; 61% (w/w) | Alleviation of cognitive impairment | [87] |
| Crude polysaccharide extracts | Fresh whole-mushroom; 63.98% (w/w) | Regulation of dislipidemia | [88] | ||
| Homogeneous polysaccharides, fractions POMP1, POMP2, POMP3 | Mycelia; yield n.s. | Anti-tumor | [89] |
| Mushrooms | Common Names | Bioactive Compounds/Substances * | Bioactivities | References |
|---|---|---|---|---|
| Agaricus bisporus | Button mushroom | Lectin | Immunomodulatory | [96] |
| Cerrena unicolor | Mossy maze polypore | Laccase | Anti-tumor | [97] |
| Coprinus comatus | Shaggy mane/chicken drumstick mushroom | Laccase | Anti-viral | [98] |
| Flammulina velutipes | Enoki/Golden needle mushroom | FIP | Anti-inflammatory | [99] |
| RIP | Anti-viral | [100] | ||
| Ganoderma applanatum | Artist’s conk | Lectin | Anti-tumor | [101] |
| Ganoderma lucidum | Lingzhi | Laccase | Anti-viral | [102] |
| Ganoderma tsugae | Hemlock reishi | FIP | Immunomodulatory | [103] |
| Hypsizygus marmoreus | Jade mushroom | RIPs (hypsin, marmorin) | Anti-fungal, anti-tumor | [104,105] |
| Inonotus baumii | Sanghuang | Laccase | Anti-tumor | [106] |
| Macrolepiota procera | Parasol mushroom | Lectin | Anti-tumor | [107] |
| Pleurotus cornucopiae | Golden oyster | Laccase | Anti-viral, anti-tumor | [108] |
| Pleurotus eryngii | King oyster mushroom | Laccase | Anti-viral | [109] |
| Pleurotus ostreatus | Oyster mushroom | Lectin | Immunomodulatory | [110] |
| Sparassis latifolia | Cauliflower mushroom | Lectin | Anti-fungal, anti-bacteria | [111] |
| Mushrooms | Common Name | Total SFA (% of Total FA) | Total MUFA (% of Total FA) | Total PUFA (% of Total FA) | Measurement Techniques | References |
|---|---|---|---|---|---|---|
| Agaricus blazei | Almond mushroom | 24.4 | 2.0 | 73.6 | GC-FID | [83] |
| Agaricus bisporus | White button mushroom | 20.3 | 1.4 | 78.3 | Capillary GLC-FID | [70] |
| Brown button mushroom | 18.4 | 1.8 | 79.8 | |||
| Agrocybe cylindracea | Poplar mushroom | 28.1 | 2.83 | 69.1 | Capillary GLC-FID | [124] |
| Boletus reticulatus | Summer cep | 21.1 | 40.3 | 38.4 | GLC-FID | [118] |
| Coprinus comatus | Shaggy mane/Lawyer’s cap | 23.8 | 11.4 | 64.8 | Capillary GLC-FID | [124] |
| Flammulina velutipes | Enoki/Golden needle mushroom | 18.5 | 7.2 | 74.3 | Capillary GLC-FID | [70] |
| 20.7 | 18.6 | 60.7 | GLC-FID | [118] | ||
| Lactarius deliciocus | Saffron milkcap | 20.8 | 42.0 | 37.3 | Capillary GLC-FID | [124] |
| Lactarius salmonicolor | Salmon milkcap | 19.0 | 19.6 | 61.6 | GLC-FID | [118] |
| Lentinus edodes | Shiitake mushroom | 16.7 | 3.5 | 79.8 | GC-FID | [83] |
| 15.1 | 2.9 | 82.0 | Capillary GLC-FID | [70] | ||
| Pleurotus eryngii | King oyster mushroom | 17.4 | 13.1 | 69.4 | Capillary GLC-FID | [70] |
| Pleurotus ostreatus | Oyster mushroom | 17.0 | 13.6 | 69.4 | Capillary GLC-FID | [70] |
| 21.8 | 11.4 | 66.5 | GLC-FID | [118] | ||
| Polyporus squamosus | Dryad’s saddle | 25.2 | 34.3 | 40.6 | GLC-FID | [118] |
| Russula anthracina | - | 23.7 | 53.3 | 22.9 | GLC-FID | [118] |
| Laetiporus sulphureus | Sulphur polypore | 21.6 | 17.6 | 60.8 | GC-FID, TLC-FID | [119] |
| Suillus collinitus | - | 17.5 | 34.4 | 47.4 | Capillary GLC-FID | [124] |
| Tricholoma myomyces | Grey knight mushroom | 15.8 | 46.3 | 37.8 | Capillary GLC-FID | [124] |
| Phenolic Compound Categories | Phenolic Compounds | Mushroom Sources | References |
|---|---|---|---|
| Phenolic acids | Ferulic acid | Agaricus brasiliensis, Agrocybe aegerita, Calocybe indica, Cantharellus cibarius | [126,127,132,133,134] |
| Gallic acid | Agaricus brasiliensis, Agrocybe aegerita, Calocybe indica, Cantharellus cibarius, Ganoderma lucidum, Pleurotus citrinopileatus, Pleurotus pulmonarius, Russula aurora | [126,130,132,133,134,135,136,137,138] | |
| Cinnamic acid | Amanita crocea, Ganoderma lucidum, Pleurotus ostreatus, Suilus belinii | [135,139,140,141] | |
| Caffeic acid | Calocybe indica, Cantharellus cibarius, Hyphodontia paradoxa, Inonotus obliquus, Pleurotus citrinopileatus, Pleurotus pulmonarius, | [127,130,133,134,142,143] | |
| p-Coumaric acid | Agaricus brasiliensis, Agaricus subrufescens, Amanita crocea, Hyphodontia paradoxa, Laccaria amethystea, Melanoleuca cognate, Pleurotus ostreatus | [56,126,129,139,140,142,144] | |
| p-Hydroxybenzoic acid | Agaricus brasilensis, Amanita crocea, Cantharellus cibarius, Lactarius indigo, Lentinus edodes, Melanoleuca cognate, Suillus belinii | [126,129,134,138,139,141] | |
| Fumaric acid | Agaricus brasiliensis | [126] | |
| Vanillic acid | Morchella esculenta (L.) Pers., Russula emetic | [136,137] | |
| Syringic acid | Hyphodontia paradoxa, Morchella esculenta (L.) Pers. | [129,130,136,142] | |
| Protocatechuic acid | Agrocybe aegerita, Calocybe indica, Cantharellus cibarius, Hyphodontia paradoxa, Inonotus obliquus, Melanoleuca, Morchella esculenta (L.) Pers., Suillus belinii, Russula emetic | [129,130,132,133,134,136,137,141,142] | |
| Rosmarinic acid | Hyphodontia paradoxa, Russula aurora, Russula emetic | [137,142,145] | |
| Flavonoids | Quercetin | Ganoderma lucidum, Laccaria amethystea, Pleurotus citrinopileatus, | [135,143] |
| Kaempferol | Ganoderma lucidum, Lactarius indigo | [135,146] | |
| Hesperetin | Calocybe indica, Ganoderma lucidum | [133,135] | |
| Naringenin | Calocybe indica, Ganoderma lucidum | [133,135] | |
| Catechin | Laccaria amethystea, Russula emetic | [137,144] | |
| Myricetin | Cantharellus cibarius, Lactarius indigo | [134,146] | |
| Procyanidin | Lactarius indigo | [146] | |
| Rutin | Pleurotus citrinopileatus, Russula emetic | [137,143] | |
| Tannins | Tannic acid | Agaricus silvaticus, Hydnum rufescens, Meripilus giganteus, Pleurotus citrinopileatus, Pleurotus ostreatus, Pleurotus tuber-regium(fries) | [147,148,149] |
| Tocopherols | α-Tocopherol | Agaricus bisporus, Boletus badius, Lepista inversa, Pleurotus ostreatus, Russula delica | [150,151] |
| β-Tocopherol | Laccaria laccata | [150] | |
| γ-Tocopherol | Clitocybe alexandri | [150] | |
| δ-Tocopherol | Lepista inversa | [150] |
| Properties | Mushroom Species | Bioactive Compounds | Study Type/Model/Effective Dosage | Mechanisms of Action | References |
|---|---|---|---|---|---|
| Immunomodulatory | Agaricus blazei | β-glucans (from pure AbM extracts or commercial mushroom extracts mixture AndoSan™ containing 85% of AbM) | Ex vivo/human whole blood/0.1–15% for 6 h; In vivo/human/20 mL thrice per day orally for 12 days | Anti-oxidant activities, enhance immune cells function and innate immune responses, trigger release of cytokines, chemokines, and leukocyte growth factors | [173] |
| Pleurotus cornucopiae | β-glucans | Clinical trial/human/24 mg per meal for 8 weeks | Th1 phenotype potentiation via macrophage-IL-12-IFN-γ pathway, up-regulation of NK cell activity | [176] | |
| Latiporus sulphureus | Lectin (LSL4) | In vitro/RAW264.7 cells/0–650 μg·mL−1 (IC50 = 1004.6 μg·mL−1) | Cell phagocytosis via TLR4 signaling pathway, triggers release of NO, iNOS, TNF-α, IL-1β, IL-6, and IL-10 | [177] | |
| Inonotus sanghuang | Extract containing polysaccharides and amino acids | In vivo/mice/4 and 8 mg·kg−1 once a day orally for 12 days | Stimulation of T lymphocytes, natural killer cells, and B cells; inhibition of cytochrome P450 isozymes | [178] | |
| Ganoderma lucidum | Polysaccharides extract (Ganoderan, heteroglycan, mannoglucan, glycopeptide) | In vivo/mice/2.5 mg·kg−1 intraperitoneal injection once per day for 7 days | Stimulation of TNF-α, IL-1, IFN-γ production, activate NF-κB | [205] | |
| Ganoderma Microsporum | FIP | In vitro/human alveolar epithelial A549 cells/4 and 16 μg·mL−1 | Down-regulation of TNF-α via NF-κB pathway | [206] | |
| Anti-cardiovascular diseases | Tricholoma matsutake | Functional peptides | In vivo/rats/50 mg·kg−1 acute oral dose | Alleviated hypertension via inhibition of angiotensin I converting enzyme | [183] |
| Pleurotus florida | Aqueous extract containing 80% soluble fiber, 44% protein, 1.4% soluble sugars, 0.2% polyphenols (w/w dry weight) | In vivo/rats/5 and 7.5% of 100 g basal diet for 4 weeks | Suppression of hepatic biosynthesis of cholesterol by inhibiting activity of liver enzyme HMG-CoA | [186] | |
| Cordyceps sinensis | Aqueous extract containing 83.9% carbohydrates (glucose, mannose, galactose, arabinose), 11.8% protein, w/w dry weight | In vivo/mice/150 and 300 mg·kg−1 per day orally for 7 days | Suppression of hepatic biosynthesis of cholesterol by inhibiting activity of liver enzyme HMG-CoA | [188] | |
| Neuroprotective | Agaricus blazei | Extract, composition not specified | In vivo/rats/50 mg·kg−1 per day intragastrically at the age of 7–23 months | Free-radical scavenging ability, cytoprotective action, antioxidation reaction | [189] |
| Hericium erinaceus | Aqueous and ehthanol extracts, composition not specified | In vitro/HT22 mouse hippocampal neurons/ethanol extracts at 400 μg·mL−1 | Inhibition of mitochondria-dependent apoptotic cellular signals activation; elevated CAT activity and GSH content; up-regulation of MAPK and PI3K/Akt pathway | [191] | |
| Extract containing erinacine A, hericenones C and D | in vivo/mice/1 mg (solubilized in water) per day for 2 months | Promoting hippocampal neurogenesis; up-regulation of lipoxin A4 and modulation of stress responsive proteins | [194] | ||
| Auricularia polytricha | Ethanolic extract containing flavonoids, phenols, linoleic acid | In vitro/HT22 mouse hippocampal cells/5, 10, 20, and 40 μg·mL−1; In vivo/Caenorhabditis elegans/20, 40 μg·mL−1 | Anti-oxidant activity via Nrf2 signaling pathway; up-regulation of Sod1 and Gpx gene expressions | [190] | |
| Grifola frondose | Aqueous extract containing, β-glucan, chitin, amino acids, unsaturated fatty acids, monosaccharides | In vivo/Saccharomyces cerevisiae/0.2 and 0.5% in culture medium; In vivo/Drosophila melanogaster/0.2% in culture medium | Increase of heat shock proteins expression by inhibition of Ras/PKA pathway; reduce levels of ROS | [192] | |
| Antidiabetic | Tremella fuciformis, Phellinus baumii | Exopolysaccharides, composition not specified | In vivo/mice/200 mg·kg−1 per day orally for 52 days | Improve insulin sensitivity via regulating PPAR-γ-mediated lipid metabolism | [197] |
| Agaricus bisporus | Not specified | In vivo/rats/200 mg·kg−1 per day orally for 3 weeks | Stimulate secretion of insulin from pancreatic beta cells | [198] | |
| Lectins | In vivo/mice/10 mg·kg−1 for 2 weeks | Induce beta-cell proliferation | [201] | ||
| Phellinus linteus | Aqueous extract containing 13.2% peptide, 82.5% carbohydrates (w/w dry weight) | In vivo/mice/30 mg·kg−1 intraperitoneally daily from 8 to 24 weeks of age | Inhibit expression of inflammatory cytokines (IFN-γ, IL-2, and TNF-α); up-regulation of IL-4 expression | [199] | |
| Agaricus blazei Murill | Isoflavovoids (genistein, genistin, daidzein, daidzin) | In vivo/rats/400 mg·kg−1 per day orally for 2 weeks | Improve beta-cell function; increase lipid peroxidation via enhanced fatty acyl CoA activity | [200] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Ganesan, K.; Xu, B. Unlocking the Power: New Insights into the Anti-Aging Properties of Mushrooms. J. Fungi 2024, 10, 215. https://doi.org/10.3390/jof10030215
Luo J, Ganesan K, Xu B. Unlocking the Power: New Insights into the Anti-Aging Properties of Mushrooms. Journal of Fungi. 2024; 10(3):215. https://doi.org/10.3390/jof10030215
Chicago/Turabian StyleLuo, Jing, Kumar Ganesan, and Baojun Xu. 2024. "Unlocking the Power: New Insights into the Anti-Aging Properties of Mushrooms" Journal of Fungi 10, no. 3: 215. https://doi.org/10.3390/jof10030215
APA StyleLuo, J., Ganesan, K., & Xu, B. (2024). Unlocking the Power: New Insights into the Anti-Aging Properties of Mushrooms. Journal of Fungi, 10(3), 215. https://doi.org/10.3390/jof10030215






