Checklist of Macrofungi Associated with Nine Different Habitats of Taburno-Camposauro Massif in Campania, Southern Italy
Abstract
1. Introduction
2. Materials and Methods
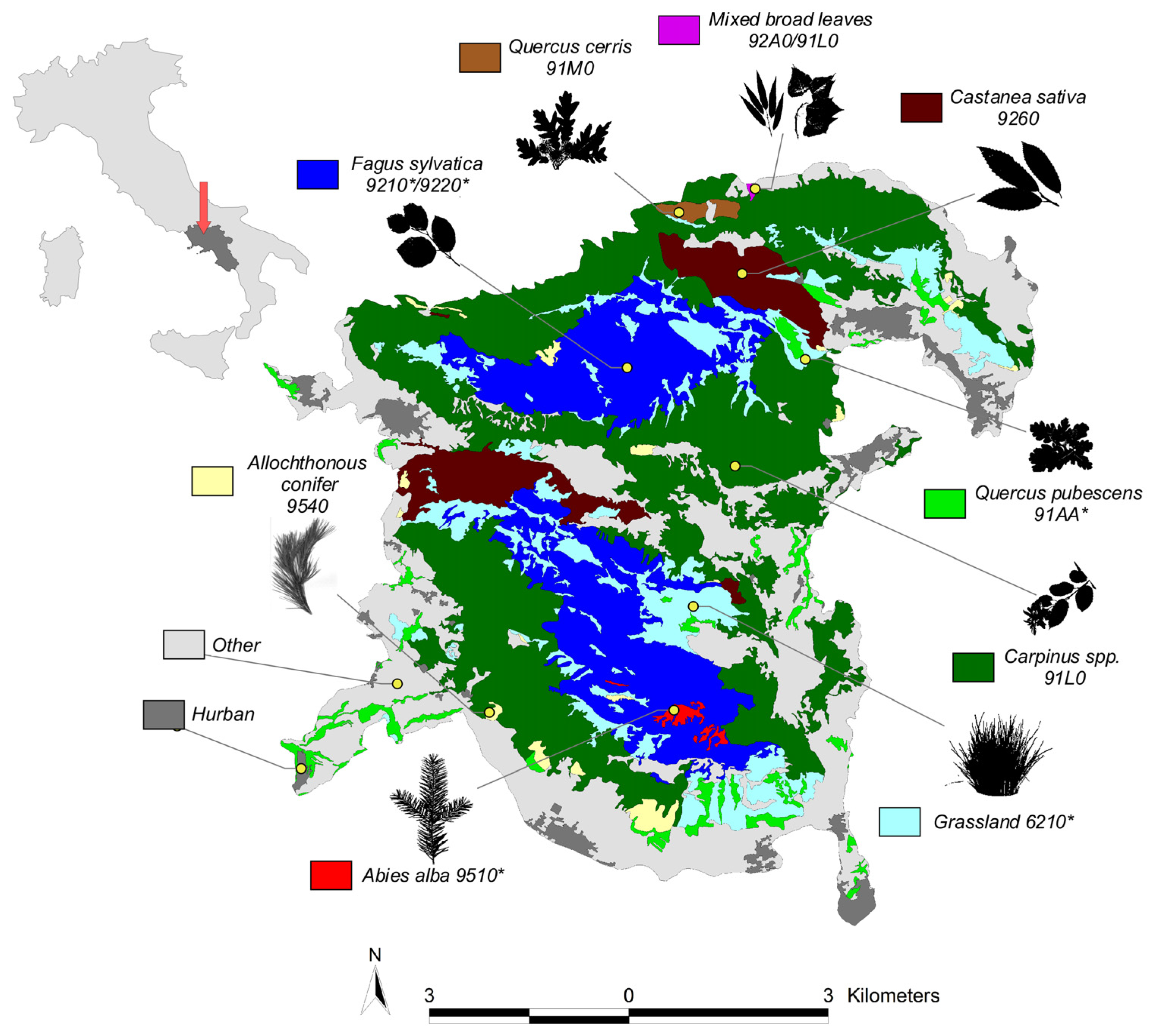
2.1. Study-Site Description and Vegetation Types
- Forests of Fagus sylvatica, encompassing around 21.15 km2 of regional park land and constituting the highest vegetation belt, with a minimum elevation of 800 m a.s.l. In the Taburno-Camposauro massif, the F. sylvatica forests include the habitats 9210 “Apennine beech forests with Abies alba” (Taburno) and 9220 “Apennine beech forests with Taxus and Ilex” (Camposauro). Both are considered priority habitats.
- Mixed forests with a prevalence of Carpinus spp. That encompass 48.80 km2 of land and are considered the most representative vegetation belt in the massif ranging from piedmont to 1000 m a.s.l. The area includes the habitat 91L0, corresponding to “Illyrian oak-hornbeam forests” and characterized by the cooccurrence of several deciduous tree species alongside the Carpinus spp., Fraxinus, Acer, and Quercus tree species; they cooccur with a scattered distribution.
- Grasslands of around 13.4 km2. This habitat type encompasses semi-natural dry grasslands that develop on calcareous (lime-rich) substrates at an elevation high above the woody vegetation and managed lowland prairies. This habitat is a proritary one and is mainly recognized in Natura 2000 as 6210, “Semi-natural dry grasslands and scrubland facies on calcareous substrates (Festuco-Brometalia)”.
- Woodlands of Castanea sativa, (7.1 km2) occupying the foothills vegetation belt and strongly favored by humans for chestnuts and wood production. The Natura 2000 habitat within the area is recognized as 9260, “Castanea sativa woods”.
- Quercus pubescens forests, encompassing 3.71 km2 of land, are represented by patches of vegetation that are mostly localized in arid south-facing sloped areas favoring thermophilus vegetation. In that area, the priority habitat 91AA is observed and described as “wooded areas dominated by various species of white oaks”.
- Allochthonous conifers refers to a reforested area with mostly two-needle pine trees (Pinus nigra, P. pinaster, P. halepensis., P. pinea) but also several Cupressus species, Pseudotsuga menziesi and Larix spp. This category is present in the park as result of reforestation policies enacted in the area in the 1970s. Those habitats are distributed at different altitudes within the park area. The actual surface of this category is around 1.38 km2 and includes the 9540 habitat, “Mediterranean formations dominated by Aleppo pine”.
- Forests of Quercus cerris account for a surface area of around 0.48 km2 and are mostly found in the form of monodominated patches in mesophilic environments. The Natura 2000 habitat within the area is recognized as 91M0 and defined as “Pannonian-Balkanic turkey oak-sessile oak forests”.
- The forest of Abies alba is localized in mount Taburno and measures approximately 0.42 km2. The area hosts the priority habitat 9510 “Southern Apennine silver fir forests”.
- Mixed broad-leaved forests are considered areas with a presence of several tree species, mostly riparian, and cover 0.03 km2. Those areas are identified as small patches of vegetation in valleys and along water bodies and are mainly dominated by the presence of Salix spp., Populus spp. mixed with Carpinus spp. Quercus spp., Acer spp. and Fraxinus spp. In the area, habitat 92A0 and 91L0, known as “Salix alba and Populus alba galleries” and “Illyrian oak-hornbeam forests” are present.
2.2. Sampling Methodology and Taxa Determination
2.3. Map Production and Data Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mueller, G.M.; Schmit, J.P.; Leacock, P.R.; Buyck, B.; Cifuentes, J.; Desjardin, D.E.; Halling, R.E.; Hjortstam, K.; Iturriaga, T.; Larsson, K.-H. Global diversity and distribution of macrofungi. Biodivers. Conserv. 2007, 16, 37–48. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, T.; Skidmore, A.; Heurich, M.; Bässler, C. 50 Years of Cumulative Open-Source Data Confirm Stable and Robust Biodiversity Distribution Patterns for Macrofungi. J. Fungi 2022, 8, 981. [Google Scholar] [CrossRef] [PubMed]
- Compagno, R.; La Rosa, A.; Sammarco, I.; Gargano, M.L.; Saitta, A.; Venturella, G. The check-list of fungi in Sicily (southern Italy): Current survey status. In Bollettino dei Musei e degli Istituti Biologici dell’Università di Genova; Giuseppina Barberis e Maria Angela Guido: Genova, Italy, 2011; p. 197. [Google Scholar]
- Ferraro, V.; Venturella, G.; Cirlincione, F.; Mirabile, G.; Gargano, M.L.; Colasuonno, P. The checklist of Sicilian macrofungi. J. Fungi 2022, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Ndifon, E.M. Systematic appraisal of macrofungi (Basidiomycotina: Ascomycotina) biodiversity of Southern Africa: Uses, distribution, checklists. J. Asia-Pac. Biodivers. 2022, 15, 80–85. [Google Scholar] [CrossRef]
- Hawksworth, D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991, 95, 641–655. [Google Scholar] [CrossRef]
- Pradhan, P.; Dutta, A.K.; Roy, A.; Basu, S.K.; Acharya, K. Macrofungal diversity and habitat specificity: A case study. Biodiversity 2013, 14, 147–161. [Google Scholar] [CrossRef]
- Ambrosio, E.; Cecchi, G.; Zotti, M.; Mariotti, M.G.; Di Piazza, S.; Boccardo, F. An annotated checklist of macrofungi in broadleaf Mediterranean forests (NW Italy). Acta Mycol. 2018, 53, 1109. [Google Scholar] [CrossRef]
- Angelini, P.; Bistocchi, G.; Arcangeli, A.; Venanzoni, R. Preliminary check-list of the macromycetes from collestrada forest ecosystems in perugia (Italy). Mycotaxon 2012, 120, 505–506. [Google Scholar]
- Senn-Irlet, B.; Heilmann-Clausen, J.; Genney, D.; Dahlberg, A. Guidance for Conservation of Macrofungi in Europe; ECCF: Strasbourg, France, 2007. [Google Scholar]
- Onofri, S.; Bernicchia, A.; Valeria, F.M.; Perini, C.; Savino, E.; Venturella, G.; Zucconi, L.; Padovan, F.; Ripa, C.; Salerni, E. Checklist dei Funghi Italiani; Carlo Delfino Editore: Sassari, Italy, 2005. [Google Scholar]
- Zotti, M.; Bonanomi, G.; Mancinelli, G.; Barquero, M.; De Filippis, F.; Giannino, F.; Mazzoleni, S.; González-Andrés, F. Riding the wave: Response of bacterial and fungal microbiota associated with the spread of the fairy ring fungus Calocybe gambosa. Appl. Soil Ecol. 2021, 163, 103963. [Google Scholar] [CrossRef]
- Bonanomi, G.; Idbella, M.; Abd-ElGawad, A.M.; Motti, R.; Ippolito, F.; Santorufo, L.; Adamo, P.; Agrelli, D.; De Marco, A.; Maisto, G. Impact of prescribed burning, mowing and abandonment on a Mediterranean grassland: A 5-year multi-kingdom comparison. Sci. Total Environ. 2022, 834, 155442. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Idbella, M.; Stinca, A.; Maisto, G.; De Marco, A.; Giusso Del Galdo, G.P.; Guarino, R.; Zotti, M. Nitrogen-fixing cushion Astragalus siculus modulates soil fertility, microclimate, plant facilitation, bacterial and fungal microbiota along an elevation gradient. J. Veg. Sci. 2023, 34, e13193. [Google Scholar] [CrossRef]
- Onofri, S.; Bernicchia, A.; Filipello Marchisio, V.; Perini, C.; Venturella, G.; Zucconi, L.; Ripa, C. The Check-list of Italian Fungi, Part I (Basidiomycetes, Basidiomycota). Bocconea 2003, 16, 1083–1089. [Google Scholar]
- Venturella, G.; Altobelli, E.; Bernicchia, A.; Di Piazza, S.; Donnini, D.; Gargano, M.; Gorjòn, S.; Granito, V.; Lantieri, A.; Lunghini, D. Fungal biodiversity and in situ conservation in Italy. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2011, 145, 950–957. [Google Scholar] [CrossRef]
- Perini, C.; Venturella, G. Fungi Conservation in Italy: Case Study from Tuscany and Sicily. Australas. Plant Conserv. J. Aust. Netw. Plant Conserv. 2005, 14, 10–12. [Google Scholar] [CrossRef]
- Aleffi, M. New check-list of the Hepaticae and Anthocerotae of Italy. Flora Mediterr. 2005, 15, 485–566. [Google Scholar]
- Violante, U.; Roca, E.; Violante, M.; Soriente, S.; Pizzolongo, F. Micoflora della Campania: Check-list dei macrofungi. Inf. Bot. Ital. 2002, 34, 3–34. [Google Scholar]
- Corazzi, G. Contributo alla conoscenza della flora del Sannio: Il complesso montuoso del Camposauro (Benevento, Campania). Webbia 2008, 63, 215–250. [Google Scholar] [CrossRef]
- Valva, V.L. Aspetti corologici della flora di interesse fitogeografico nell’Apennino Meridionale. Plant Biosyst. 1992, 126, 131–144. [Google Scholar] [CrossRef]
- Guarino, C.; Napolitano, F. Community habitats and biodiversity in the Taburno-Camposauro Regional Park. Woodland, rare species, endangered species and their conservation. For. J. Silvic. For. Ecol. 2006, 3, 527. [Google Scholar] [CrossRef]
- Biondi, E.; Zivkovic, L.; Esposito, L.; Pesaresi, S. Vegetation, plant landscape and habitat analyses of a fluvial ecosystem in central Italy. Acta Bot. Gall. 2009, 156, 571–587. [Google Scholar] [CrossRef]
- Angelini, P.; Bianco, P.; Cardillo, A.; Francescato, C.; Oriolo, G. Gli Habitat in Carta della Natura; ISPRA: Rome, Italy, 2009. [Google Scholar]
- Bagnaia, R.; Viglietti, S.; Laureti, L.; Giacanelli, V.; Ceralli, D.; Bianco, P.M.; Loreto, A.; Luce, E.; Fusco, L. Carta della Natura della Regione Campania: Carta degli Habitat alla Scala 1:25.000; ISPRA: Rome, Italy, 2017. [Google Scholar]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.I.; Merckx, V.; Tedersoo, L. FungalRoot: Global online database of plant mycorrhizal associations. New Phytol. 2020, 227, 955–966. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Resolving the mycorrhizal status of important northern hemisphere trees. Plant Soil 2020, 454, 3–34. [Google Scholar] [CrossRef]
- Arnolds, E. The role of macrofungi in environmental conservation. Plant Biosyst. 1992, 126, 779–795. [Google Scholar] [CrossRef]
- Allegrezza, M.; Bonanomi, G.; Zotti, M.; Carteni, F.; Moreno, M.; Olivieri, L.; Garbarino, M.; Tesei, G.; Giannino, F.; Mazzoleni, S. Biogeography and shape of fungal fairy rings in the Apennine mountains, Italy. J. Biogeogr. 2022, 49, 353–363. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Allegrezza, M. Assessing the impact of land abandonment, nitrogen enrichment and fairy-ring fungi on plant diversity of Mediterranean grasslands. Biodivers. Conserv. 2013, 22, 2285–2304. [Google Scholar] [CrossRef]
- Zotti, M.; Persiani, A.; Ambrosio, E.; Vizzini, A.; Venturella, G.; Donnini, D.; Angelini, P.; Di Piazza, S.; Pavarino, M.; Lunghini, D. Macrofungi as ecosystem resources: Conservation versus exploitation. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2013, 147, 219–225. [Google Scholar] [CrossRef]
- Yun, W.; Hall, I.R.; Evans, L.A. Ectomycorrhizal fungi with edible fruiting bodies 1. Tricholoma matsutake and related fungi. In Economic Botany; Springer: Berlin/Heidelberg, Germany, 1997; pp. 311–327. [Google Scholar]
- Benucci, G.M.N.; Bonito, G.; Falini, L.B.; Bencivenga, M.; Donnini, D. Truffles, timber, food, and fuel: Sustainable approaches for multi-cropping truffles and economically important plants. In Edible Ectomycorrhizal Mushrooms: Current Knowledge and Future Prospects; Springer: Berlin/Heidelberg, Germany, 2012; pp. 265–280. [Google Scholar]
- Saulino, L.; Rita, A.; Allegrezza, M.; Zotti, M.; Mogavero, V.; Tesei, G.; Montecchiari, S.; Allevato, E.; Borghetti, M.; Bonanomi, G. Clonality drives structural patterns and shapes the community assemblage of the Mediterranean Fagus sylvatica subalpine belt. Front. Plant Sci. 2022, 13, 3525. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Mogavero, V.; Cesarano, G.; Saulino, L.; Rita, A.; Tesei, G.; Allegrezza, M.; Saracino, A.; Allevato, E. Climatic and anthropogenic factors explain the variability of Fagus sylvatica treeline elevation in fifteen mountain groups across the Apennines. For. Ecosyst. 2020, 7, 5. [Google Scholar] [CrossRef]
- Komonen, A.; Niemi, M.E.; Junninen, K. Lakeside riparian forests support diversity of wood fungi in managed boreal forests. Can. J. For. Res. 2008, 38, 2650–2659. [Google Scholar] [CrossRef]
- Frankland, J.C. Fungal succession—Unravelling the unpredictable. Mycol. Res. 1998, 102, 1–15. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Cesarano, G.; La Storia, A.; Zotti, M.; Mazzoleni, S.; Incerti, G. Linking bacterial and eukaryotic microbiota to litter chemistry: Combining next generation sequencing with 13C CPMAS NMR spectroscopy. Soil Biol. Biochem. 2019, 129, 110–121. [Google Scholar] [CrossRef]
- Gil-Martínez, M.; Navarro-Fernández, C.M.; Murillo, J.M.; Domínguez, M.T.; Marañón, T. Trace elements and C and N isotope composition in two mushroom species from a mine-spill contaminated site. Sci. Rep. 2020, 10, 6434. [Google Scholar] [CrossRef]
- Santos-Silva, C.; Louro, R. Assessment of the diversity of epigeous Basidiomycota under different soil-management systems in a montado ecosystem: A case study conducted in Alentejo. Agrofor. Syst. 2016, 90, 117–126. [Google Scholar] [CrossRef]
- Zotti, M.; Bonanomi, G.; Saulino, L.; Allevato, E.; Saracino, A.; Mazzoleni, S.; Idbella, M. Shifts of Leaf Litter-Induced Plant-Soil Feedback from Negative to Positive Driven by Ectomycorrhizal Symbiosis between Quercus ilex and Pisolithus arrhizus. Microorganisms 2023, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.; Henkel, T.W.; Smith, M.E. Ectomycorrhizal associations in the tropics–biogeography, diversity patterns and ecosystem roles. New Phytol. 2018, 220, 1076–1091. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Bonanomi, G.; Incerti, G.; Chiusano, M.L.; Termolino, P.; Mingo, A.; Senatore, M.; Giannino, F.; Cartenì, F.; Rietkerk, M. Inhibitory and toxic effects of extracellular self-DNA in litter: A mechanism for negative plant–soil feedbacks? New Phytol. 2015, 205, 1195–1210. [Google Scholar] [CrossRef]
- Tedersoo, L.; Brundrett, M.C. Evolution of ectomycorrhizal symbiosis in plants. In Biogeography of Mycorrhizal Symbiosis; Springer: Cham, Switzerland, 2017; pp. 407–467. [Google Scholar]
- Horton, T.R. Spore dispersal in ectomycorrhizal fungi at fine and regional scales. In Biogeography of Mycorrhizal Symbiosis; Springer: Cham, Switzerland, 2017; pp. 61–78. [Google Scholar]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Fernandez, C.W.; Kennedy, P.G. Revisiting the ‘Gadgil effect’: Do interguild fungal interactions control carbon cycling in forest soils? New Phytol. 2016, 209, 1382–1394. [Google Scholar] [CrossRef]
- Gadgil, P.D.; Gadgil, R.L. Suppression of Litter Decomposition by Mycorrhizal Roots of Pinus Radiata; New Zealand Forest Service: Rotorua, New Zealand, 1975.
- Zotti, M.; De Filippis, F.; Cesarano, G.; Ercolini, D.; Tesei, G.; Allegrezza, M.; Giannino, F.; Mazzoleni, S.; Bonanomi, G. One ring to rule them all: An ecosystem engineer fungus fosters plant and microbial diversity in a Mediterranean grassland. New Phytol. 2020, 227, 884–898. [Google Scholar] [CrossRef] [PubMed]

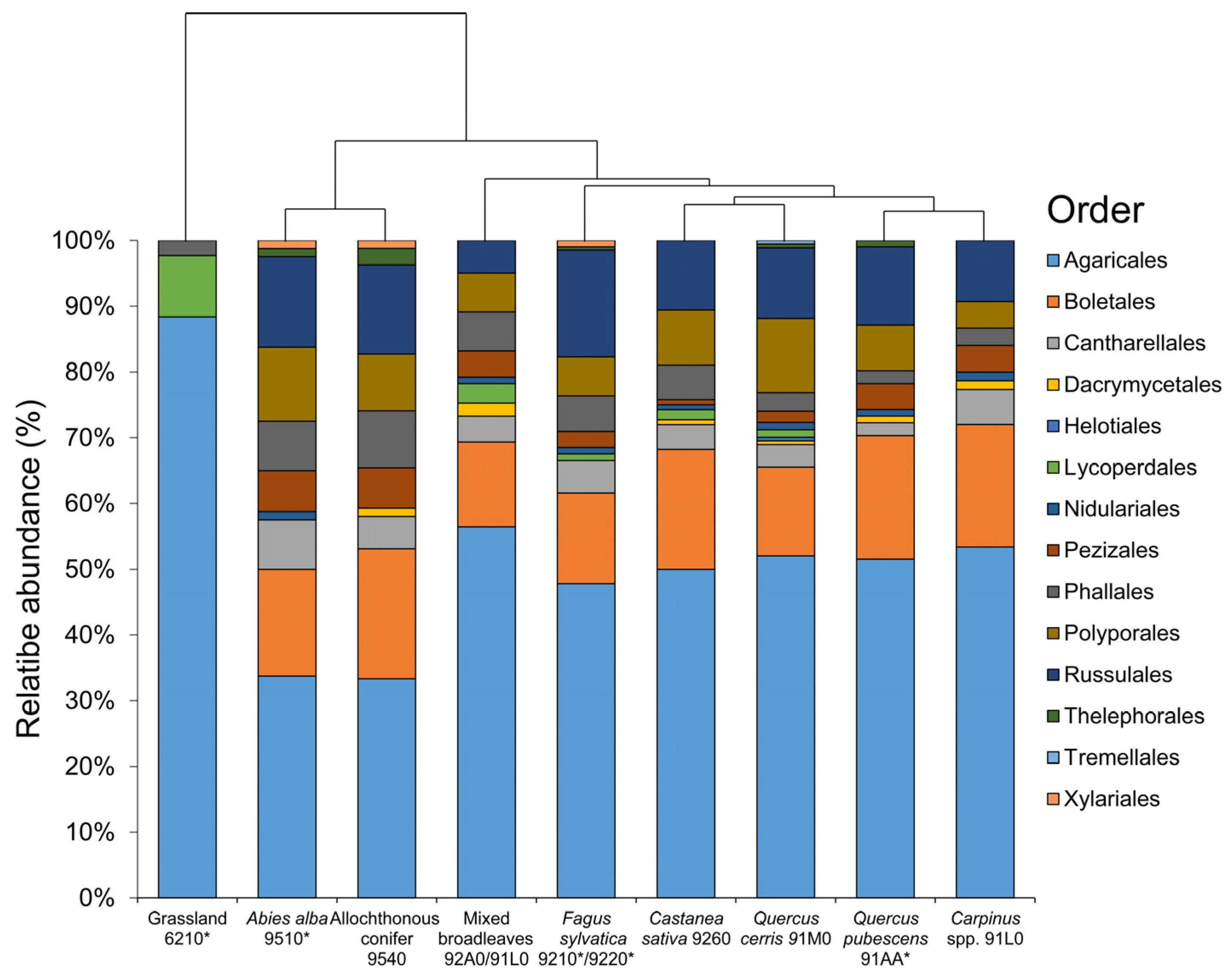
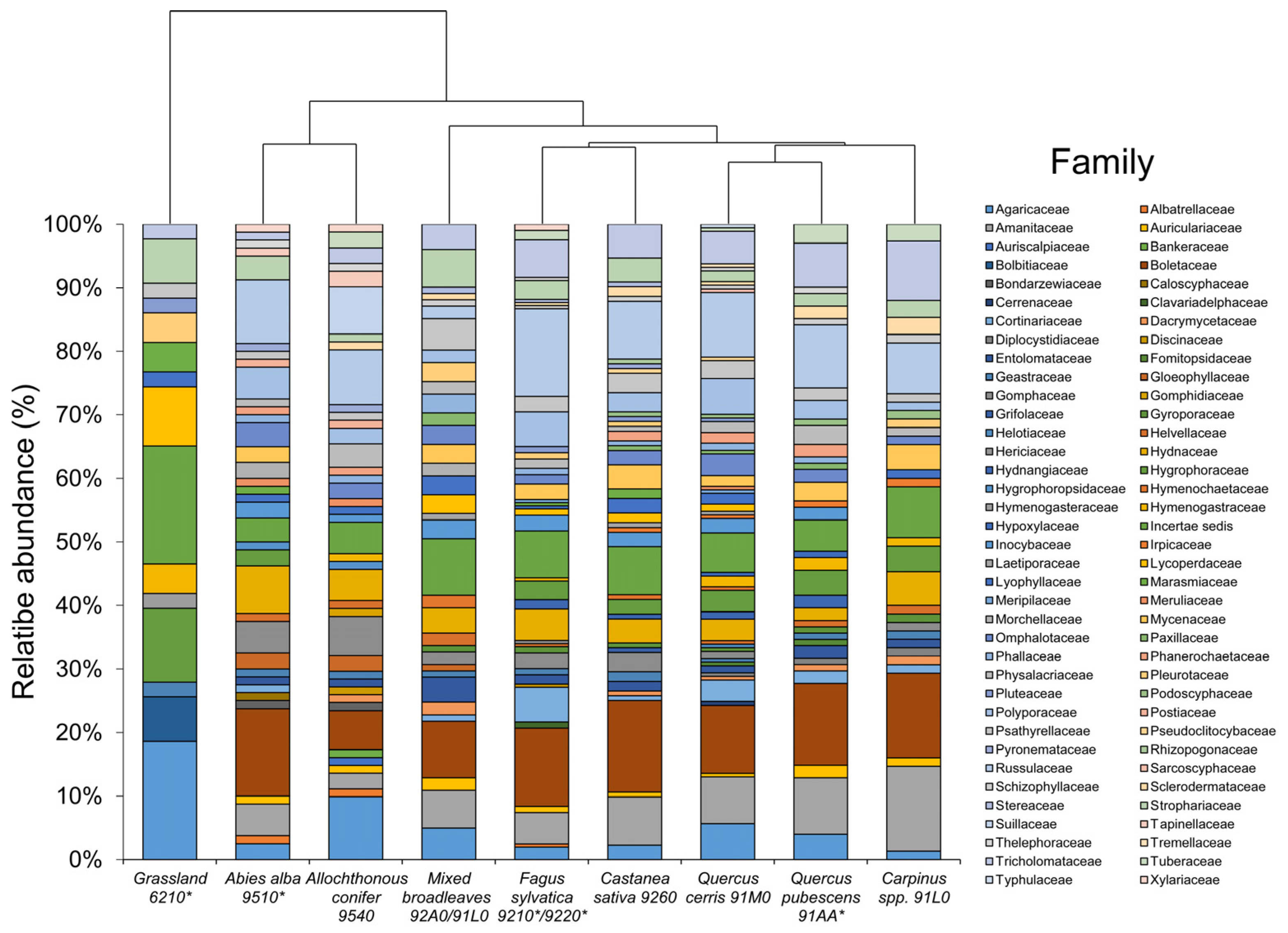
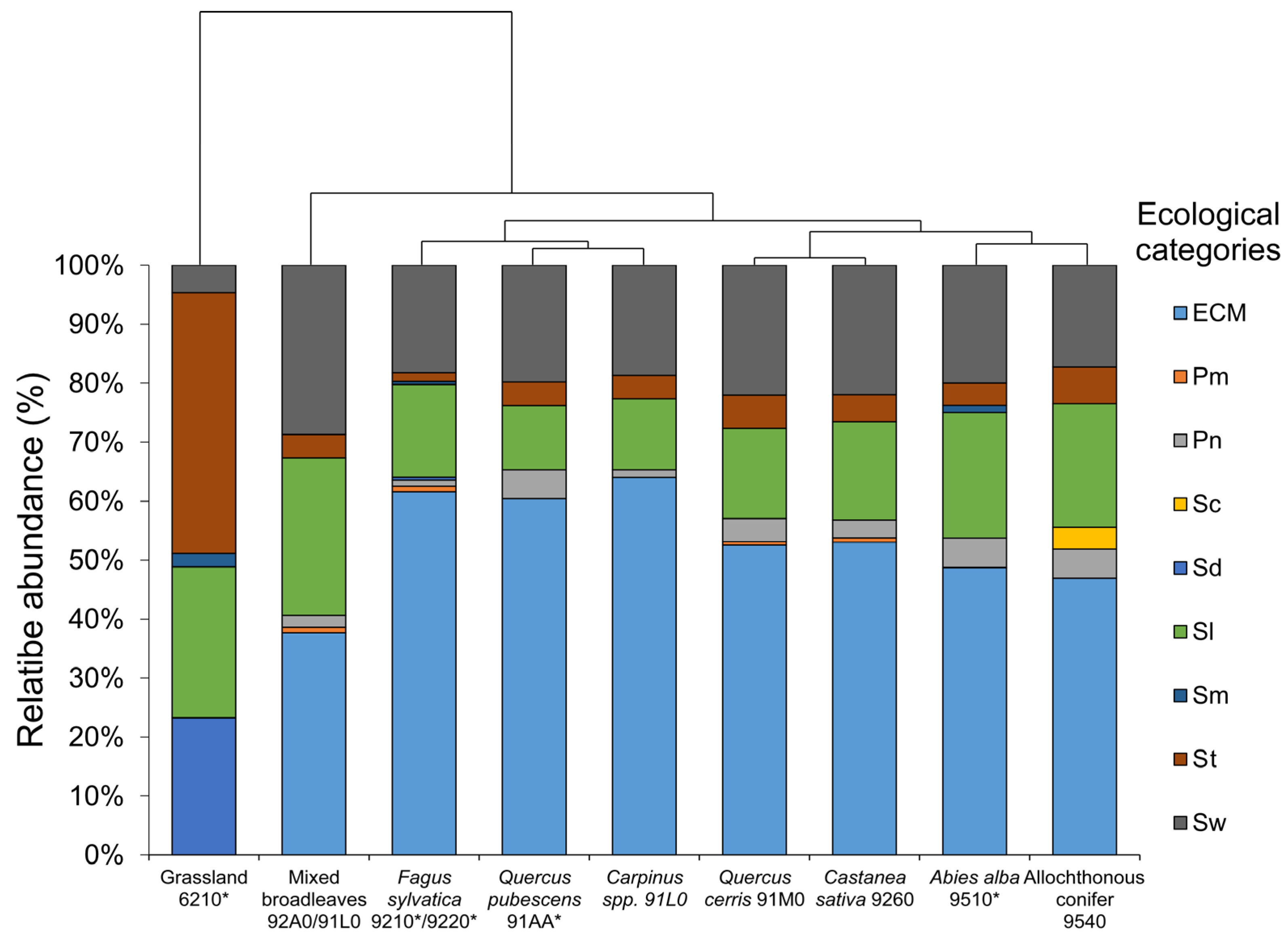



| Taxon | Fagus sylvatica 9210 */9220 * | Abies alba 9510 * | Allochthonous Conifers 9540 | Quercus cerris 91M0 | Quercus pubescens 91AA * | Carpinus spp. 91L0 | Castanea sativa 9260 | Mixed Broadleaves 92A0/91L0 | Grassland 6210 * | Order (.ales) | Family (.aceae) | Eco |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abortiporus biennis (Bull.) Singer | x | x | x | x | Polypor. | Podoscyph. | Sw | |||||
| Agaricus arvensis Schaeff. * | x | x | x | Agaric. | Agaric. | St | ||||||
| Agaricus augustus Fr. var. augustus * | x | x | Agaric. | Agaric. | St | |||||||
| Agaricus bisporus (J. E. Lange) Imbach | x | Agaric. | Agaric. | St | ||||||||
| Agaricus bresadolanus Bohus | x | x | Agaric. | Agaric. | St | |||||||
| Agaricus campestris L. * | x | Agaric. | Agaric. | St | ||||||||
| Agaricus moelleri Wasser * | x | Agaric. | Agaric. | St | ||||||||
| Agaricus sylvaticus Schaeff. * | x | x | Agaric. | Agaric. | St | |||||||
| Agaricus sylvicola (Vittad.) Peck * | x | Agaric. | Agaric. | St | ||||||||
| Agaricus xanthodermus Genev. subsp. xanthodermus * | x | Agaric. | Agaric. | St | ||||||||
| Agrocybe molesta (Lasch) Singer | x | Agaric. | Strophari. | St | ||||||||
| Agrocybe praecox (Pers.) Fayod * | x | x | Agaric. | Strophari. | St | |||||||
| Albatrellus ovinus (Schaeff.) Kotl. & Pouzar * | x | x | Russul. | Albatrell. | Ec | |||||||
| Aleuria aurantia (Pers.) Fuckel | x | x | Peziz. | Pyronemat. | St | |||||||
| Amanita caesarea (Scop.) Pers. * | x | x | x | x | x | Agaric. | Amanit. | Ec | ||||
| Amanita ceciliae (Berk. & Broome) Bas * | x | x | x | Agaric. | Amanit. | Ec | ||||||
| Amanita citrina Pers. * | x | x | x | x | x | x | x | x | Agaric. | Amanit. | Ec | |
| Amanita crocea (Quél.) Singer * | x | x | x | Agaric. | Amanit. | Ec | ||||||
| Amanita echinocephala (Vittad.) Quél. * | x | x | Agaric. | Amanit. | Ec | |||||||
| Amanita eliae Quél. * | x | x | x | x | Agaric. | Amanit. | Ec | |||||
| Amanita excelsa (Fr.) Bertill. * | x | Agaric. | Amanit. | Ec | ||||||||
| Amanita franchetii (Boud.) Fayod | x | x | x | x | Agaric. | Amanit. | Ec | |||||
| Amanita fulva Fr. * | x | Agaric. | Amanit. | Ec | ||||||||
| Amanita muscaria (L.) Lam. * | x | Agaric. | Amanit. | Ec | ||||||||
| Amanita pantherina (DC.) Krombh. * | x | x | x | x | x | x | x | Agaric. | Amanit. | Ec | ||
| Amanita phalloides (Vaill. ex Fr.) Link * | x | x | x | x | x | x | Agaric. | Amanit. | Ec | |||
| Amanita porphyria Alb. & Schwein. | x | Agaric. | Amanit. | Ec | ||||||||
| Amanita rubescens Pers. * | x | x | x | x | x | x | x | Agaric. | Amanit. | Ec | ||
| Amanita vaginata (Bull.) Lam. * | x | x | x | x | x | x | Agaric. | Amanit. | Ec | |||
| Amanita verna Bull. ex Lam. * | x | x | x | Agaric. | Amanit. | Ec | ||||||
| Amaropostia stiptica (Pers.) B.K. Cui, L.L. | x | x | Polypor. | Posti. | Sw | |||||||
| Armillaria cepistipes Velen. | x | x | Agaric. | Physalacri. | Pn | |||||||
| Armillaria gallica Marxm. & Romagn. | x | x | Agaric. | Physalacri. | Pn | |||||||
| Armillaria mellea (Vahl) P. Kumm. * | x | x | x | x | x | x | Agaric. | Physalacri. | Pn | |||
| Arrhenia spathulata (Fr.) Redhead | x | x | Agaric. | Hygrophor. | Sm | |||||||
| Aspropaxillus candidus (Bres.) M.M. Moser | x | x | Agaric. | Tricholomat. | St | |||||||
| Aspropaxillus giganteus (Sowerby) Kühner & Maire * | x | Agaric. | Tricholomat. | St | ||||||||
| Asterophora lycoperdoides (Bull.) Ditmar | x | x | x | x | Agaric. | Lyophyll. | Pm | |||||
| Astraeus hygrometricus (Pers.) Morgan | x | x | x | Bolet. | Diplocystidi. | St | ||||||
| Aureoboletus gentilis (Quél.) Pouzar * | x | Bolet. | Bolet. | Ec | ||||||||
| Auricularia auricula-judae (Bull.) Quél. | x | x | x | x | x | x | x | Russul. | Auriculari. | Sw | ||
| Auricularia mesenterica (Dicks.) Pers. * | x | x | x | x | Russul. | Auriculari. | Sw | |||||
| Auriscalpium vulgare Gray * | x | Russul. | Auriscalpi. | Sc | ||||||||
| Baorangia emileorum (Barbier) Vizzini, Simonini & Gelardi | x | Bolet. | Bolet. | Ec | ||||||||
| Bjerkandera adusta (Willd.) P. Karst. * | x | x | x | x | Polypor. | Phanerochaet. | Pn | |||||
| Bjerkandera fumosa (Pers.) P. Karst. * | x | x | x | Polypor. | Phanerochaet. | Pn | ||||||
| Bolbitius titubans (Bull.) Fr. | x | Agaric. | Bolbiti. | Sd | ||||||||
| Boletopsis grisea (Peck) Bondartsev & Singer | x | Thelephor. | Banker. | Ec | ||||||||
| Boletus aereus Bull. * | x | x | x | x | x | Bolet. | Bolet. | Ec | ||||
| Boletus edulis Bull. * | x | Bolet. | Bolet. | Ec | ||||||||
| Boletus pinophilus Pilát & Dermek * | x | Bolet. | Bolet. | Ec | ||||||||
| Boletus reticulatus Schaeff. * | x | x | x | x | x | x | Bolet. | Bolet. | Ec | |||
| Bovista plumbea Pers. * | x | Lycoperd. | Lycoperd. | St | ||||||||
| Bovistella utriformis (Bull.) Demoulin & Rebriev | x | Lycoperd. | Lycoperd. | St | ||||||||
| Butyriboletus appendiculatus (Schaeff.) D. Arora & J.L. Frank * | x | x | x | Bolet. | Bolet. | Ec | ||||||
| Butyriboletus fechtneri (Velen.) D. Arora & J.L. Frank * | x | Bolet. | Bolet. | Ec | ||||||||
| Butyriboletus pseudoregius (Heinr. Huber) D. Arora & J.L. Frank | x | x | Bolet. | Bolet. | Ec | |||||||
| Butyriboletus regius (Krombh.) D. Arora & J.L. Frank * | x | x | x | Bolet. | Bolet. | Ec | ||||||
| Byssomerulius corium (Pers.) Parmasto | x | x | x | x | Polypor. | Irpic. | Sw | |||||
| Caloboletus calopus (Pers.) Vizzini * | x | x | x | x | x | Bolet. | Bolet. | Ec | ||||
| Caloboletus radicans (Pers.) Vizzini * | x | x | x | Bolet. | Bolet. | Ec | ||||||
| Calocera cornea (Batsch) Fr. * | x | x | x | x | x | Dacrymycet. | Dacrymycet. | Sw | ||||
| Calocera viscosa (Pers.) Fr. * | x | x | Dacrymycet. | Dacrymycet. | Sw | |||||||
| Calocybe gambosa (Fr.) Donk * | x | x | x | x | x | x | Agaric. | Lyophyll. | st | |||
| Caloscypha fulgens (Pers.: Fr.) Boud. | x | Peziz. | Caloscyph. | Sl | ||||||||
| Calvatia cyathiformis (Bosc) Morgan * | x | Lycoperd. | Lycoperd. | St | ||||||||
| Candolleomyces candolleanus (Fr.) D. Wächt. & A. Melzer | x | x | x | x | x | x | x | Agaric. | Psathyrell. | Sw | ||
| Cantharellus alborufescens (Malençon) Papetti & S. Alberti | x | x | x | x | x | x | Cantharell. | Hydn. | Ec | |||
| Cantharellus amethysteus (Quél.) Sacc. | x | x | Cantharell. | Hydn. | Ec | |||||||
| Cantharellus cibarius Fr. var. cibarius * | x | x | x | x | x | Cantharell. | Hydn. | Ec | ||||
| Cantharellus cinereus (Pers.) Fr. | x | x | Cantharell. | Hydn. | Ec | |||||||
| Cantharellus ferruginascens P.D. Orton | x | x | Cantharell. | Hydn. | Ec | |||||||
| Cantharellus friesii Quél. * | x | x | Cantharell. | Hydn. | Ec | |||||||
| Cantharellus pallens Pilát | x | x | x | x | x | x | x | x | Cantharell. | Hydn. | Ec | |
| Cerioporus meridionalis (A. David) Zmitr. & Kovalenko | x | x | Polypor. | Polypor. | Sw | |||||||
| Cerioporus squamosus (Huds.) Quél. | x | x | x | Polypor. | Polypor. | Sw | ||||||
| Cerrena unicolor (Bull.) Murrill | x | Polypor. | Cerren. | Sw | ||||||||
| Chalciporus piperatus (Bull.) Bataille * | x | Bolet. | Bolet. | Ec | ||||||||
| Chroogomphus rutilus (Schaeff.) O.K. Mill. * | x | Bolet. | Gomphidi. | Ec | ||||||||
| Clathrus ruber P. Micheli ex Pers. * | x | x | x | x | x | Phall. | Phall. | Sl | ||||
| Clavariadelphus pistillaris (L.) Donk | x | Phall. | Clavariadelph. | Ec | ||||||||
| Clavariadelphus truncatus Donk * | x | Phall. | Clavariadelph. | Ec | ||||||||
| Clavulina coralloides (L.) J. Schröt. * | x | x | Cantharell. | Hydn. | Sw | |||||||
| Clavulina rugosa (Bull.) J. Schröt. | x | Cantharell. | Hydn. | Sl | ||||||||
| Clitocybe costata Kühner & Romagn. * | x | x | Agaric. | Incertae sedis | Sl | |||||||
| Clitocybe dealbata (Sowerby) P. Kumm. * | x | x | Agaric. | Incertae sedis | Sl | |||||||
| Clitocybe infundibuliformis (Schaeff.) Quél. | x | Agaric. | Incertae sedis | Sl | ||||||||
| Clitocybe metachroa (Fr.) P. Kumm. * | x | x | Agaric. | Incertae sedis | Sl | |||||||
| Clitocybe nebularis (Batsch) P. Kumm. * | x | x | x | x | x | x | Agaric. | Incertae sedis | Sl | |||
| Clitocybe odora (Bull.) P. Kumm. * | x | x | Agaric. | Incertae sedis | Sl | |||||||
| Clitocybe phaeophthalma (Pers.) Kuyper | x | Agaric. | Incertae sedis | Sl | ||||||||
| Clitocybe phyllophila (Pers.) P. Kumm. * | x | x | Agaric. | Incertae sedis | Sl | |||||||
| Clitocybe rivulosa (Pers.) P. Kumm. | x | Agaric. | Incertae sedis | Sl | ||||||||
| Clitopilus geminus (Paulet)Noordel. & Co-David | x | x | Agaric. | Entolomat. | Ec | |||||||
| Clitopilus prunulus (Scop.) P. Kumm. * | x | x | x | x | x | Agaric. | Entolomat. | Ec | ||||
| Collybia butyracea (Bull.) P. Kumm. * | x | x | x | x | x | x | Agaric. | Omphalot. | Sl | |||
| Conocybe aporos Kits van Wav. | x | Agaric. | Bolbiti. | Sd | ||||||||
| Conocybe tenera (Schaeff.) Fayod | x | Agaric. | Bolbiti. | Sd | ||||||||
| Coprinellus disseminatus (Pers.) J.E. Lange * | x | x | x | x | Agaric. | Psathyrell. | Sw | |||||
| Coprinellus domesticus (Bolton) Vilgalys, Hopple & Jacq. Johnson | x | Agaric. | Psathyrell. | Sw | ||||||||
| Coprinellus micaceus (Bull.) Vilgalys, Hopple & Jacq. Johnson * | x | x | x | Agaric. | Psathyrell. | Sw | ||||||
| Coprinellus radians (Desm.) Vilgalys, Hopple & Jacq. Johnson * | x | Agaric. | Psathyrell. | Sw | ||||||||
| Coprinellus xanthothrix (Romagn.) Vilgalys, Hopple & Jacq. Johnson | x | x | Agaric. | Psathyrell. | Sw | |||||||
| Coprinopsis atramentaria (Bull.) Redhead, Vilgalys & Moncalvo * | x | x | x | x | Agaric. | Psathyrell. | Sw | |||||
| Coprinopsis picacea (Bull.) Redhead, Vilgalys & Moncalvo * | x | x | Agaric. | Psathyrell. | Sl | |||||||
| Coprinus comatus (O.F. Müll.) Pers. * | x | x | x | x | Agaric. | Agaric. | Sl | |||||
| Cortinarius anserinus (Velen.) Rob. Henry * | x | Agaric. | Cortinari. | Ec | ||||||||
| Cortinarius atrocaeruleus M.M. Moser * | x | x | x | Agaric. | Cortinari. | Ec | ||||||
| Cortinarius balteatocumatilis Rob. Henry | x | x | Agaric. | Cortinari. | Ec | |||||||
| Cortinarius camphoratus (Fr.) Fr. | x | Agaric. | Cortinari. | Ec | ||||||||
| Cortinarius cinnabarinus Fr. | x | Agaric. | Cortinari. | Ec | ||||||||
| Cortinarius cotoneus Fr. * | x | Agaric. | Cortinari. | Ec | ||||||||
| Cortinarius elegantior (Fr.) Fr. * | x | Agaric. | Cortinari. | Ec | ||||||||
| Cortinarius elegantissimus Rob. Henry | x | Agaric. | Cortinari. | Ec | ||||||||
| Cortinarius infractus (Pers.) Fr. | x | x | x | Agaric. | Cortinari. | Ec | ||||||
| Cortinarius rapaceus Fr. | x | Agaric. | Cortinari. | Ec | ||||||||
| Cortinarius splendens Rob. Henry * | x | x | x | Agaric. | Cortinari. | Ec | ||||||
| Cortinarius varius (Schaeff.) Fr. * | x | Agaric. | Cortinari. | Ec | ||||||||
| Craterellus cornucopioides (L.) Pers. * | x | x | Cantharell. | Hydn. | Ec | |||||||
| Craterellus lutescens (Fr.) Fr. | x | Cantharell. | Hydn. | Ec | ||||||||
| Craterellus tubaeformis (Fr.) Quél. | x | x | Cantharell. | Hydn. | Ec | |||||||
| Crucibulum laeve (Huds.) Kambly | x | x | x | x | Nidulari. | Incertae sedis | Sw | |||||
| Cuphophyllus pratensis (Pers.) Bon | x | Agaric. | Hygrophor. | Sl | ||||||||
| Cuphophyllus virgineus (Wulfen) Kovalenko | x | Agaric. | Hygrophor. | Sl | ||||||||
| Cupreoboletus poikilochromus (Pöder, Cetto & Zuccher.) Simonini, Gelardi & Vizzini | x | Bolet. | Bolet. | Ec | ||||||||
| Cyanoboletus pulverulentus (Opat.) Gelardi, Vizzini & Simonini * | x | x | x | x | Bolet. | Bolet. | Ec | |||||
| Cyathus olla (Batsch) Pers. * | x | x | Nidulari. | Incertae sedis | Sl | |||||||
| Cyathus striatus (Huds.) Willd. * | x | x | x | Nidulari. | Incertae sedis | Sw | ||||||
| Cyclocybe cylindracea (DC.) Vizzini & Angelini * | x | Agaric. | Strophari. | Sw | ||||||||
| Cystoderma amianthinum (Scop.) Fayod | x | x | x | x | Agaric. | Incertae sedis | Sl | |||||
| Cystoderma carcharias (Pers.) Fayod | x | Agaric. | Incertae sedis | Sl | ||||||||
| Daedalea quercina (L.) Pers. * | x | x | Agaric. | Fomitopsid. | Sw | |||||||
| Daedaleopsis confragosa (Bolton) J. Schröt. * | x | x | Polypor. | Polypor. | Sw | |||||||
| Daedaleopsis nitida (Durieu & Mont.) Zmitr. & Malysheva | x | Polypor. | Polypor. | Sw | ||||||||
| Daldinia concentrica (Bolton) Ces. & De Not. * | x | x | Bolet. | Hypoxyl. | Sw | |||||||
| Deconica coprophila (Bull.) P. Karst. | x | Agaric. | Strophari. | Sd | ||||||||
| Desarmillaria tabescens (Scop.) R.A.Koch & Aime * | x | x | Agaric. | Physalacri. | Pn | |||||||
| Echinoderma asperum (Pers.) Bon | x | x | x | Agaric. | Agaric. | Sl | ||||||
| Echinoderma echinaceum (J.E. Lange) Bon | x | Agaric. | Agaric. | Sl | ||||||||
| Entoloma aprile (Britzelm.) Sacc. * | x | Agaric. | Entolomat. | Ec | ||||||||
| Entoloma clypeatum (L.) P. Kumm. * | x | Agaric. | Entolomat. | Ec | ||||||||
| Entoloma lividoalbum (Kühner &Romagn.) Kubicka * | x | Agaric. | Entolomat. | Ec | ||||||||
| Entoloma sinuatum (Bull.) P. Kumm. * | x | x | x | x | x | x | Agaric. | Entolomat. | Ec | |||
| Fistulina hepatica (Schaeff.) With. * | x | Agaric. | Incertae sedis | Sw | ||||||||
| Fomes fomentarius (L.) Fr. * | x | Polypor. | Polypor. | Pn | ||||||||
| Fomitopsis pinicola (Sw.) P. Karst. | x | x | Polypor. | Polypor. | Sw | |||||||
| Fuscoporia torulosa (Pers.) T. Wagner & M. Fisch. | x | x | Polypor. | Hymenochaet. | Pn | |||||||
| Galerina marginata (Batsch) Kühner | x | Agaric. | Hymenogastr. | Sw | ||||||||
| Ganoderma applanatum (Pers.) Pat. * | x | x | Polypor. | Polypor. | Pn | |||||||
| Ganoderma lucidum (Curtis) P. Karst. * | x | x | Polypor. | Polypor. | Pn | |||||||
| Geastrum campestre Morgan | x | Phall. | Geastr. | Sl | ||||||||
| Geastrum fimbriatum Fr. * | x | x | Phall. | Geastr. | Sl | |||||||
| Geastrum triplex Jungh. * | x | x | x | x | x | x | x | x | Phall. | Geastr. | Sl | |
| Geopora sumneriana (Cooke ex W. Phillips) M. Torre | x | Peziz. | Pyronemat. | Sl | ||||||||
| Gliophorus psittacinus (Schaeff.) Herink | x | Agaric. | Hygrophor. | Sl | ||||||||
| Gloeophyllum abietinum (Bull.) P. Karst. | x | x | Polypor. | Gloeophyll. | Sw | |||||||
| Gloeophyllum sepiarium (Wulfen) P. Karst. * | x | x | Polypor. | Gloeophyll. | Sw | |||||||
| Grifola frondosa (Dicks.) Gray * | x | Polypor. | Grifol. | Sw | ||||||||
| Gymnopilus junonius (Fr.) P.D. Orton * | x | Agaric. | Hymenogastr. | Sw | ||||||||
| Gymnopus dryophilus (Bull.) Murrill | x | x | Agaric. | Omphalot. | Sw | |||||||
| Gymnopus foetidus (Sowerby) P.M. Kirk * | x | x | x | Agaric. | Omphalot. | Sw | ||||||
| Gymnopus fusipes (Bull.) Gray * | x | x | x | Agaric. | Omphalot. | Sw | ||||||
| Gyromitra esculenta (Pers.) Fr. * | x | Peziz. | Discin. | Sl | ||||||||
| Gyromitra gigas (Krombh.) Cooke * | x | Peziz. | Discin. | Sl | ||||||||
| Gyroporus castaneus (Bull.) Quél. * | x | x | x | x | x | x | Bolet. | Gyropor. | Ec | |||
| Gyroporus cyanescens (Bull.) Quél. * | x | Bolet. | Gyropor. | Ec | ||||||||
| Hapalopilus rutilans (Pers.) Murrill | x | x | Polypor. | Phanerochaet. | Sw | |||||||
| Hebeloma crustuliniforme (Bull.) Quél. * | x | x | x | Agaric. | Hymenogastr. | Ec | ||||||
| Hebeloma sacchariolens Quél. | x | Agaric. | Hymenogastr. | Ec | ||||||||
| Hebeloma sinapizans (Paulet) Gillet * | x | x | Agaric. | Hymenogastr. | Ec | |||||||
| Helvella crispa (Scop.) Fr. * | x | x | x | x | x | x | Peziz. | Helvell. | Sl | |||
| Helvella elastica Bull. * | x | x | Peziz. | Helvell. | Sl | |||||||
| Hemileccinum depilatum (Redeuilh) Šutara | x | x | Bolet. | Bolet. | Ec | |||||||
| Hemileccinum impolitum (Fr.) Šutara * | x | x | Bolet. | Bolet. | Ec | |||||||
| Hericium erinaceus (Bull.) Pers. | x | Russul. | Herici. | Sw | ||||||||
| Heterobasidion annosum (Fr.) Bref. * | x | x | Russul. | Bondarzewi. | Pn | |||||||
| Hohenbuehelia petaloides (Bull.) Schulzer | x | x | x | Agaric. | Pleurot. | Sw | ||||||
| Hortiboletus engelii (Hlavá?cek)Biketova & Wasser | x | Bolet. | Bolet. | Ec | ||||||||
| Hydnum repandum L. * | x | x | x | x | x | x | Cantharell. | Hydn. | Ec | |||
| Hygrocybe acutoconica (Clem.) Singer | x | Agaric. | Hygrophor. | St | ||||||||
| Hygrocybe coccinea (Schaeff.) P. Kumm. | x | Agaric. | Hygrophor. | St | ||||||||
| Hygrocybe conica (Schaeff.) P. Kumm. | x | Agaric. | Hygrophor. | St | ||||||||
| Hygrocybe nigrescens (Quél.) Kühner | x | Agaric. | Hygrophor. | St | ||||||||
| Hygrophoropsis aurantiaca (Wulfen) Maire | x | x | Bolet. | Hygrophoropsid. | Ec | |||||||
| Hygrophorus chrysodon (Batsch) Fr. * | x | x | x | x | Agaric. | Hygrophor. | Ec | |||||
| Hygrophorus cossus (Sowerby) Fr. | x | x | Agaric. | Hygrophor. | Ec | |||||||
| Hygrophorus discoxanthus (Fr.) Rea | x | Agaric. | Hygrophor. | Ec | ||||||||
| Hygrophorus eburneus (Bull.) Fr. * | x | Agaric. | Hygrophor. | Ec | ||||||||
| Hygrophorus erubescens (Fr.) Fr. | x | x | Agaric. | Hygrophor. | Ec | |||||||
| Hygrophorus marzuolus (Fr.) Bres. * | x | x | Agaric. | Hygrophor. | Ec | |||||||
| Hygrophorus nemoreus (Pers.) Fr. | x | Agaric. | Hygrophor. | Ec | ||||||||
| Hygrophorus penarioides Jacobson & E. Larss. | x | Agaric. | Hygrophor. | Ec | ||||||||
| Hygrophorus penarius Fr. | x | Agaric. | Hygrophor. | Ec | ||||||||
| Hygrophorus persoonii Arnolds * | x | x | x | Agaric. | Hygrophor. | Ec | ||||||
| Hygrophorus russula (Schaeff. ex Fr.) Kauffman * | x | x | Agaric. | Hygrophor. | Ec | |||||||
| Hymenopellis radicata (Relhan)R.H. Petersen * | x | Agaric. | Physalacri. | Sw | ||||||||
| Hymenoscyphus calyculus (Fr.) W. Phillips * | x | Heloti. | Heloti. | Sw | ||||||||
| Hypholoma fasciculare (Huds.) P. Kumm. * | x | x | x | x | x | x | x | Agaric. | Strophari. | Sw | ||
| Hypholoma lateritium (Schaeff.) P. Kumm. | x | x | x | x | x | x | x | Agaric. | Strophari. | Sw | ||
| Hypsizygus ulmarius (Bull.) Redhead | x | Agaric. | Lyophyll. | Sw | ||||||||
| Imperator luteocupreus (Bertéa & Estadès) Assyov, Bellanger et al. | x | Bolet. | Bolet. | Ec | ||||||||
| Imperator rhodopurpureus (Smotl.) Assyov, Bellanger, Bertéa et al. * | x | x | x | x | Bolet. | Bolet. | Ec | |||||
| Infundibulicybe geotropa (Bull.) Harmaja * | x | x | x | Agaric. | Incertae sedis | St | ||||||
| Infundibulicybe gibba (Pers.) Harmaja * | x | x | x | x | x | x | Agaric. | Incertae sedis | Sl | |||
| Infundibulicybe meridionalis (Bon) Pérez-De-Greg. | x | x | x | x | x | x | Agaric. | Incertae sedis | Sl | |||
| Inocybe amethystina Kuyper | x | x | Agaric. | Inocyb. | Ec | |||||||
| Inocybe assimilata Britzelm. * | x | Agaric. | Inocyb. | Ec | ||||||||
| Inocybe asterospora Quél. * | x | x | Agaric. | Inocyb. | Ec | |||||||
| Inocybe geophylla (Sowerby) P. Kumm. * | x | x | Agaric. | Inocyb. | Ec | |||||||
| Inocybe lanuginosa (Bull.) Kalchbr. | x | Agaric. | Inocyb. | Ec | ||||||||
| Inocybe pyriodora (Pers.) P. Kumm. | x | x | Agaric. | Inocyb. | Ec | |||||||
| Inocybe whitei (Berk. & Broome) Sacc. * | x | Agaric. | Inocyb. | Ec | ||||||||
| Inonotus hispidus (Bull.) P. Karst. * | x | Polypor. | Hymenochaet. | Pn | ||||||||
| Inosperma bongardii (Weinm.) Matheny &Esteve-Rav. | x | Agaric. | Inocyb. | Ec | ||||||||
| Laccaria amethystina Cooke * | x | x | x | x | Agaric. | Hydnangi. | Ec | |||||
| Laccaria bicolor (Maire) P.D. Orton * | x | Agaric. | Hydnangi. | Ec | ||||||||
| Laccaria laccata (Scop.) Cooke * | x | x | x | Agaric. | Hydnangi. | Ec | ||||||
| Lacrymaria lacrymabunda (Bull.) Pat. * | x | x | x | x | x | x | x | Agaric. | Inocyb. | Sl | ||
| Lactarius acerrimus Britzelm. * | x | Russul. | Russul. | Ec | ||||||||
| Lactarius atlanticus Bon | x | Russul. | Russul. | Ec | ||||||||
| Lactarius blennius (Fr.) Fr. * | x | Russul. | Russul. | Ec | ||||||||
| Lactarius chrysorrheus Fr. * | x | x | Russul. | Russul. | Ec | |||||||
| Lactarius deliciosus (L.) Gray * | x | Russul. | Russul. | Ec | ||||||||
| Lactarius fuliginosus (Fr.) Fr. * | x | Russul. | Russul. | Ec | ||||||||
| Lactarius mairei Malençon | x | Russul. | Russul. | Ec | ||||||||
| Lactarius salmonicolor R. Heim & Leclair * | x | Russul. | Russul. | Ec | ||||||||
| Lactarius sanguifluus (Paulet) Fr. * | x | Russul. | Russul. | Ec | ||||||||
| Lactarius semisanguifluus R. Heim & Leclair * | x | Russul. | Russul. | Ec | ||||||||
| Lactarius subumbonatus Lindgr. | x | Russul. | Russul. | Ec | ||||||||
| Lactarius zonarius (Bull.) Fr. * | x | x | x | x | Russul. | Russul. | Ec | |||||
| Lactifluus bertillonii (Neuhoff ex Z. Schaef.) Verbeken | x | x | Russul. | Russul. | Ec | |||||||
| Lactifluus piperatus (L.) Roussel * | x | Russul. | Russul. | Ec | ||||||||
| Lactifluus vellereus (Fr.) Kuntze * | x | x | x | Russul. | Russul. | Ec | ||||||
| Lactifluus volemus (Fr.) Kuntze * | x | x | x | Russul. | Russul. | Ec | ||||||
| Laetiporus sulphureus (Bull.) Murrill * | x | x | x | Polypor. | Laetipor. | Sw | ||||||
| Lanmaoa fragrans (Vittad.) Vizzini, Gelardi & Simonini * | x | Bolet. | Bolet. | Ec | ||||||||
| Leccinellum crocipodium (Letell.) Della Magg. & Trassin. * | x | Bolet. | Bolet. | Ec | ||||||||
| Leccinellum pseudoscabrum (Kallenb.) Mikšík * | x | Bolet. | Bolet. | Ec | ||||||||
| Leccinum aurantiacum (Bull.) Gray * | x | x | Bolet. | Bolet. | Ec | |||||||
| Leccinum duriusculum (Schulzer exKalchbr.) Singer | x | Bolet. | Bolet. | Ec | ||||||||
| Leccinum versipelle (Fr. & Hök) Snell | x | Bolet. | Bolet. | Ec | ||||||||
| Lentinus arcularius (Batsch) Zmitr. | x | x | x | Polypor. | Polypor. | Sw | ||||||
| Lentinus tigrinus (Bull.) Fr. | x | Polypor. | Polypor. | Sw | ||||||||
| Lepiota clypeolaria (Bull.) P. Kumm. * | x | x | x | x | Agaric. | Agaric. | Sl | |||||
| Lepiota cristata (Bolton) P. Kumm. * | x | x | Agaric. | Agaric. | Sl | |||||||
| Lepiota helveola Bres. * | x | Agaric. | Agaric. | Sl | ||||||||
| Lepiota ignivolvata Bousset & Joss. ex Joss. | x | x | Agaric. | Agaric. | Sl | |||||||
| Lepiota lilacea Bres. | x | x | Agaric. | Agaric. | Sl | |||||||
| Lepista irina (Fr.) H.E. Bigelow * | x | Agaric. | Incertae sedis | Sl | ||||||||
| Lepista nuda (Bull.) Cooke * | x | x | x | x | Agaric. | Incertae sedis | Sl | |||||
| Lepista panaeolus (Fr.) P. Karst. | x | Agaric. | Incertae sedis | Sl | ||||||||
| Lepista sordida (Schumach.) Singer | x | x | x | Agaric. | Incertae sedis | Sl | ||||||
| Leratiomyces ceres (Cooke & Massee) Spooner & Bridge | x | x | Agaric. | Strophari. | Sl | |||||||
| Leucoagaricus americanus (Peck) Vellinga | x | Agaric. | Agaric. | St | ||||||||
| Leucoagaricus leucothites (Vittad.) Wasser | x | x | Agaric. | Agaric. | St | |||||||
| Leucocortinarius bulbiger (Alb.& Schwein.) Singer | x | Agaric. | Incertae sedis | Ec | ||||||||
| Leucopaxillus gentianeus (Quél.) Kotl. | x | Agaric. | Tricholomat. | Sl | ||||||||
| Limacellopsis guttata (Pers.) Zhu L. Yang, Q. Cai & Y.Y. Cui | x | x | Agaric. | Amanit. | Sl | |||||||
| Lycoperdon echinatum Pers. | x | x | x | Lycoperd. | Lycoperd. | Sl | ||||||
| Lycoperdon excipuliforme (Scop.) Pers. | x | Lycoperd. | Lycoperd. | Sl | ||||||||
| Lycoperdon mammiforme Pers. | x | x | Lycoperd. | Lycoperd. | Sl | |||||||
| Lycoperdon perlatum Pers. * | x | x | Lycoperd. | Lycoperd. | St | |||||||
| Lycoperdon pratense Pers. | x | Lycoperd. | Lycoperd. | St | ||||||||
| Lycoperdon pyriforme Schaeff. * | x | Lycoperd. | Lycoperd. | Sw | ||||||||
| Lyophyllum decastes (Fr.) Singer * | x | x | Agaric. | Lyophyll. | St | |||||||
| Lyophyllum infumatum (Bres.) Kühner | x | Agaric. | Lyophyll. | St | ||||||||
| Macrolepiota excoriata (Schaeff.) Wasser | x | Agaric. | Agaric. | Sl | ||||||||
| Macrolepiota mastoidea (Fr.) Singer | x | x | Agaric. | Agaric. | Sl | |||||||
| Macrolepiota procera (Scop.) Singer * | x | x | x | x | x | Agaric. | Agaric. | Sl | ||||
| Marasmiellus candidus (Fr.) Singer | x | Agaric. | Omphalot. | Sw | ||||||||
| Marasmius collinus (Scop.) Singer * | x | Agaric. | Marasmi. | St | ||||||||
| Marasmius oreades (Bolton) Fr. * | x | Agaric. | Marasmi. | St | ||||||||
| Marasmius rotula (Scop.) Fr. * | x | Agaric. | Marasmi. | Sw | ||||||||
| Marasmius wynneae Berk. & Broome * | x | x | Agaric. | Marasmi. | Sw | |||||||
| Megacollybia platyphylla (Pers.) Kotl. & Pouzar * | x | Agaric. | Incertae sedis | Sl | ||||||||
| Melanoleuca brevipes (Bull.) Pat. * | x | Agaric. | Incertae sedis | Sl | ||||||||
| Melanoleuca cognata (Fr.) Konrad & Maubl. | x | Agaric. | Incertae sedis | Sl | ||||||||
| Melanoleuca grammopodia (Bull.) Murrill * | x | Agaric. | Incertae sedis | Sl | ||||||||
| Melanoleuca melaleuca (Pers.) Murrill | x | Agaric. | Incertae sedis | Sl | ||||||||
| Meripilus giganteus (Pers.) P. Karst. * | x | x | Polypor. | Meripil. | Sw | |||||||
| Morchella costata Pers. * | x | Peziz. | Morchell. | Sl | ||||||||
| Morchella deliciosa Fr. * | x | Peziz. | Morchell. | Sl | ||||||||
| Morchella esculenta (L.) Pers. * | x | Peziz. | Morchell. | Sl | ||||||||
| Mucidula mucida (Schrad.) Pat. | x | Agaric. | Physalacri. | Sw | ||||||||
| Mutinus caninus (Huds.) Fr. * | x | x | x | Phall. | Phall. | Sl | ||||||
| Mycena arcangeliana Bres. | x | Agaric. | Mycen. | Sw | ||||||||
| Mycena epipterygia (Scop.) Gray * | x | Agaric. | Mycen. | St | ||||||||
| Mycena haematopus (Pers.) P. Kumm. | x | x | x | x | Agaric. | Mycen. | Sw | |||||
| Mycena meliigena (Berk. & Cooke) Sacc. * | x | x | Agaric. | Mycen. | St | |||||||
| Mycena pelianthina (Fr.) Quél. * | x | Agaric. | Mycen. | Sl | ||||||||
| Mycena pura (Pers.) P. Kumm. * | x | x | x | x | x | x | Agaric. | Mycen. | Sl | |||
| Mycena renati Quél. * | x | x | x | Agaric. | Mycen. | Sw | ||||||
| Mycena rosea Gramberg * | x | x | x | x | x | x | Agaric. | Mycen. | Sl | |||
| Mycetinis alliaceus (Jacq.) Earle ex A.W. Wilson & Desjardin * | x | Agaric. | Omphalot. | Sl | ||||||||
| Mycetinis scorodonius (Fr.) A.W. Wilson & Desjardin * | x | Agaric. | Marasmi. | Sl | ||||||||
| Neoboletus praestigiator (R. Schulz) Svetash., Gelardi, Simonini & Vizzini * | x | x | x | x | Bolet. | Bolet. | Ec | |||||
| Neoboletus xanthopus (Klofac & A. Urb.) Klofac & A. Urb. | x | Bolet. | Bolet. | Ec | ||||||||
| Neofavolus alveolaris (DC.) Sotome & T. Hatt | x | Polypor. | Polypor. | Sw | ||||||||
| Neolentinus cyathiformis (Schaeff.) DellaMagg. & Trassin. | x | Polypor. | Gloeophyll. | Sw | ||||||||
| Omphalotus olearius (DC.) Singer * | x | x | x | x | Agaric. | Omphalot. | Sw | |||||
| Panaeolus acuminatus (P. Kumm.) Quél. * | x | Agaric. | Incertae sedis | Sd | ||||||||
| Panaeolus papilionaceus (Bull.) Quél. * | x | x | Agaric. | Incertae sedis | Sd | |||||||
| Panaeolus semiovatus (Sowerby) S. Lundell & Nannf. | x | Agaric. | Incertae sedis | Sd | ||||||||
| Paragymnopus perforans (Hoffm.) J.S. Oliveira * | x | Agaric. | Omphalot. | Sw | ||||||||
| Paralepista flaccida (Sowerby) Vizzini * | x | Agaric. | Incertae sedis | Sl | ||||||||
| Parasola plicatilis (Curtis) Redhead, Vilgalys & Hopple | x | Agaric. | Psathyrell. | Sl | ||||||||
| Paxillus involutus (Batsch) Fr. * | x | x | x | x | Bolet. | Paxill. | Ec | |||||
| Paxillus rubicundulus P.D. Orton | x | bolet. | Paxill. | Ec | ||||||||
| Phallus impudicus L. * | x | x | x | Phall. | Phall. | Sl | ||||||
| Phellinus pomaceus (Pers.) Maire | x | Polypor. | Hymenochaet. | Sw | ||||||||
| Phlebia rufa (Pers.: Fr.) M.P. Christ. | x | x | x | Polypor. | Meruli. | Sw | ||||||
| Pholiota adiposa (Batsch) P. Kumm. | x | Agaric. | Strophari. | Sw | ||||||||
| Pholiota gummosa (Lasch) Singer | x | Agaric. | Strophari. | Sw | ||||||||
| Pholiota squarrosa (Vahl) P. Kumm. | x | x | Agaric. | Strophari. | Sw | |||||||
| Phylloporus pelletieri (Lév.) Quél. | x | Bolet. | Bolet. | Ec | ||||||||
| Picipes badius (Persoon) Zmitr. & Kovalenko | x | x | Polypor. | Polypor. | Sw | |||||||
| Picipes melanopus (Pers.) Zmitr. & Kovalenko | x | Polypor. | Polypor. | Sw | ||||||||
| Pisolithus arhizus (Scop.) Rauschert | x | x | Bolet. | Sclerodermat. | Ec | |||||||
| Pleurotus cornucopiae (Paulet) Rolland * | x | Agaric. | Pleurot. | Sw | ||||||||
| Pleurotus eryngii (DC.) Quél. var. eryngii * | x | Agaric. | Pleurot. | Sw | ||||||||
| Pleurotus eryngii (DC.) Quél. var. ferulae (Lanzi) Sacc. * | x | Agaric. | Pleurot. | Sw | ||||||||
| Pleurotus ostreatus (Jacq.) P. Kumm. * | x | x | x | Agaric. | Pleurot. | Sw | ||||||
| Pluteus cervinus (Schaeff.) P. Kumm. * | x | x | Agaric. | Plute. | Sw | |||||||
| Polyporus tuberaster (Jacq. ex Pers.) Fr. * | x | x | Polypor. | Polypor. | Sw | |||||||
| Porphyriellus porphyrosporus (Fr. & Hok) A.H. Sm. & Thiers | x | x | Bolet. | Bolet. | Ec | |||||||
| Porpoloma macrorhizum (Quél.) Bon Pseudoclitocybaceae | x | x | Agaric. | Pseudoclitocyb. | Sw | |||||||
| Protostropharia semiglobata (Batsch) Redhead, Moncalvo & Vilgalys | x | Agaric. | Strophari. | Sd | ||||||||
| Pseudosperma rimosum (Bull.) Matheny & Esteve-Rav. * | x | Agaric. | Inocyb. | Ec | ||||||||
| Psilocybe coronilla (Bull.) Noordel. * | x | Agaric. | Hymenogaster. | st | ||||||||
| Psilocybe semilanceata (Fr.) P. Kumm. | x | Agaric. | Hymenogastr. | Sd | ||||||||
| Psilocybe serbica M.M. Moser & E. Horak | x | Agaric. | Hymenogastr. | Sd | ||||||||
| Ramaria aurea (Schaeff.) Quél. | x | x | Phall. | Gomph. | Ec | |||||||
| Ramaria botrytis (Pers.) Bourdot * | x | x | x | x | x | Phall. | Gomph. | Ec | ||||
| Ramaria flava (Schaeff.) Quél. * | x | x | x | x | Phall. | Gomph. | Ec | |||||
| Ramaria formosa (Pers.) Quél. * | x | x | x | Phall. | Gomph. | Ec | ||||||
| Ramaria gracilis (Pers.) Quél. * | x | Phall. | Gomph. | Ec | ||||||||
| Ramaria pallida (Schaeff.) Ricken * | x | Phall. | Gomph. | Ec | ||||||||
| Ramaria stricta (Pers.) Quél. * | x | x | x | x | x | x | x | Phall. | Gomph. | Ec | ||
| Rheubarbariboletus armeniacus (Quél.) Vizzini, Simonini & Gelardi * | x | x | Bolet. | Bolet. | Ec | |||||||
| Rheubarbariboletus persicolor (H. Engel, Klofac, H. Grünert & R. Grünert) Vizzini, Simonini & Gelardi | x | x | Bolet. | Bolet. | Ec | |||||||
| Rhizopogon roseolus (Corda) Th. Fr. | x | Bolet. | Rhizopogon. | Ec | ||||||||
| Rhodocollybia maculata (Alb.& Schwein.) Singer * | x | x | Agaric. | Omphalot. | Sl | |||||||
| Rhodotus palmatus (Bull.) Maire | x | Agaric. | Physalacri. | Sw | ||||||||
| Rubroboletus legaliae (Pilát & Dermek) DellaMagg. & Trassin. * | x | x | Bolet. | Bolet. | Ec | |||||||
| Rubroboletus lupinus (Fr.) Costanzo, Gelardi, Simonini & Vizzini * | x | x | Bolet. | Bolet. | Ec | |||||||
| Rubroboletus pulchrotinctus (Alessio) Kuan Zhao & Zhu L. Yang | x | Bolet. | Bolet. | Ec | ||||||||
| Rubroboletus rhodoxanthus (Krombh.) KuanZhao & Zhu L. Yang | x | x | x | Bolet. | Bolet. | Ec | ||||||
| Rubroboletus rubrosanguineus (Cheype) Kuan Zhao & Zhu L. Yang | x | x | Bolet. | Bolet. | Ec | |||||||
| Rubroboletus satanas (Lenz) Kuan Zhao &Zhu L. Yang * | x | x | x | x | Bolet. | Bolet. | Ec | |||||
| Russula acrifolia Romagn. * | x | Russul. | Russul. | Ec | ||||||||
| Russula adusta (Pers.) Fr. | x | Russul. | Russul. | Ec | ||||||||
| Russula albonigra (Krombh.) Fr. * | x | x | x | Russul. | Russul. | Ec | ||||||
| Russula alutacea (Fr.) Fr. * | x | Russul. | Russul. | Ec | ||||||||
| Russula amoena Quél. | x | x | Russul. | Russul. | Ec | |||||||
| Russula amoenicolor Romagn. | x | x | Russul. | Russul. | Ec | |||||||
| Russula atropurpurea (Krombh.) Britzelm. * | x | Russul. | Russul. | Ec | ||||||||
| Russula aurea Pers. * | x | x | Russul. | Russul. | Ec | |||||||
| Russula cavipes Britzelm. | x | Russul. | Russul. | Ec | ||||||||
| Russula chloroides (Krombh.) Bres. | x | x | x | x | x | x | x | Russul. | Russul. | Ec | ||
| Russula curtipes F.H. Møller & Jul. Schäff. | x | x | x | x | Russul. | Russul. | Ec | |||||
| Russula delica Fr. * | x | x | x | x | x | Russul. | Russul. | Ec | ||||
| Russula densifolia Secr. ex Gillet * | x | Russul. | Russul. | Ec | ||||||||
| Russula faginea Romagn. | x | Russul. | Russul. | Ec | ||||||||
| Russula foetens Pers. * | x | x | Russul. | Russul. | Ec | |||||||
| Russula heterophylla (Fr.) Fr. * | x | x | x | Russul. | Russul. | Ec | ||||||
| Russula illota Romagn. | x | Russul. | Russul. | Ec | ||||||||
| Russula insignis Quél. | x | x | Russul. | Russul. | Ec | |||||||
| Russula luteotacta Rea | x | Russul. | Russul. | Ec | ||||||||
| Russula nigricans Fr. * | x | Russul. | Russul. | Ec | ||||||||
| Russula nobilis Velen. | x | Russul. | Russul. | Ec | ||||||||
| Russula ochroleuca Fr. * | x | Russul. | Russul. | Ec | ||||||||
| Russula olivacea (Schaeff.) Fr. * | x | Russul. | Russul. | Ec | ||||||||
| Russula pallidospora J. Blum ex Romagn. | x | Russul. | Russul. | Ec | ||||||||
| Russula praetervisa Sarnari | x | Russul. | Russul. | Ec | ||||||||
| Russula risigallina (Batsch) Sacc. * | x | x | Russul. | Russul. | Ec | |||||||
| Russula romellii Maire | x | Russul. | Russul. | Ec | ||||||||
| Russula rosea Pers. | x | x | x | Russul. | Russul. | Ec | ||||||
| Russula rubroalba (Singer) Romagn. | x | x | x | Russul. | Russul. | Ec | ||||||
| Russula sanguinea Fr. * | x | Russul. | Russul. | Ec | ||||||||
| Russula solaris Ferd. & Winge | x | Russul. | Russul. | Ec | ||||||||
| Russula sororia (Fr.) Romell * | x | Russul. | Russul. | Ec | ||||||||
| Russula torulosa Bres. * | x | Russul. | Russul. | Ec | ||||||||
| Russula vesca Fr. * | x | x | Russul. | Russul. | Ec | |||||||
| Russula violeipes Quél. * | x | Russul. | Russul. | Ec | ||||||||
| Russula virescens (Schaeff.) Fr. * | x | x | x | Russul. | Russul. | Ec | ||||||
| Sarcoscypha coccinea (Gray) Boud. * | x | Peziz. | Sarcoscyph. | Sl | ||||||||
| Schizophyllum commune Fr. * | x | x | x | x | x | x | Agaric. | Schizophyll. | Sw | |||
| Scleroderma citrinum Pers. * | x | Bolet. | Sclerodermat. | Ec | ||||||||
| Scleroderma meridionale Demoulin & Malençon | x | x | Bolet. | Sclerodermat. | Ec | |||||||
| Scleroderma polyrhizum (J.F. Gmel.) Pers. * | x | x | Bolet. | Sclerodermat. | Ec | |||||||
| Scleroderma verrucosum (Bull.) Pers. * | x | x | x | Bolet. | Sclerodermat. | Ec | ||||||
| Scutiger pes-caprae (Pers.) Bondartsev & Singer * | x | Russul. | Albatrell. | Ec | ||||||||
| Stereum hirsutum (Willd.) Pers. Stereaceae | x | x | x | Russul. | Stere. | Sw | ||||||
| Strobilomyces strobilaceus (Scop.) Berk. * | x | Bolet. | Bolet. | Ec | ||||||||
| Strobilurus esculentus (Wulfen) Singer | x | Agaric. | Physalacri. | Sc | ||||||||
| Strobilurus tenacellus (Pers.) Singer | x | Agaric. | Physalacri. | Sc | ||||||||
| Stropharia aeruginosa (Curtis) Quél. * | x | x | Agaric. | Strophari. | Sl | |||||||
| Stropharia caerulea Kreisel * | x | x | Agaric. | Strophari. | Sl | |||||||
| Stropharia rugosoannulata Farl. ex Murrill | x | Agaric. | Strophari. | Sl | ||||||||
| Suillellus luridus (Schaeff.) Murrill * | x | x | x | x | x | Bolet. | Bolet. | Ec | ||||
| Suillellus mendax (Simonini & Vizzini) Vizzini, Simonini & Gelardi | x | Bolet. | Bolet. | Ec | ||||||||
| Suillellus queletii (Schulzer) Vizzini, Simonini & Gelardi * | x | x | x | Bolet. | Bolet. | Ec | ||||||
| Suillus bellinii (Inzenga) Kuntze | x | Bolet. | Suill. | Ec | ||||||||
| Suillus bovinus (L.) Roussel * | x | Bolet. | Suill. | Ec | ||||||||
| Suillus collinitus (Fr.) Kuntze * | x | Bolet. | Suill. | Ec | ||||||||
| Suillus granulatus (L.) Roussel * | x | Bolet. | Suill. | Ec | ||||||||
| Suillus lakei (Murrill) A.H. Sm. & Thiers | x | Bolet. | Suill. | Ec | ||||||||
| Suillus mediterraneensis (Jacquet. & J. Blum) Redeuilh | x | Bolet. | Suill. | Ec | ||||||||
| Tapinella atrotomentosa (Batsch) Šutara | x | x | Bolet. | Tapinell. | Sw | |||||||
| Tapinella panuoides (Fr.) E.-J. Gilbert | x | Bolet. | Tapinell. | Sw | ||||||||
| Thaxterogaster purpurascens (Fr.) Niskanen & Liimat. * | x | x | x | x | Agaric. | Cortinari. | Ec | |||||
| Thelephora palmata (Scop.) Fr. * | x | x | Thelephor. | Thelephor. | Ec | |||||||
| Thelephora terrestris Ehrh. | x | x | x | Thelephor. | Thelephor. | Ec | ||||||
| Trametes gibbosa (Pers.) Fr. * | x | x | Polypor. | Polypor. | Sw | |||||||
| Trametes hirsuta (Wulfen) Lloyd * | x | x | x | Polypor. | Polypor. | Sw | ||||||
| Trametes trogii Berk. | x | Polypor. | Polypor. | Sw | ||||||||
| Trametes versicolor (L.) Lloyd * | x | x | x | x | x | x | Polypor. | Polypor. | Sw | |||
| Tremella mesenterica Retz. * | x | Tremell. | Tremell. | Sw | ||||||||
| Tricholoma acerbum (Bull.) Quél. * | x | Agaric. | Tricholomat. | Ec | ||||||||
| Tricholoma album (Schaeff.) P. Kumm. * | x | x | x | x | x | x | Agaric. | Tricholomat. | Ec | |||
| Tricholoma argyraceum (Bull.) Gillet | x | Agaric. | Tricholomat. | Ec | ||||||||
| Tricholoma atrosquamosum Sacc. | x | Agaric. | Tricholomat. | Ec | ||||||||
| Tricholoma basirubens (Bon) A. Riva & Bon | x | Agaric. | Tricholomat. | Ec | ||||||||
| Tricholoma columbetta (Fr.) P. Kumm. * | x | x | x | Agaric. | Tricholomat. | Ec | ||||||
| Tricholoma equestre (L.) P. Kumm. * | x | Agaric. | Tricholomat. | Ec | ||||||||
| Tricholoma quercetorum Contu | x | x | Agaric. | Tricholomat. | Ec | |||||||
| Tricholoma saponaceum (Fr.) P. Kumm. * | x | x | x | x | x | x | Agaric. | Tricholomat. | Ec | |||
| Tricholoma scalpturatum (Fr.) Quél. | x | x | x | x | Agaric. | Tricholomat. | Ec | |||||
| Tricholoma sciodes (Pers.) C. Martín | x | Agaric. | Tricholomat. | Ec | ||||||||
| Tricholoma sejunctum (Sowerby) Quél. * | x | x | x | x | x | x | Agaric. | Tricholomat. | Ec | |||
| Tricholoma sulphureum (Bull.) P. Kumm. * | x | x | x | Agaric. | Tricholomat. | Ec | ||||||
| Tricholoma terreum (Schaeff.) P. Kumm. * | x | Agaric. | Tricholomat. | Ec | ||||||||
| Tricholoma ustale (Fr.) P. Kumm. * | x | x | x | x | x | Agaric. | Tricholomat. | Ec | ||||
| Tricholoma ustaloides Romagn. | x | x | x | x | Agaric. | Tricholomat. | Ec | |||||
| Tricholomopsis rutilans (Schaeff.) Singer * | x | x | Agaric. | Incertae sedis | Sw | |||||||
| Tuber aestivum Vittad. | x | x | Peziz. | Tuber. | Ec | |||||||
| Tuber borchii Vittad. * | x | x | x | Peziz. | Tuber. | Ec | ||||||
| Tuber brumale Vittad. * | x | x | Peziz. | Tuber. | Ec | |||||||
| Tuber excavatum Vittad. * | x | x | Peziz. | Tuber. | Ec | |||||||
| Tuber mesentericum Vittad. * | x | x | Peziz. | Tuber. | Ec | |||||||
| Tulostoma brumale Pers. * | x | Agaric. | Agaric. | Sm | ||||||||
| Typhula fistulosa (Holmsk.) Olariaga | x | Agaric. | Typhul. | Sw | ||||||||
| Verpa conica (O.F. Müll.) Sw. | x | Peziz. | Morchell. | Sl | ||||||||
| Volvariella surrecta (Knapp) Singer | x | Agaric. | Plute. | Pm | ||||||||
| Volvariella volvacea (Bull.) Singer * | x | Agaric. | Plute. | Sl | ||||||||
| Volvopluteus gloiocephalus (DC.) Vizzini, Contu & Justo * | x | Agaric. | Plute. | St | ||||||||
| Xerocomellus chrysenteron (Bull.) Šutara | x | x | x | Bolet. | Bolet. | Ec | ||||||
| Xerocomellus porosporus (Imler exWatling) Šutara | x | Bolet. | Bolet. | Ec | ||||||||
| Xerocomellus pruinatus (Fr. & Hök) Šutara | x | x | x | x | x | Bolet. | Bolet. | Ec | ||||
| Xerocomus rubellus Quél. * | x | x | Bolet. | Bolet. | Ec | |||||||
| Xerocomus subtomentosus (L.) Quél. * | x | x | x | x | x | x | x | x | Bolet. | Bolet. | Ec | |
| Xylaria hypoxylon (L.: Fr.) Grev. | x | x | x | Xylari. | Xylari. | Sw | ||||||
| Xylaria polymorpha (Pers.: Fr.) Grev. * | x | Xylari. | Xylari. | Sw |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotti, M. Checklist of Macrofungi Associated with Nine Different Habitats of Taburno-Camposauro Massif in Campania, Southern Italy. J. Fungi 2024, 10, 275. https://doi.org/10.3390/jof10040275
Zotti M. Checklist of Macrofungi Associated with Nine Different Habitats of Taburno-Camposauro Massif in Campania, Southern Italy. Journal of Fungi. 2024; 10(4):275. https://doi.org/10.3390/jof10040275
Chicago/Turabian StyleZotti, Maurizio. 2024. "Checklist of Macrofungi Associated with Nine Different Habitats of Taburno-Camposauro Massif in Campania, Southern Italy" Journal of Fungi 10, no. 4: 275. https://doi.org/10.3390/jof10040275
APA StyleZotti, M. (2024). Checklist of Macrofungi Associated with Nine Different Habitats of Taburno-Camposauro Massif in Campania, Southern Italy. Journal of Fungi, 10(4), 275. https://doi.org/10.3390/jof10040275






