The Phylogeny and Taxonomy of Cryptothecia (Arthoniaceae, Ascomycota) and Myriostigma (Arthoniaceae, Ascomycota), including Three New Species and Two New Records from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphology and Anatomy
2.2. Colour Reaction
2.3. Chemical Analysis
2.4. DNA Extraction, PCR Amplification and Sequencing
2.5. Sequence Alignment and Phylogenetic Analysis
3. Results
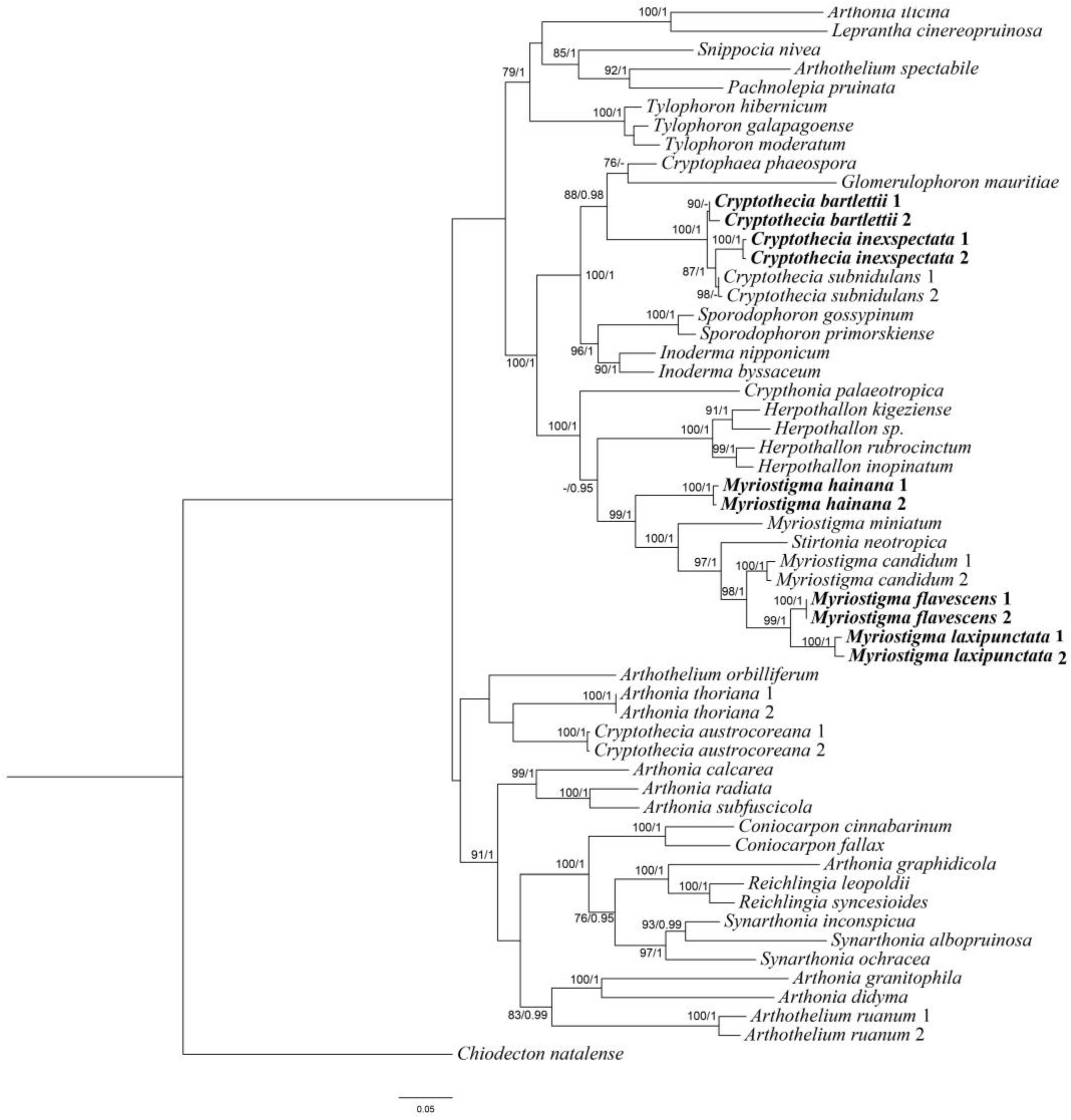
3.1. Phylogenetic Analyses
3.2. Taxonomy
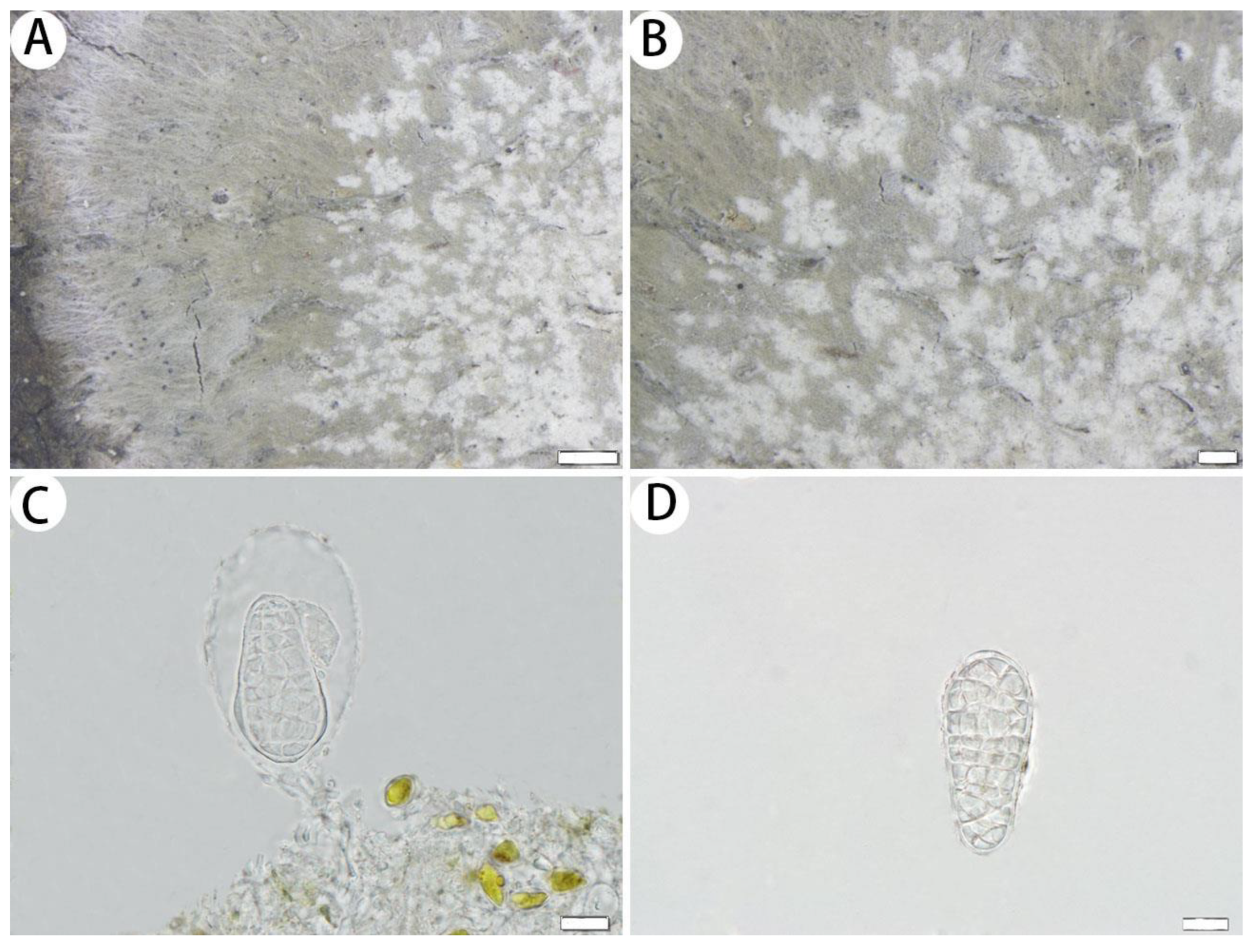
3.2.1. The New Species
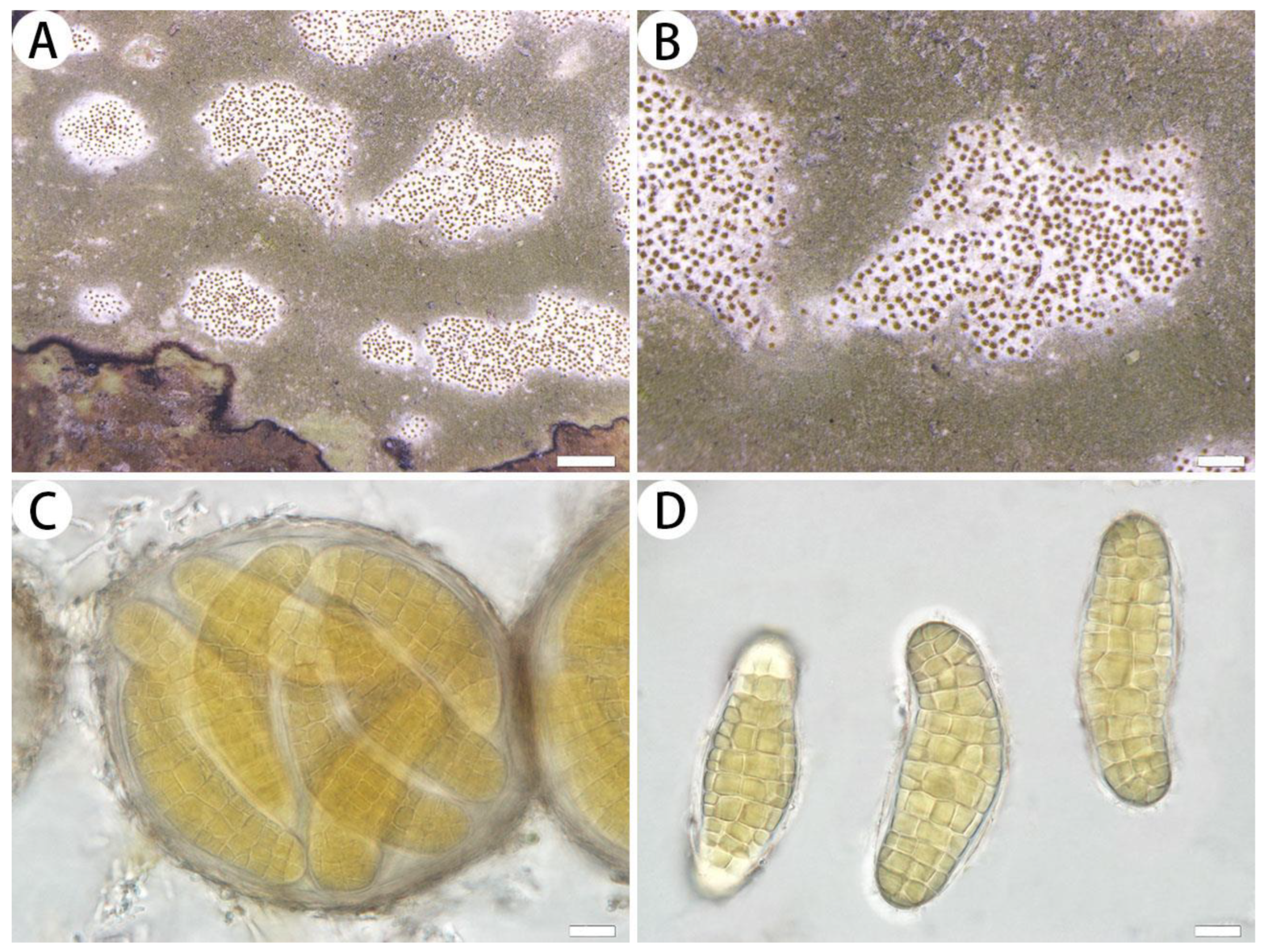
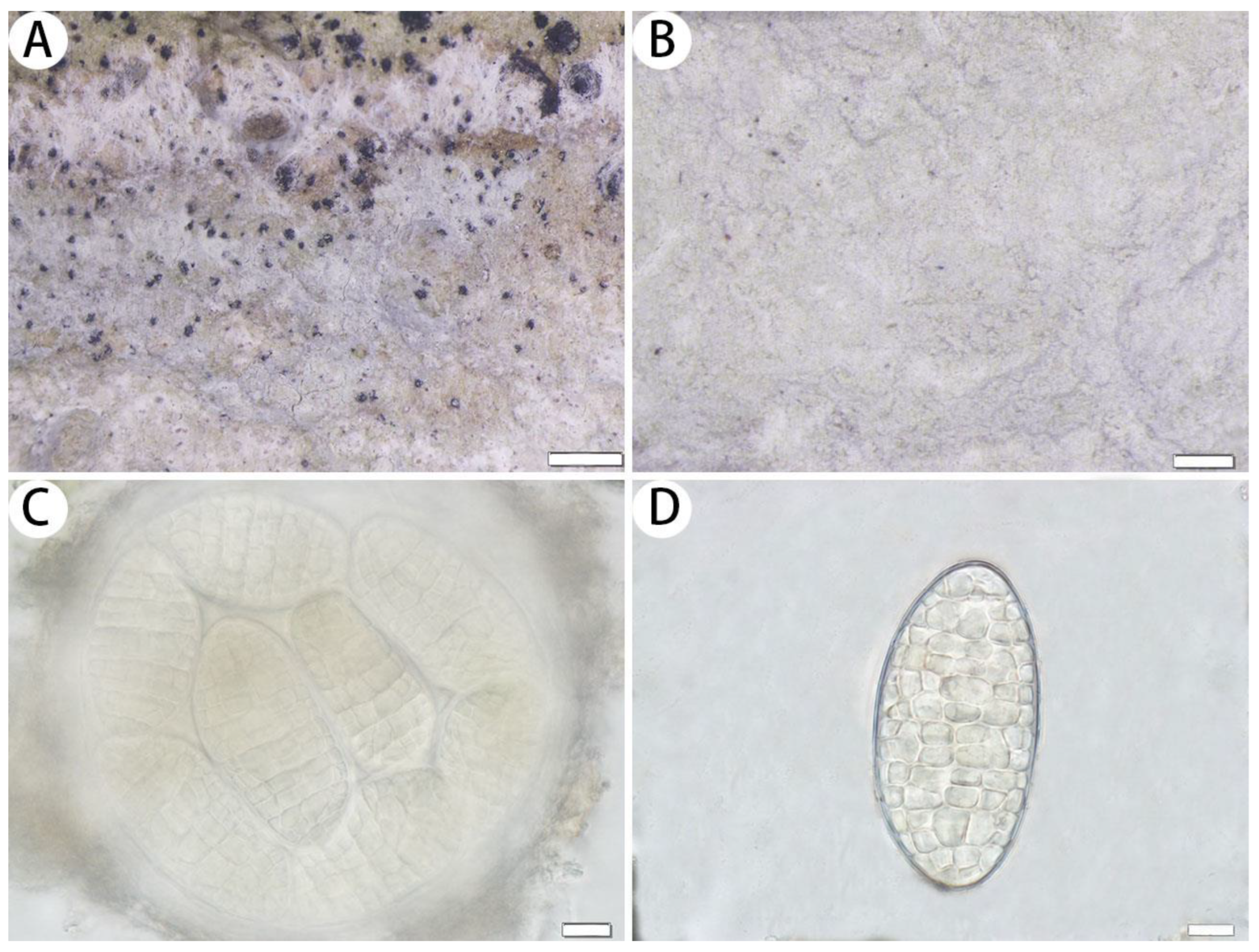
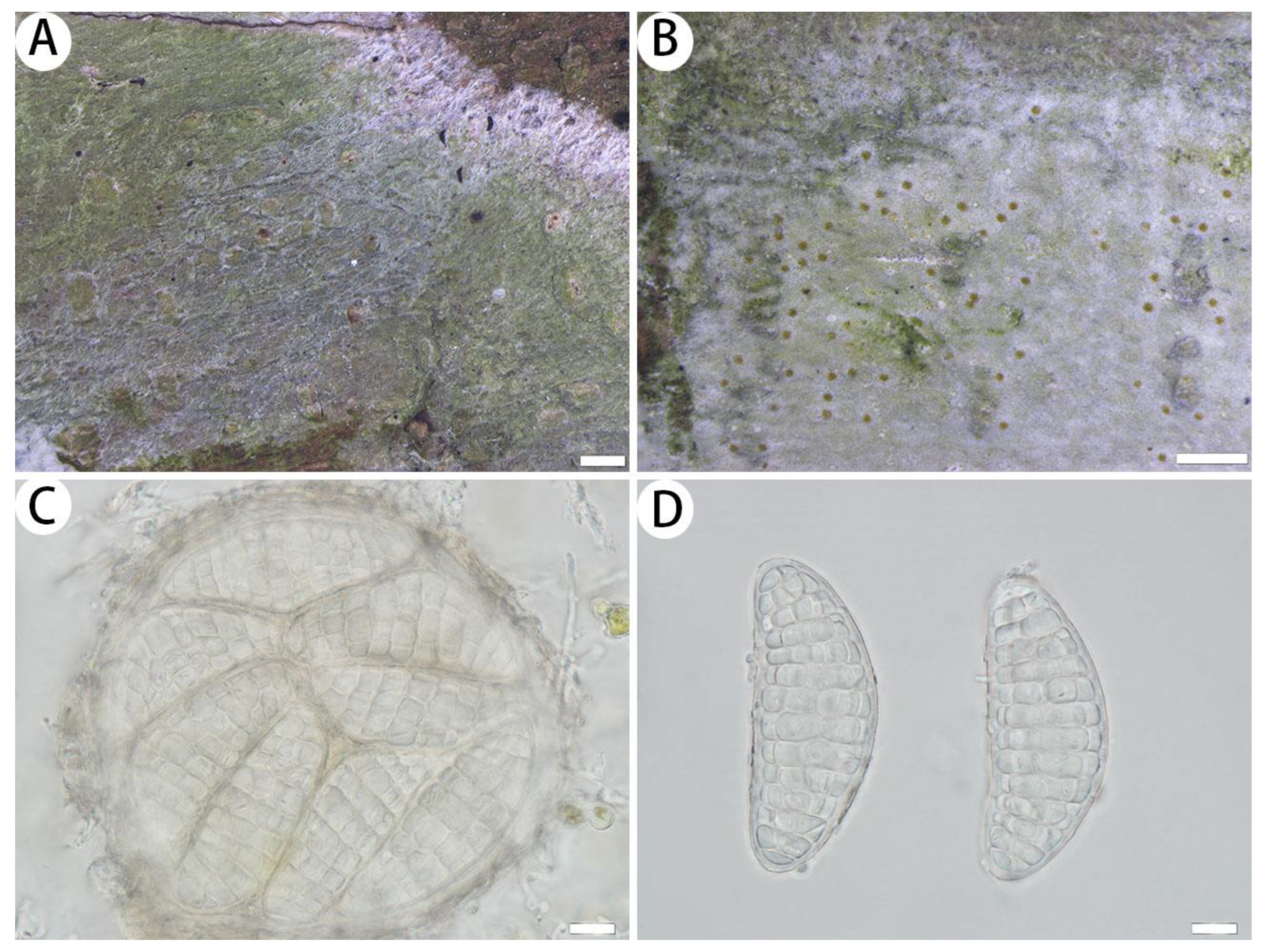
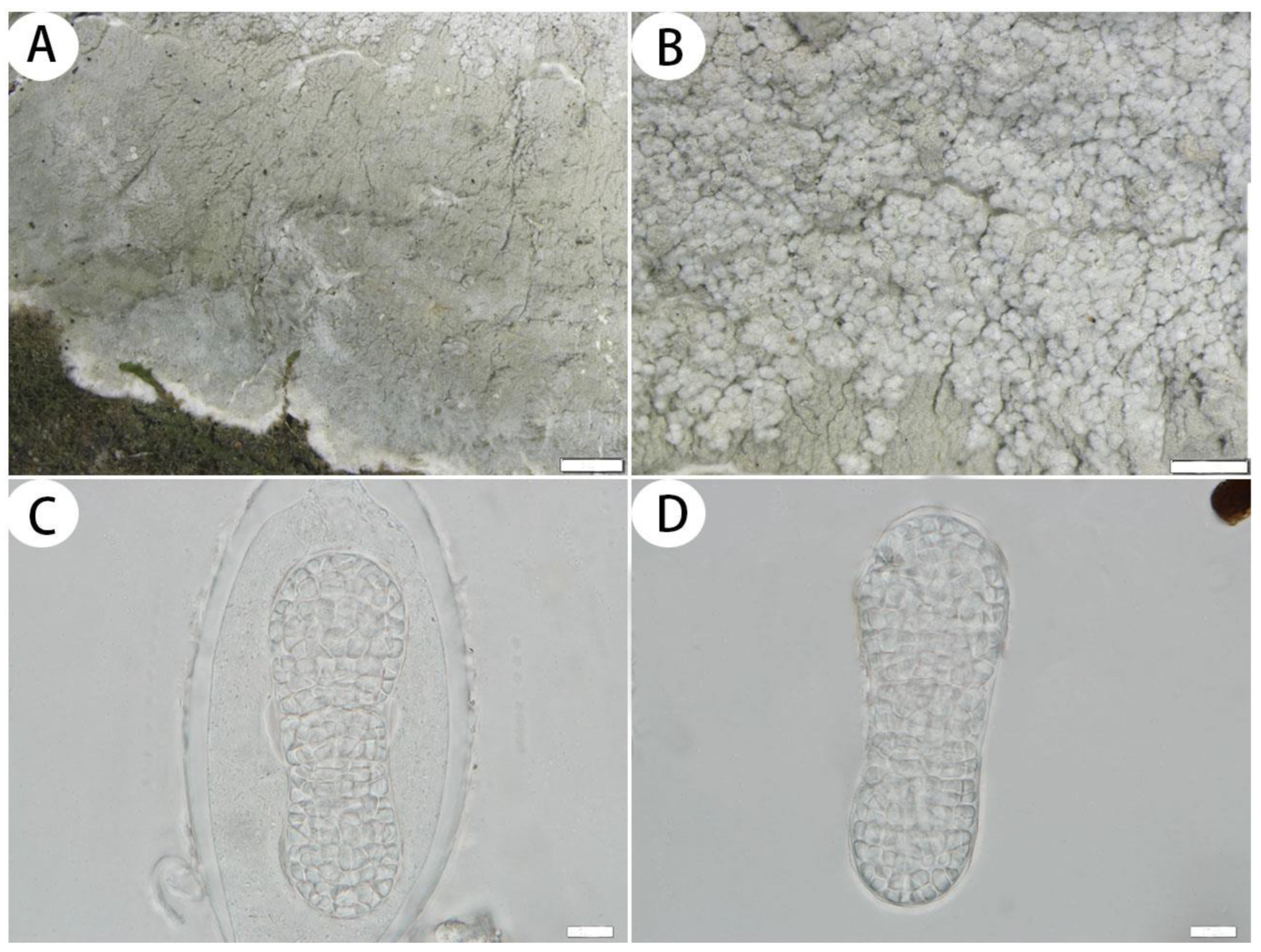
3.2.2. The New Records
| 1a. | Asci 1–2-spored—2 |
| 1b. | Asci 8-spored—4 |
| 2a. | Thallus P+ yellow; with psoromic acid—C. subnidulans |
| 2b. | Thallus P–; without psoromic acid—3 |
| 3a. | Ascigerous areas whitish and usually radiately elongated; ascospores 33–50 × 16–22 µm—C. inexspectata |
| 3b. | Ascigerous areas generally covered with globose isidia-like structures; ascospores (49–)68–100(–105) × (18–)23–36(–42) µm—C. bartlettii |
| 4a. | Thallus without lichen substances—5 |
| 4b. | Thallus with lichen substances—6 |
| 5a. | Ascospores narrow; 60–76 × 17–30 µm—C. aleurella |
| 5b. | Ascospores broad; 65–108 × 42–50 µm—C. aleurocarpa |
| 6a. | Thallus C+ red; with gyrophoric or 2′-O-methylanziaic acids—7 |
| 6b. | Thallus C–; without gyrophoric or 2′-O-methylanziaic acids—10 |
| 7a. | Thallus with gyrophoric acid and additional substances—8 |
| 7b. | Thallus with 2′-O-methylanziaic and 2′-O-methylperlatolic acids; ascospores 40–65 × 12–25 µm—M. candidum |
| 8a. | Ascigerous areas indistinct; additionally with methyl 2′-O-methylmicrophyllinate; ascospores 52–88 × 24–47 µm—M. hainana |
| 8b. | Ascigerous areas distinct; additionally with confluentic acid—9 |
| 9a. | Ascigerous areas white with dense brown dots; ascospores yellow; 58–76 × 19–28 µm—M. flavescens |
| 9b. | Ascigerous areas pale greenish with loose brown dots; ascospores hyaline; 57–78 × 24–33 µm—M. laxipunctata |
| 10a. | Thallus P+ yellow; with psoromic acid; ascospores 50–70 × 30–37 µm—C. polymorpha |
| 10b. | Thallus P–; without psoromic acid; ascospores 27–40 × 12–20 µm—C. subtecta |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eriksson, O.; Hawksworth, D.L. Outline of the ascomycetes. Syst. Ascomycetum 1993, 12, 51–257. [Google Scholar]
- Thor, G. The genus Cryptothecia in Australia and New Zealand and the circumscription of the genus. Symb. Bot. Ups. 1997, 32, 267–289. [Google Scholar]
- Aptroot, A.; Feuerstein, S.C.; Cunha-Dias, I.P.R.; de Lucena Nunes, Á.R.; Honorato, M.E.; Cáceres, M.E.S. New lichen species and lichen reports from Amazon forest remnants and Cerrado vegetation in the Tocantina Region, northern Brazil. Bryologist 2017, 120, 320–328. [Google Scholar] [CrossRef]
- Aptroot, A.; Souza, M.F. New crustose lichens from a tropical coastal area in Paraná (Brazil). Cryptogam. Mycol. 2021, 42, 191–197. [Google Scholar] [CrossRef]
- Schumm, F.; Aptroot, A. Brazilian Lichens; Books on Demand GmbH: Norderstedt, Germany, 2024; pp. 1–564. [Google Scholar]
- Aptroot, A.; Cáceres, M.E.S.; Santos, L.A. The taxonomy of sterile Arthoniaceae from Brazil: White crusts on overhanging tropical trees can be named. Lichenologist 2024, 56, 1–13. [Google Scholar] [CrossRef]
- Frisch, A.; Thor, G.; Ertz, D.; Grube, M. The Arthonialean challenge: Restructuring Arthoniaceae. Taxon 2014, 63, 727–744. [Google Scholar] [CrossRef]
- Aptroot, A.; Spier, J.L. The lichen genus Cryptothecia (Arthoniaceae) in Java. Australas. Lichenol. 2010, 66, 50–57. [Google Scholar]
- Bungartz, F.; Dutan-Patino, V.L.; Elix, J.A. The lichen genera Cryptothecia, Herpothallon and Helminthocarpon (Arthoniales) in the Galapagos Islands, Ecuador. Lichenologist 2013, 45, 739–762. [Google Scholar] [CrossRef]
- Ertz, D.; Flakus, A.; Oset, M.; Sipman, J.M.H.; Kukwa, M. A first assessment of lichenized Arthoniales in Bolivia with descriptions of two new species. Phytotaxa 2015, 217, 1–25. [Google Scholar] [CrossRef]
- Jagadeesh Ram, T.A.M.; Sinha, G.P. A world key to Cryptothecia and Myriostigma (Arthoniaceae), with new species and new records from the Andaman and Nicobar Islands, India. Phytotaxa 2016, 266, 103–114. [Google Scholar]
- Woo, J.J.; LőKös, L.; Farkas, E.; Park, C.H.; Hur, J.S. Cryptothecia austrocoreana (Arthoniales, Arthoniaceae), a New Species from South Korea. Mycobiology 2017, 45, 338–343. [Google Scholar] [CrossRef][Green Version]
- Singh, P. A new species of Cryptothecia (Arthoniales, Arthoniaceae) from the Western Ghats biodiversity hotspot, India. Phytotaxa 2019, 409, 101–104. [Google Scholar] [CrossRef]
- Borgato, L.; Ertz, D. Cryptothecia aleurodes (Arthoniaceae), a misunderstood species. Phytotaxa 2020, 449, 90–94. [Google Scholar] [CrossRef]
- Aptroot, A.; de Souza, M.F.; dos Santos, L.A.; Junior, I.O.; Cardoso Barbosa, B.M.; da Silva, M.E.C. New species of lichenized fungi from Brazil, with a record report of 492 species in a small area of the Amazon Forest. Bryologist 2022, 125, 433–465. [Google Scholar] [CrossRef]
- Silva, T.E.F.; Silva, M.S.R.C.; Santos, M.A.L.; Cáceres, M.E.S.; Aptroot, A.; Vitória, N.S. Two new Arthoniales species (Lichenized Ascomycota) from Brazil. RGSA–Rev. Gestão Soc. Ambient. 2023, 18, 1–13. [Google Scholar] [CrossRef]
- Aptroot, A. Annotated checklist of Hongkong Lichens. Trop. Bryol. 1999, 17, 57–101. [Google Scholar]
- Aptroot, A.; Sparrius, L.B. New microlichens from Taiwan. Fungal Divers. 2003, 14, 1–50. [Google Scholar]
- Orange, A.; James, P.W.; White, F.J. Microchemical Methods for the Identification of Lichens, 2nd ed.; British Lichen Society: London, UK, 2010; pp. 1–101. [Google Scholar]
- Elix, J.A. A Catalogue of Standardized Thin-Layer Chromatographic Data and Biosynthetic Relationships for Lichen Substances, 3rd ed.; Australian National University: Canberra, Australia, 2014. [Google Scholar]
- Zoller, S.; Scheidegger, C.; Sperisen, C. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 1999, 31, 511–516. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Miadlikowska, J.; Lutzoni, F. Phylogenetic revision of the genus Peltigera (lichen-forming ascomycetes) based on morphological, chemical and large subunit nuclear ribosomal DNA data. Int. J. Plant Sci. 2000, 161, 925–985. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Stamatakis, A. RaxML version 8: A tool for phylogenetic analysis and postanalysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree, v. 1.4.0. Institute of Evolutionary Biology, University of Edinburgh, 2012. Available online: http://tree.bio.ed.ac.uk/software/Figtree/ (accessed on 2 March 2024).
- Aptroot, A. A revision of the lichen genus Stirtonia. Lichenologist 2009, 41, 1–11. [Google Scholar] [CrossRef]
- Lücking, R.; Thor, G.; Aptroot, A.; Kalb, K.; Elix, J.A. The Cryptothecia candida complex revisited. Lichenologist 2006, 38, 235–240. [Google Scholar] [CrossRef]
- Aptroot, A.; Rodrigues, A.F. New lichen records for the Azores, with the report of some tropical species new to Europe. Cryptogam. Mycol. 2005, 26, 273–280. [Google Scholar]
- Das, K.; Rossi, W.; Leonardi, M.; Ghosh, A.; Bera, I.; Hembrom, M.E.; Bajpai, R.; Joseph, S.; Nayaka, S.; Upreti, D.K.; et al. Fungal Biodiversity Profiles 61-70. Cryptogam. Mycol. 2018, 39, 381–418. [Google Scholar] [CrossRef]
- Aptroot, A.; Ertz, D.; Silva, J.R.; Grube, M.; Cáceres, M.E.S. The phylogenetic position of Coniarthonia and the transfer of Cryptothecia miniata to Myriostigma (Arthoniaceae, lichenized ascomycetes). Phytotaxa 2015, 218, 128–136. [Google Scholar] [CrossRef]
- Neuwirth, G.; Aptroot, A. Cryptothecia stockeri (Arthoniales, Arthoniaceae), a New Corticolous Lichen Species from the Seychelles. Herzogia 2016, 29, 97–102. [Google Scholar] [CrossRef]
- Thor, G. The Placement of Chiodecton sanguineum (syn. Chiodecton rubrocinctum), and Cryptothecia striata sp. nov. Bryologist 1991, 94, 278–283. [Google Scholar]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota–Approaching one thousand genera. Bryologist 2016, 119, 361–416. [Google Scholar] [CrossRef]
- Thiyagaraja, V.; Lücking, R.; Ertz, D.; Wanasinghe, D.N.; Karunarathna, S.C.; Camporesi, E.; Hyde, K.D. Evolution of non-lichenized, saprotrophic species of Arthonia (Ascomycota, Arthoniales) and resurrection of Naevia, with notes on Mycoporum. Fungal Divers. 2020, 102, 205–224. [Google Scholar] [CrossRef]
- Zhu, H. The tropical forests of Southern China and conservation of biodiversity. Bot. Rev. 2017, 83, 87–105. [Google Scholar] [CrossRef]
- Wei, J.C. The Enumeration of Lichenized Fungi in China; China Forestry Publishing House: Beijing, China, 2020; pp. 1–606. [Google Scholar]
| Species Name | Voucher Specimen | GenBank Accession Number | ||
|---|---|---|---|---|
| mtSSU | RPB2 | nLSU | ||
| Arthonia calcarea | Thor 11/6a (UPS) | KJ850974 | KJ851105 | – |
| Arthonia didyma | Ertz 7587 (BR) | EU704047 | EU704010 | EU704083 |
| Arthonia granitophila | Frish 10/Se74 (UPS) | KJ850981 | KJ851107 | KJ851049 |
| Arthonia graphidicola | Frisch 10/Jp102 (UPS) | KJ850980 | – | KJ851034 |
| Arthonia ilicina | McCune 31067 | KJ850982 | – | – |
| Arthonia radiata | Frisch 10/Se29 (UPS) | KJ850968 | KJ851108 | – |
| Arthonia subfuscicola | Thor 11/1 (UPS) | KJ850971 | KJ851110 | – |
| Arthonia thoriana 1 | Sanderson 2176 (BR) | MG207687 | – | – |
| Arthonia thoriana 2 | Sanderson 2174 (herb.Sanderson) | MG207685 | – | – |
| Arthothelium orbilliferum | TRH-L-15449 | KY983977 | – | – |
| Arthothelium ruanum 1 | KoLRI 038018 | MF616609 | MF616619 | – |
| Arthothelium ruanum 2 | KoLRI 038261 | MF616611 | MF616621 | – |
| Arthothelium spectabile | Frisch 12Jp179a (TNS) | KP870144 | KP870160 | – |
| Chiodecton natalense | Ertz 6576 (BR) | EU704051 | EU704014 | EU704085 |
| Coniocarpon cinnabarinum | Johnsen 111003 (UPS) | KJ850976 | KJ851103 | KJ851083 |
| Coniocarpon fallax | LD:L10075 | KJ850979 | KJ851101 | – |
| Crypthonia palaeotropica | Frisch 11/Ug457 (UPS) | KJ850961 | KJ851084 | – |
| Cryptophaea phaeospora | Van den Broeck 5809 (BR) | KX077541 | – | – |
| Cryptothecia austrocoreana 1 | KolRI No. 041892 | MF769375 | – | – |
| Cryptothecia austrocoreana 2 | KolRI No. 044721 | MF769374 | – | – |
| Cryptothecia bartlettii 1 | Zhang et al. 20220297 (SDNU) | PP051262 | – | – |
| Cryptothecia bartlettii 2 | Zhang et al. 20220275 (SDNU) | PP051261 | – | – |
| Cryptothecia inexspectata 1 | Liu et al. 20230668 (SDNU) | PP051263 | PP109371 | – |
| Cryptothecia inexspectata 2 | Liu et al. 20230639 (SDNU) | PP051264 | PP109370 | – |
| Cryptothecia subnidulans 1 | v.d.Boom 40613 (hd v.d. Boom) | KJ850952 | KJ851087 | – |
| Cryptothecia subnidulans 2 | Joensson Guyana 6a (UPS) | KJ850953 | KJ851088 | – |
| Glomerulophoron mauritiae | Ertz 19164 (BR) | KP870153 | KP870167 | – |
| Herpothallon inopinatum | Rudolphi 12 (UPS) | KJ850964 | KJ851099 | – |
| Herpothallon kigeziense | Frisch 11/Ug26 (UPS) | KF707644 | KF707654 | – |
| Herpothallon rubrocinctum | Rudolphi 5 (UPS) | KF707643 | KF707655 | – |
| Herpothallon sp. | Frisch 11/Ug401 (UPS) | KF707645 | KF707653 | – |
| Inoderma byssaceum | Thor 25952 (UPS) | KJ850962 | KJ851089 | KJ851040 |
| Inoderma nipponicum | Frisch 12Jp227 (TNS) | KP870146 | KP870162 | – |
| Leprantha cinereopruinosa | Kukwa 17127 & Lubek (BR) | MG207692 | – | – |
| Myriostigma candidum 1 | Ertz 9260 (BR) | EU704052 | EU704015 | HQ454520 |
| Myriostigma candidum 2 | Frisch 11/Ug125 (UPS) | KJ850959 | KJ851096 | – |
| Myriostigma flavescens 1 | Liu et al. 20230612 (SDNU) | PP051268 | PP130144 | – |
| Myriostigma flavescens 2 | Liu et al. 20230641 (SDNU) | PP051267 | – | – |
| Myriostigma hainana 1 | Xue et al. 20230061 (SDNU) | PP051271 | PP101845 | – |
| Myriostigma hainana 2 | Xue et al. 20230050 (SDNU) | PP051272 | PP109365 | – |
| Myriostigma laxipunctata 1 | Liu et al. 20231231 (SDNU) | PP051266 | PP109369 | PP033944 |
| Myriostigma laxipunctata 2 | Liu et al. 20231052 (SDNU) | PP051265 | PP109368 | PP033943 |
| Myriostigma miniatum | Silva T2A29 (ISE—epitype) | KP843606 | – | – |
| Pachnolepia pruinata | Frisch 11/Se34 (UPS) | KJ850967 | KJ851098 | – |
| Reichlingia leopoldii | Ertz 13293 | JF830773 | HQ454722 | HQ454581 |
| Reichlingia syncesioides | Frisch 11/Ug14 (UPS) | KF707651 | KF707656 | KF707636 |
| Snippocia nivea | Ertz 17437 (BR) | MG207695 | – | – |
| Stirtonia neotropica | Cáceres & Aptroot 11112 (ISE) | KP843611 | – | – |
| Sporodophoron gossypinum | Frisch 12Jp186 (TNS) | KP870154 | KP870168 | – |
| Sporodophoron primorskiense | Ohmura 10509 (TNS) | KP870157 | KP870169 | – |
| Synarthonia albopruinosa | VDB 6086 (BR<BEL>) | MH251873 | – | – |
| Synarthonia inconspicua | VDB 7013B (BR<BEL>) | MH251881 | – | – |
| Synarthonia ochracea | VDB 6653 (BR<BEL>) | MH251884 | – | – |
| Tylophoron galapagoense | Bungartz 8749 (CDS) | JF830776 | – | JF295078 |
| Tylophoron hibernicum | Frisch 11/Ug220 (UPS) | KJ850966 | KJ851097 | KJ851065 |
| Tylophoron moderatum | Ertz 14504 (BR) | JF830780 | – | JF295085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Cai, Y.; Zhang, L. The Phylogeny and Taxonomy of Cryptothecia (Arthoniaceae, Ascomycota) and Myriostigma (Arthoniaceae, Ascomycota), including Three New Species and Two New Records from China. J. Fungi 2024, 10, 274. https://doi.org/10.3390/jof10040274
Xue J, Cai Y, Zhang L. The Phylogeny and Taxonomy of Cryptothecia (Arthoniaceae, Ascomycota) and Myriostigma (Arthoniaceae, Ascomycota), including Three New Species and Two New Records from China. Journal of Fungi. 2024; 10(4):274. https://doi.org/10.3390/jof10040274
Chicago/Turabian StyleXue, Junxia, Yutong Cai, and Lulu Zhang. 2024. "The Phylogeny and Taxonomy of Cryptothecia (Arthoniaceae, Ascomycota) and Myriostigma (Arthoniaceae, Ascomycota), including Three New Species and Two New Records from China" Journal of Fungi 10, no. 4: 274. https://doi.org/10.3390/jof10040274
APA StyleXue, J., Cai, Y., & Zhang, L. (2024). The Phylogeny and Taxonomy of Cryptothecia (Arthoniaceae, Ascomycota) and Myriostigma (Arthoniaceae, Ascomycota), including Three New Species and Two New Records from China. Journal of Fungi, 10(4), 274. https://doi.org/10.3390/jof10040274





