Abstract
This study aims to report three new species of Conoideocrella and Moelleriella from Yunnan Province, Southwestern China. Species of Conoideocrella and Moelleriella parasitize scale insects (Coccidae and Lecaniidae, Hemiptera) and whiteflies (Aleyrodidae, Hemiptera). Based on the phylogenetic analyses of the three-gene nrLSU, tef-1α, and rpb1, it showed one new record species (Conoideocrella tenuis) and one new species (Conoideocrella fenshuilingensis sp. nov.) in the genus Conoideocrella, and two new species, i.e., Moelleriella longzhuensis sp. nov. and Moelleriella jinuoana sp. nov. in the genus Moelleriella. The three new species were each clustered into separate clades that distinguished themselves from one another. All of them were distinguishable from their allied species based on their morphology. Morphological descriptions, illustrations, and comparisons of the allied taxa of the four species are provided in the present paper. In addition, calculations of intraspecific and interspecific genetic distances were performed for Moelleriella and Conoideocrella.
1. Introduction
In the family Clavicipitaceae, there are some genera that have been found to parasitize scale insects or whiteflies, such as Aschersonia Mont., Dussiella Pat., Helicocollum Luangsaard, Mongkols., Noisrip. & Thanak, Hyperdermium J.F. White, R.F. Sullivan, Bills & Hywel-Jones, Hypocrella Sacc., Moelleriella Bres., Orbiocrella D. Johnson, G.H. Sung, Hywel-Jones & Spatafora, Regiocrella Chaverri & K.T. Hodge, and Samuelsia P. Chaverri & K.T. Hodge [1,2,3,4,5]. Among them, Aschersonia, Hypocrella, and Moelleriella are relatively old genera and have more species. A taxonomic revision was undertaken by Chaverri et al. (2008) for the species formerly belonging to Hypocrella Sacc. s. l. (anamorph Aschersonia Mont. s. l.). They used three-gene phylogenetic analyses and morphological characters to classify Hypocrella Sacc. s. l. into Hypocrella s. str. (anamorph Aschersonia), Moelleriella (anamorph aschersonia-like), and Samuelsia (anamorph aschersonia-like) [3]. Moelleriella was distinguished from Hypocrella s. str. and Samuelsia by the fact that their ascospores can be disarticulated at the septa within the ascus, whereas those of the latter two cannot [3].
The genus Moelleriella was erected by Bresadola in 1896 to accommodate the type species M. sulphurea, which is currently regarded as a synonym of M. phyllogena (Mont.) P. Chaverri & K.T. Hodge (basionym Hypocrella phyllogena (Mont.) Speg.) [3]. There are currently 65 species in this genus, all of which have brightly colored stromata; obpyriform to subglobose perithecia; cylindrical asci; filiform multiseptate ascospores that disarticulate at the septa inside the ascus; and aschersonia-like anamorphs with fusoid conidia (according to the Index Fungorum, which is available online at http://www.indexfungorum.org; accessed on 11 December 2023 [6]). Among them, there are 30 species from the New World and 35 species from the Old World (according to the Index Fungorum, which is available online at http://www.indexfungorum.org; accessed on 29 August 2023 [3,6,7,8,9,10,11,12,13,14,15]). In China, four new species have been reported. Two species were reported in Yunnan Province, and another two were reported in Fujian Province [11,13,14,15]. In addition to these new published species, Moelleriella has also been distributed in other provinces in China. However, previous studies have primarily focused on morphology, with few molecular data available in public databases [16,17,18,19].
Torrubiella Boud. species infect a wide range of arthropods, mainly spiders and scale insects [4,20]. The genus currently has about 80 records in the index (according to the Index Fungorum, which is available online at http://www.indexfungorum.org; accessed on 11 December 2023). However, the fact that the previous studies identified species on the basis of their morphological characteristics resulted in a lack of molecular data for most species of Torrubiella. With the advent of molecular technology and the application of multigene phylogenetic analyses, species identification methods based on phylogenetic analyses combined with morphological characteristics have gradually gained recognition. Johnson et al. (2009) found that previous phylogenetic studies had shown that the genus Torrubiella was not monophyletic, but none of them had attempted to resolve this [4,21,22]. Subsequently, a multigene phylogenetic tree covering 10 species of Torrubiella was constructed by Johnson et al. to determine the phylogenetic position of these species [4]. Phylogenetic analyses showed that these species were distributed in Clavicipitaceae, Cordycipitaceae, and Ophiocordycipitaceae [4]. Torrubiella tenuis (Petch) D. Johnson, G.-H. Sung, Hywel-Jones & Spatafora and Torrubiella luteorostrata (Zimm.) D. Johnson, G.H. Sung, Hywel-Jones & Spatafora form a statistically well-supported clade in Clavicipitaceae. Therefore, a new genus, Conoideocrella, was proposed by Johnson et al. to accommodate the species T. tenuis and T. luteorostrata, and T. luteorostrata was designated as the type species [4]. The genus Conoideocrella was named thus for its perithecium with a conical shape that is similar to that of Torrubiella [4]. It currently contains three species, all of which have elongate, conical perithecia and planar stromata [23,24]. Conoideocrella luteorostrata was shown to be distributed in Seychelles, Sri Lanka, Java, Samoa, New Zealand, the far Eastern U.S.S.R., and Thailand [23,25]. Conoideocrella tenuis was known to be distributed in Sri Lanka and Thailand, and C. krungchingensis was known only to be in Thailand [23,24,25]. All three species have been reported to be able to parasitize scale insects [23,24].
Entomopathogenic fungi are widely distributed in China, and Yunnan Province is one of the richest provinces in China in terms of biodiversity. In this study, we collected some specimens with macro-morphological similarities to Moelleriella and Conoideocrella during an investigation of entomopathogenic fungi in Yunnan. A three-gene phylogenetic analysis revealed two new species of Moelleriella, one new species, and one known species of Conoideocrella. Conoideocrella tenuis is a recently newly recorded species in China.
2. Materials and Methods
2.1. Fungal Collection and Isolation
The specimens were collected from Bampo village, Jinuo Township, Jinghong City, and the Fenshuiling National Nature Reserve, Jinping County, Yunnan Province, China. In fields, whole leaves with stromata were collected, and some bark from branches with stromata was chipped off with a pocket knife. Then they were placed in sterilized plastic boxes and brought to the laboratory. The detailed procedure to obtain axenic cultures in this study was described in Yang et al. [15]. After the isolation of pure cultures, they were transplanted to PDA slant and grown for 10 days before being stored at 4 °C. The specimens were deposited in the Yunnan Herbal Herbarium (YHH) of Yunnan University, China. The strains were deposited at the Yunnan Fungal Culture Collection (YFCC) of Yunnan University, China.
2.2. Morphological Observations
Because of the small size of the stromata, a dissecting microscope (SZ61, Olympus Corporation, Tokyo, Japan) was used to observe their macro-morphological characteristics and measure them. The stromata were sectioned with a thickness of 30~40 µm for observations of their micro-morphological features using a HM525NX freezing microtome (Thermo Fisher Scientific, Waltham, MA, USA). The sections were placed on slides dripping with water or lactic acid in cotton blue. The observations were made and measurements were taken using a light microscope (Olympus BX53, Olympus Corporation, Tokyo, Japan). In order to observe and record the color and texture of the colonies, several new plates were transferred from the purified colonies and incubated in a 25 °C incubator for three weeks. The growth rate of colonies was used according to the method of Liu and Hodge [26] and was categorized as follows: fast growing (30–35 mm in diameter), moderately growing (20–30 mm in diameter), and slow growing (<20 mm in diameter).
2.3. DNA Extraction, PCR, and Sequencing
The specimens were washed with 75% alcohol, and the genomic DNA was extracted using the Genomic DNA Purification Kit (Qiagen GmbH, Hilden, Germany). The DNA of the cultures was extracted using cetyltrimethyl ammonium bromide (CTAB) following the procedure described by Liu et al. [26]. Three genes (nrLSU, tef-1α, and rpb1) were sequenced, and the following primer pairs were used for PCR amplification. LR5 (5′-ATCCTGAGGGAAACTTC-3′) and LR0R (5′-GTACCCGCTGAACTTAAGC-3′) were used to amplify the nuclear ribosomal large subunit (nrLSU) [27,28]. EF1α-EF (5′-GCTCCYGGHCAYCGTGAYTTYAT-3′) and EF1α-ER (5′-ATGACACCRACRGCRACRGTYTG-3′) were used to amplify the translation elongation factor 1α (tef-1α) [22,29]. RPB1-5′F (5′-CAYCCWGGYTTYATCAAGAA-3′) and RPB1-5′R (5′-CCNGCDATNTCRTTRTCCATRTA-3′) were used to amplify the largest subunits of RNA polymerase II (rpb1) [22,29]. The polymerase chain reaction (PCR) matrix and the PCR reactions were performed as described by Wang et al. [30]. A BIORAD T100TM thermal cycler (BIO-RAD Laboratories, Hercules, CA, USA) was used to perform amplification reactions. Then the PCR products were sequenced by the Beijing Genomics Institute (Chongqing, China).
2.4. Phylogenetic Analyses
Datasets of three genes (nrLSU, tef-1α, and rpb1) used to construct a phylogenetic tree were downloaded from GenBank and combined with the newly generated data in this study. The sequences downloaded were based on previous studies by Mongkolsamrit et al. [24] and Yang et al. [15]. Names, voucher information, and corresponding GenBank accession numbers of the taxa are listed in Table 1. Sequences were aligned, and poorly aligned regions were removed with MEGA v.6.06 [31]. The aligned three-gene sequences were concatenated using Phylosuite v1.2.2 [32]. Phylogenetic analyses were performed using BI and ML methods [33,34]. A maximum likelihood (ML) tree was created using IQ-tree v.2.1.3, and a Bayesian inference (BI) tree was created using MrBayes v.3.2.2 [35,36]. Modelfinder was used to select the best-fitting likelihood model [37]. The optimal model for the ML analyses was the TIM2+F+I+G4 model, with 5000 rapid bootstraps in a single run [38]. The optimal model for the BI analysis was the GTR+F+I+G4 model. The four Markov chain Monte Carlo simulations ran for 2 million generations from a random start tree with a sampling frequency of 100 generations. Twenty-five percent of initial sampled data were discarded as burn-in. Phylogenetic trees were viewed and edited in Figtree v.1.4.3 and visualized in Adobe Illustrator CS6. The interspecies and intraspecies genetic distances for the three genes (tef-1α, rpb1, and nrLSU) in Moelleriella and Conoideocrella were calculated using MEGAE v.6.06. Genetic distances were calculated by selecting the maximum composite likelihood model.

Table 1.
Names, voucher information, and corresponding GenBank accession numbers of the taxa used in this study.
3. Results
3.1. Sequencing and Phylogenetic Analyses
Three-gene (nrLSU, tef-1α, and rpb1) sequences were generated from eight specimens and two living cultures (see Table 1). Three-gene sequences of 129 samples from 14 genera in the family Clavicipitaceae were used for the ML and BI phylogenetic analyses. Pleurocordyceps aurantiaca MFLUCC 17-2113 and Pleurocordyceps marginaliradians MFLU 17-1582 were used as the outgroups. The concatenated three-gene sequences contained 2726 bp (nrLSU: 935 bp, tef-1α: 1024 bp, and rpb1: 767 bp). Both the ML and BI analyses exhibited nearly consistent overall topologies. The results of the phylogenetic analysis showed three highly supported clades, viz., the Pulvinate clade (BP = 100%, PP = 1), the Globose clade (BP = 100%, PP = 1), and the Effuse clade (BP = 100%, PP = 1) (Figure 1). The Effuse clade was segregated into two sister clades, subclade I and subclade II. Moelleriella contains the Effuse clade and the Globose clade. Two new species of Moelleriella were distributed in the Effuse clade (M. longzhuensis) and the Globose clade (M. jinuoana). Three samples of M. longzhuensis were clustered closely with M. rhombispora (M. Liu & K.T. Hodge) M. Liu & P. Chaverri and formed a monophyletic clade in the Effuse clade with a high level of statistical support (BP = 100%, PP = 1). Three samples of M. jinuoana formed a monophyletic clade in the Globose clade with a high level of statistical support (BP = 91%, PP = 0.8). The genus Conoideocrella was clustered with Orbiocrella and had a new species (C. fenshuilingensis) and one known species (C. tenuis). Two samples of C. fenshuilingensis formed a monophyletic clade in Conoideocrella with a high level of statistical support (BP = 100%, PP = 1).
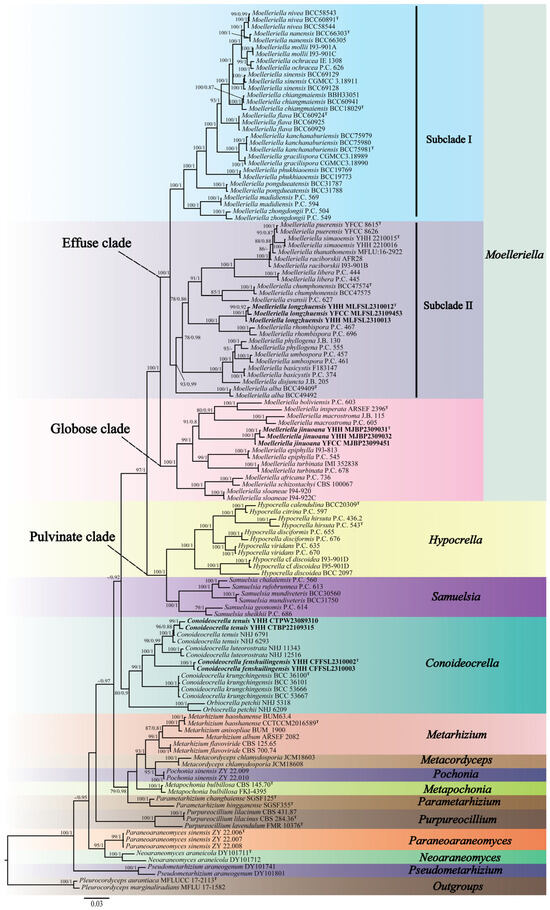
Figure 1.
Phylogenetic relationships of 14 genera in Clavicipitaceae are based on the maximum likelihood (ML) and the Bayesian inference (BI) analyses using nrLSU, tef-1α, and rpb1 sequences. Statistical support values greater than 70% are shown at the nodes for the BI posterior probabilities/the ML bootstrap proportions. The new taxa are highlighted in bold and T for ex-type material.
The genetic distances calculated based on the three genes (nrLSU, tef-1α, and rpb1) among interspecies and intraspecies in Moelleriella and Conoideocrella are shown in Tables S1–S6. The intraspecific genetic distances for nrLSU, tef-1α, and rpb1 in Moelleriella were 0–0.0276, 0–0.0428, and 0–0.0168, respectively. The interspecific genetic distances for nrLSU, tef-1α, and rpb1 between the known species and M. longzhuensis were 0.03–0.08, 0.09–0.16, and 0.08–0.24, respectively, and those between the known species and M. jinuoana were 0.04–0.08, 0.10–0.15, and 0.15–0.25, respectively. In Conoideocrella, the intraspecific genetic distances of nrLSU, tef-1α, and rpb1 were 0–0.0028, 0–0.0052, and 0.0019–0.0027, respectively, and the genetic distances between C. fenshuilingensis and the known species were 0.01–0.02, 0.06–0.09, and 0.08, respectively.
| DICHOTOMOUS KEYS TO CONOIDEOCRELLA SPECIES |
| 1a.Stromata flattened pulvinate to discoid, planar, pulvinate, almost planar.............................................................................2 |
| 1b.Stromata scutate or hemi-globose....................................................................................................................C. fenshuilingensis |
| 2a.Perithecia < 600 µm long................................................................................................................................................................3 |
| 2b.Perithecia > 600 µm long.........................................................................................................................................C. luteorostrata |
| 3a.Stromata pale yellow, orange to reddish brown; Asci < 180 µm long; Conidia 8–15 × 2–4 μm............................................... |
| ...................................................................................................................................................................................C. krungchingensis |
| 3b.Stromata white to orangish-pink; Asci > 180 µm long; Conidia 6.1–12.5 × 1.3–2.3 μm............................................................ |
| ...................................................................................................................................................................................................C. tenuis |
| DICHOTOMOUS KEYS TO MOELLERIELLA SPECIES |
| Based on teleomorphic characters |
| 1a.Part-ascospores > 16 µm long.……................................................................................................................................................2 |
| 1b.Part-ascospores < 16 µm long…..…...............................................................................................................................................3 |
| 2a.Perithecia embedded in stroma and scattered.............................................................................................................................4 |
| 2b.Perithecia embedded in top part of stroma, number perithecia per stroma > 30................................................M. phyllogena |
| 3a.Part-ascospores with rounded, blunt, or acute ends...................................................................................................................5 |
| 3b.Part-ascospores with truncated ends.................................................................................................................................M. flava |
| 4a.Part-ascospores ventricose cylindrical or curved with rounded ends and usually inflated in the middle; Part-ascospores |
| < 20 µm long.......................................................................................................................................................................M. basicystis |
| 4b.Part-ascospores ventricose cylindrical or curved with rounded ends and usually inflated in the middle; Part-ascospores |
| > 20 µm long.....................................................................................................................................................................M. umbospora |
| 5a.Stromata not thin-umbonate..........................................................................................................................................................6 |
| 5b.Stromata thin-umbonate, raised, with globose to subglobose base...............................................................M. chiangmaiensis |
| 6a.Part-ascospores 5–10 µm long........................................................................................................................................................7 |
| 6b.Part-ascospores 7–16 µm long........................................................................................................................................................8 |
| 7a.Stromata size < 2 mm.......................................................................................................................................................................9 |
| 7b.Stromata size > 2 mm.....................................................................................................................................................................10 |
| 8a.Part-ascospores < 3.5 µm width....................................................................................................................................................18 |
| 8b.Part-ascospores > 3.5 µm width.................................................................................................................................M. colliculosa |
| 9a.Stromata surface roughened..........................................................................................................................................M. castanea |
| 9b.Stromata surface not roughened..................................................................................................................................................11 |
| 10a.Stromata suface tomentose...............................................................................................................................................M. nivea |
| 10b.Stromata suface smooth..............................................................................................................................................................15 |
| 11a.Stromata yellowish white to white, pale yellow......................................................................................................................12 |
| 11b.Stromata greyish yellow or reddish brown or dark brown almost black.............................................................................13 |
| 12a.Stromata globose with head markedly constricted at base....................................................................................................14 |
| 12b.Stromata pulvinate, base slightly constricted.....................................................................................................M. zhongdongii |
| 13a.Stromata greyish yellow and reddish brown...........................................................................................................M. epiphylla |
| 13b.Stromata dark brown almost black.......................................................................................................................M. guaranitica |
| 14a.Hypothallus present...................................................................................................................................................M. disjuncta |
| 14b.Hypothallus absent...................................................................................................................................................M. boliviensis |
| 15a.Stromata obconical........................................................................................................................................................M. cornuta |
| 15b.Stromata globose or subglose....................................................................................................................................................16 |
| 16a.Stromata buff to pale greenish.............................................................................................................................M. gaertneriana |
| 16b.Stromata brownish orange, greyish brown, bark brown almost black.................................................................................17 |
| 17a.Stromata subglose...........................................................................................................................................................M. palmae |
| 17b.Stromata globose............................................................................................................................................................M. globosa |
| 18a.Part-ascospores not ventricose...................................................................................................................................................19 |
| 18b.Part-ascospores ventricose with rounded or acute ends...................................................................................M. rhombispora |
| 19a.Part-ascospores fusoid................................................................................................................................................................20 |
| 19b.Part-ascospores cylindrical with round or blunt ends............................................................................................................21 |
| 20a.Stromata surface tomentose.......................................................................................................................................................22 |
| 20b.Stromata surface not tomentose................................................................................................................................................23 |
| 21a.Perithecia embedded in stroma, scattered...............................................................................................................................26 |
| 21b.Perithecia in gregarious but well-separated tubercles or gregarious tubercles..................................................................27 |
| 22a.Perithecia in gregarious but well-separated tubercles.........................................................................................M. simaoensis |
| 22b.Perithecia embedded in stroma, scattered..............................................................................................................M. puerensis |
| 23a.Part-ascospores > 3 µm width...................................................................................................................................M. turbinata |
| 23b.Part-ascospores < 3 µm width...................................................................................................................................................24 |
| 24a.Stromata yellowish white to white, pale yellow.....................................................................................................................25 |
| 24b.Stromata yellow....................................................................................................................................................M. macrostroma |
| 25a.Hypothallus present.........................................................................................................................................................M. libera |
| 25b.Hypothallus absent........................................................................................................................................................M. evansii |
| 26a.Part-ascospores < 2 µm width......................................................................................................................................M. sinensis |
| 26b.Part-ascospores > 2 µm width....................................................................................................................................................28 |
| 27a.Hypothallus present...................................................................................................................................................................29 |
| 27b.Hypothallus absent.....................................................................................................................................................M. nanensis |
| 28a.Stromata thin pulvinate with pronounced tubercles or pulvinate with sloping side, tubercles half-embedded................ |
| ...............................................................................................................................................................................................M. sloaneae |
| 28b.Stromata flat pulvinate.......................................................................................................................................M. phukhiaoensis |
| 29a.Stromata pulvinate with sloping sides or base slightly constricted.......................................................................M. ochracea |
| 29b.Stromata flat pulvinate...............................................................................................................................................................30 |
| 30a.Stromata white, pale yellow to orange...........................................................................................................M. chumphonensis |
| 30b.Stromata white....................................................................................................................................................................M. alba |
| Based on anamorphic characters |
| 1a.Conidia > 4 µm width......................................................................................................................................................................2 |
| 1b.Conidia < 4 µm width......................................................................................................................................................................3 |
| 2a.Conidiomata number fewer than ten.............................................................................................................................................4 |
| 2b.Conidiomata number more than ten..............................................................................................................................................5 |
| 3a.Conidia < 6 µm long.........................................................................................................................................................................7 |
| 3b.Conidia > 6 µm long.........................................................................................................................................................................8 |
| 4a.Conidiomata locules simple depressions of surface without distinct rims.............................................................M. epiphylla |
| 4b.Conidiomata locules pezizoid......................................................................................................................................M. turbinata |
| 5a.Conidiomata scattered in stroma.....................................................................................................................................M. globosa |
| 5b.Conidiomata circular in stroma......................................................................................................................................................6 |
| 6a.Conidia ventricose with acute ends.............................................................................................................................M. basicystis |
| 6b.Conidia ventricose almost rhomboid with acute ends............................................................................................M. umbospora |
| 7a.Stromata greyish brown; thick pulvinate, obconical pulvinate.................................................................................M. castanea |
| 7b.Stromata pale yellow; discoid with distinct stud shape..................................................................................M. pongdueatensis |
| 8a.Conidiomata scattered or circular in stroma................................................................................................................................9 |
| 8b.Conidiomata arrangement in the central of stroma...................................................................................................................10 |
| 9a.Stromata flat pulvinate, dark orange to golden yellow.....................................................................................M. phukhiaoensis |
| 9b.Stromata not as above....................................................................................................................................................................11 |
| 10a.Conidia < 16.5 µm long................................................................................................................................................................24 |
| 10b.Conidia > 16.5 µm long......................................................................................................................................M. chumphonensis |
| 11a.Conidia > 3 µm width...................................................................................................................................................................12 |
| 11b.Conidia < 3 µm width...................................................................................................................................................................13 |
| 12a.Stromata dark brown, black.....................................................................................................................................M. guaranitica |
| 12b.Stromata yellowish white to white, pale yellow.....................................................................................................M. phyllogena |
| 13a.Conidia ventricose...................................................................................................................................................M. rhombispora |
| 13b.Conidia fusoid...............................................................................................................................................................................14 |
| 14a.Conidia > 2 µm width...................................................................................................................................................................15 |
| 14b.Conidia < 2 µm width...................................................................................................................................................................16 |
| 15a.Conidiomata circular in stroma..................................................................................................................................M. disjuncta |
| 15b.Conidiomata scattered in stroma................................................................................................................................................17 |
| 16a.Conidiomata circular in stroma........................................................................................................................................M. libera |
| 16b.Conidiomata scattered in stroma................................................................................................................................................20 |
| 17a.Conidiomata fewer than ten....................................................................................................................................................... 18 |
| 17b.Conidiomata more than ten.........................................................................................................................................................19 |
| 18a.Stromata yellowish white to white, pale yellow...................................................................................................M. madidiensis |
| 18b.Stromata yellow, orange yellow to orange.................................................................................................................M. jinuoana |
| 19a.Stromata yellow......................................................................................................................................................M. macrostroma |
| 19b.Stromata yellowish white to white, pale yellow........................................................................................................M. sloaneae |
| 20a.Cultural on PDA compact, leathery............................................................................................................................M. ochracea |
| 20b.Cultural on PDA compact, floccose/tomentose........................................................................................................................21 |
| 21a.Stromata yellowish white to white, pale yellow.......................................................................................................................22 |
| 21b.Stromata brown..................................................................................................................................................M. thanathonensis |
| 22a.Stromata tuberculate, thick pulvinate, obconical pulvinate..............................................................................M. zhongdongii |
| 22b.Stromata flat or raised, globose to subglobose.........................................................................................................................23 |
| 23a.Paraphyses present in conidioma....................................................................................................................M. chiangmaiensis |
| 23b.Paraphyses absent in conidioma................................................................................................................................M. nanensis |
| 24a.Paraphyses present in conidioma...............................................................................................................................................25 |
| 24b.Paraphyses absent in conidioma................................................................................................................................................26 |
| 25a.Stromata yellowish white to white, pale yellow.......................................................................................................................27 |
| 25b.Stromata whitish to pale brown.......................................................................................................................M. chumphonensis |
| 26a.Conidiomata more than ten.........................................................................................................................................................29 |
| 26b.Conidiomata fewer than ten........................................................................................................................................................30 |
| 27a.Stromata thin pulvinate, almost effuse......................................................................................................................................28 |
| 27b.Stromata flat to umbonate, globose to subglobose....................................................................................................M. sinensis |
| 28a.Conidia 8.8–14 × 1.6–3 μm........................................................................................................................................M. simaoensis |
| 28b.Conidia 9.7–13.4 × 1.3–2.3 μm...................................................................................................................................M. puerensis |
| 29a.Stromata scutate (a hemisphaerical central region abruptly attenuating and extending to the edge).................................. |
| ..................................................................................................................................................................................................M. evansii |
| 29b.Stromata flat to umbonate, globose to subglobose...................................................................................M. kanchanaburiensis |
| 30a.Stromata yellowish white to white, whitish to pale yellow, pale yellow, yellow................................................................31 |
| 30b.Stromata white.....................................................................................................................................................................M. alba |
| 31a.Conidia < 2 µm width..................................................................................................................................................................32 |
| 31b.Conidia > 2 µm width...........................................................................................................................................M. longzhuensis |
| 32a.Stromata flat or raised, globose to subglobose; conidia 9–14 × 1–2 μm........................................................................M. flava |
| 32b.Stromata flat to umbonate, subglobose; conidia 7–10 × 1–2 μm...................................................................................M. nivea |
3.2. Taxonomy
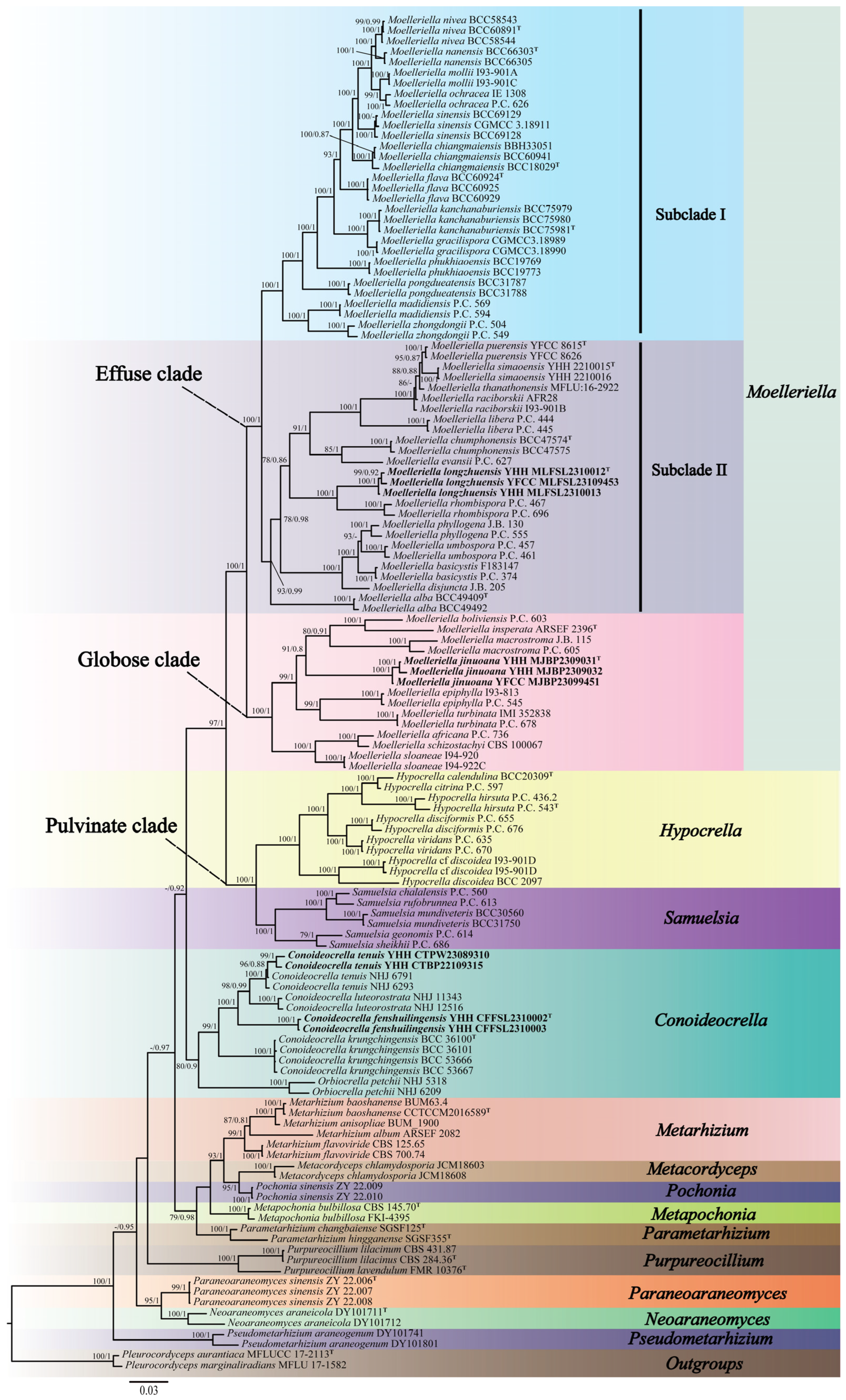
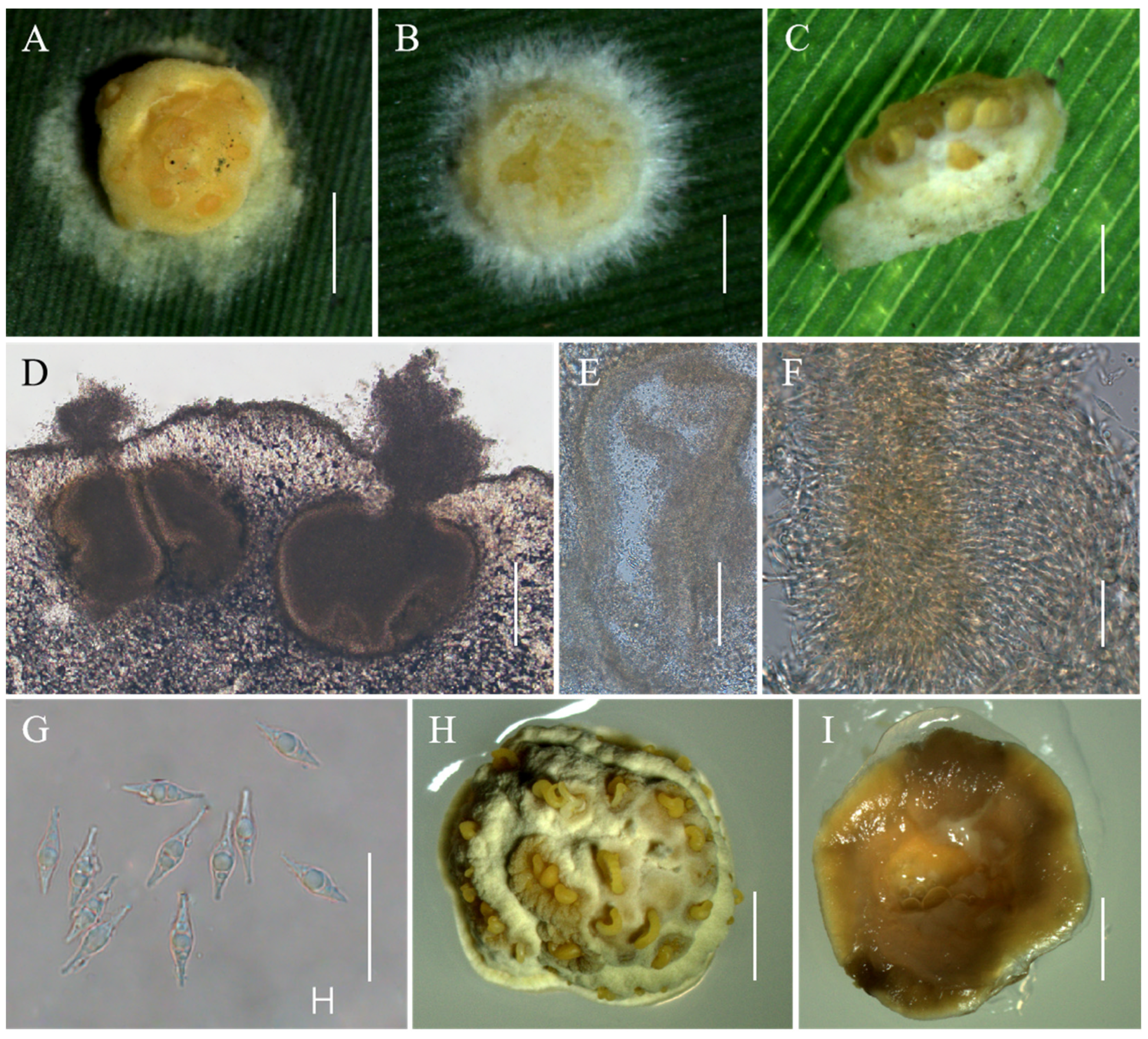
Conoideocrella tenuis (Petch) D. Johnson, G.H. Sung, Hywel-Jones & Spatafora, Mycol. Res. 113(3): 286 (2009), Figure 2.
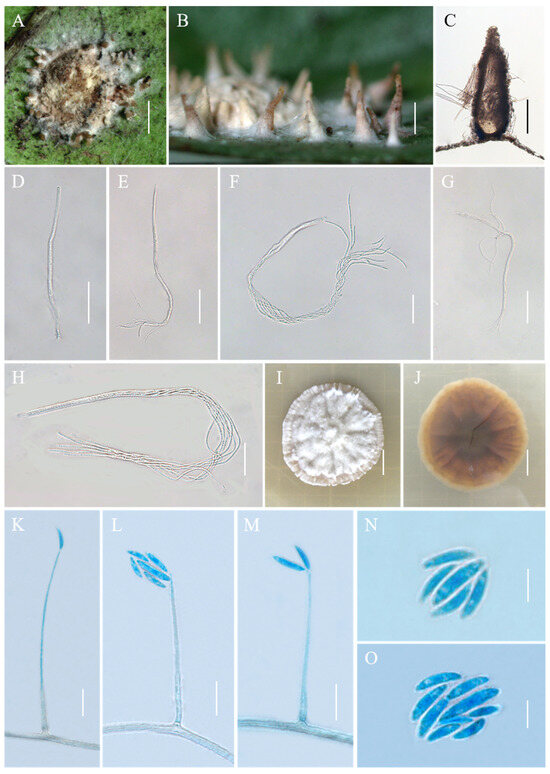
Figure 2.
Morphology of Conoideocrella tenuis. (A,B) Telemorphic stroma containing perithecia; (C) Perithecium; (D–H) Mature asci with developing asci; (I) Obverse of colonies on PDA at 25 °C after 21 days; (J) Reverse of colonies on PDA at 25 °C after 21 days; (K–O) Conidia of hirsutella-like asexual stage on PDA. Scale bars: 1 mm (A,B); 200 µm (C); 50 µm (D,E); 25 µm (F); 50 µm (G); 20 µm (H); 1 cm (I,J); 10 µm (K–M); and 5 µm (N,O).
≡Torrubiella tenuis Petch, Ann. Perad. 7, 323 (1923).
MycoBank No: MB 512029.
Description. Sexual morph: Teleomorphic stromata pulvinate, flattened pulvinate or almost planar, 2–4 mm in diam, white to orangish-pink, tomentose, rather loose internally, surrounded by a broad, fibrillose margin or hypothallus. Perithecia mostly distributed at the margin of the stroma or on the hypothallus, scattered or clustered, color deepens from the bottom to the top, white to pale brown, covered with hyphae up to two-thirds their height in mature perithecia, dozens of perithecia per stroma, elongated flask shape or elongated conic shape, 190–500 × 160–270 μm. Asci cylindrical, eight-spored, 190–480 × 3.3–5.5 μm, caps 2.5–3.5 μm thick. Ascospores whole, filiform, septate. Asexual morph: Not known.
Culture characteristics. Colonies grow slowly on PDA at 25 °C, attaining a diam of 15–17 mm in 21 days, greyish-white to cream-white mycelium at first, turning lilac with age. Colonies are loose on the surface and compact at the bottom. Hyphae smooth, septate, hyaline, 1.1–3.6 µm wide. Hirsutella-like asexual state arises from hyphae, conidiogenous structures with slender base tapering more or less evenly to a neck, hyaline, produced directly on hyphae of the stromatic colonies from ca. 5 wk onwards, 16.3–149.4 × 0.6–2.4 μm, and 0.3–1.3 μm wide at the apex. Conidia hyaline, smooth, fusiform and slightly curved, produced singly or in a group of two at the neck apex, 6.1–12.5 × 1.3–2.3 μm.
Habitat. Parasitic on Aspidiotus destructor on a jungle tree; on a black Aleyrodes on Sarcococca pruniformis; on a scale on Hedyotis lessertianan; on Aleyrodes on Lasianthus walkerianus and Psychotria elongata.
Distribution. Sri Lanka (type locality) and Thailand, China.
Other material examined. China, Yunnan Province, Jinghong City, Jinuo Township, Banpo village, 100°98′ E, 22°06′ N, alt. 1035 m, found on the underside of living leaves of dicotyledonous plants, 2 October 2022. Hong Yu and Zhi-Qin Wang (YHH CTBP221012, YHH CTBP221013, YHH CTBP22109315; YFCC CTBP22109316, living culture). Yunnan Province, Puwen Town, 100°97′ E, 22°52′ N, alt. 1020 m, found on the underside of living leaves of dicotyledonous plants, 3 August 2023, Hong Yu and Zhi-Qin Wang (YHH CTPW2308031; YHH CTPW23089310).
Commentary. The species C. tenuis, formerly in the genus Torrubiella, was reclassified by Johnson et al. [4] to Conoideocrella. In 1923, Petch described the morphological characteristics of T. tenuis, as well as its distribution sites and host insects. But there was no record of the size of the asci or ascospores. Hywel-Jones [23] collected specimens of T. tenuis in Thailand. They recorded the size of the asci and ascospores and isolated pure cultures. In our study, specimens of this species were collected in Yunnan, China, and it was found to be distributed in China. Its macromorphology and micromorphology were generally consistent with those described by Petch and Hywel-Jones and Evens, with one difference being that the materials used in this study extend the perithecium (190–900 × 160–270 μm) and asci (190–500 × 3.3–7.0 μm) size range of this species. Noteworthy, a hirsutella-like asexual state was observed on the stromatic colonies in the present study, which has not yet been observed in other studies. Unfortunately, as in the case of the Thai material, part-spores were not seen in the Chinese collection.
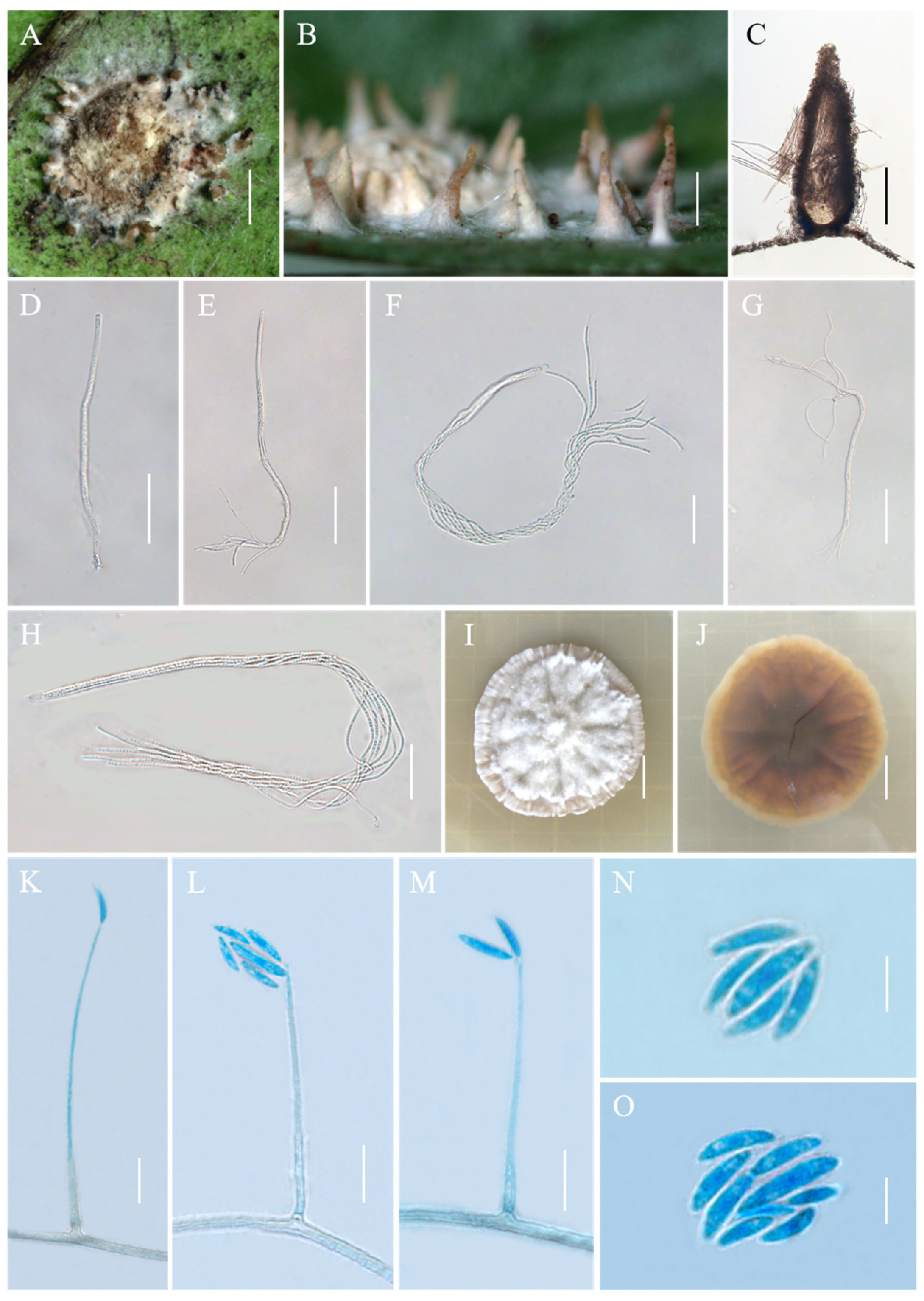
Conoideocrella fenshuilingensis Hong Yu bis, Z.L. Yang, Z.Q. Wang & J.M. Ma, sp. nov. Figure 3.
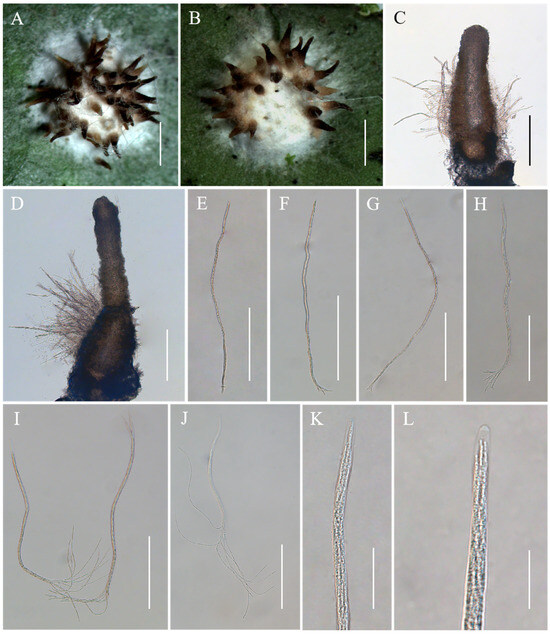
Figure 3.
Morphology of Conoideocrella fenshuilingensis. (A,B) Telemorphic stroma containing perithecia; (C,D) Perithecium; (E–L) Mature asci with developing asci. Scale bars: 1 mm (A,B); 200 µm (C–G); 100 µm (H); 150 µm (I); 100 µm (J); 30 µm (K); and 20 µm (L).
Mycobank No: 851868.
Etymology. Named after the Fenshuiling National Nature Reserve where the species was collected.
Type. China, Yunnan, Jinping County, the Fenshuiling National Nature Reserve. 103°49′ E, 22°82′ N, alt. 519 m, found on the underside of living leaves of dicotyledonous plants. 24 October 2023, Hong Yu (YHH CFFSL2310002, holotype).
Description. Sexual morph: Teleomorphic stromata scutate or hemi-globose, 3.0–3.4 mm in diam, snow-white, surrounded by a snow-white hypothallus. Perithecia densely distributed on stroma, a few distributed on hypothallus, scattered or clustered, color deepens from the bottom to the top, pale brown to black, dozens of perithecia per mature stroma, covered with hyphae up to two-thirds their height in mature perithecia, elongated flask shape or elongated conic shape, 259–795 × 144–242 μm. Asci cylindrical, eight-spored, 246–685 × 3.8–6.9 μm, caps 1.2–2.3 μm thick. Ascospores whole, filiform, septate. No anamorph was found with these stromata in nature. Asexual morph: Undetermined.
Habitat. Parasitic on scale insects (Coccidae, Sternorrhyncha, Hemiptera), found on the underside of living leaves of dicotyledonous plants.
Distribution. China, Yunnan Province, Jinping County.
Other Material Examined. China, Yunnan, Jinping County, the Fenshuiling National Nature Reserve. 103°49′ E, 22°82′ N, alt. 519 m, found on the underside of living leaves of dicotyledonous plants. 24 October 2023, Hong Yu and Zhi-Qin Wang (YHH CFFSL2310003, YHH CFFSL2310004, YHH CFFSL2310005, YHH CFFSL2310006, YHH CFFSL2310007, YHH CFFSL2310008, YHH CFFSL2310009, YHH CFFSL2310010, YHH CFFSL2310011, paratype).
Commentary. The phylogenetic analyses revealed that two samples of C. fenshuilingensis were grouped together and formed a separate clade in the genus Conoideocrella. Currently, only three species of Conoideocrella have been reported [4,24]. Conoideocrella fenshuilingensis was similar to the other three species in its elongated, conical perithecium and cylindrical asci. However, C. fenshuilingensis was particularly easy to distinguish from other species by the location of the perithecia on the stromata. Its perithecia was almost always grown on a hemispherical stroma, while that of C. krungchingensis was grown on a slight weft of hyphae, and that of C. luteorostrata was more commonly grown on the hypothallus [23,24]. Although the perithecium of C. tenuis was usually on the thicker part of the stroma, C. fenshuilingensis can be distinguished from C. tenuis by its hemispherical stroma, while C. tenuis has a flattened pulvinate or almost planar stroma [23].
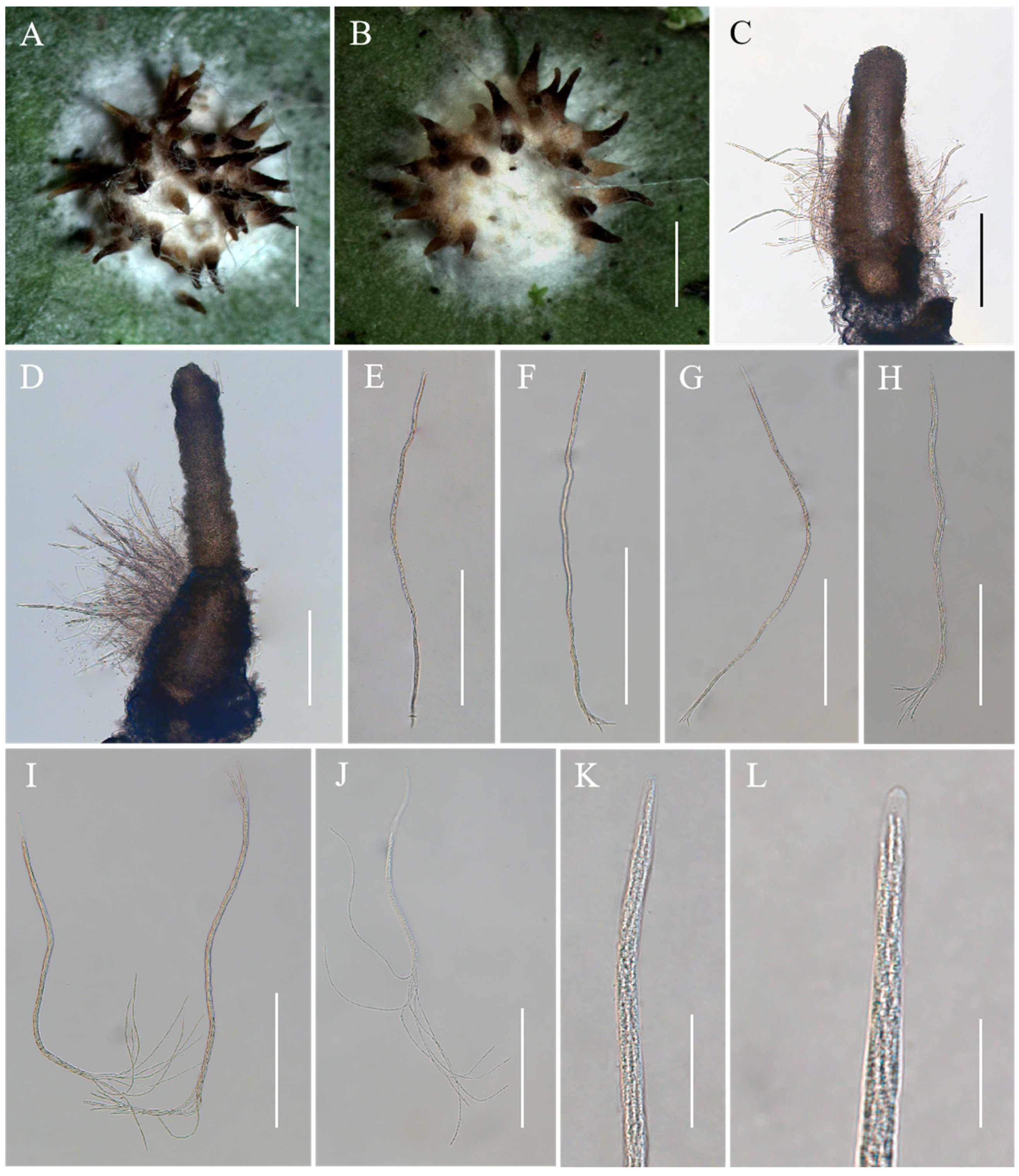
Moelleriella jinuoana Hong Yu bis, Z.L. Yang, Z.Q. Wang & J.M. Ma, sp. nov. Figure 4.
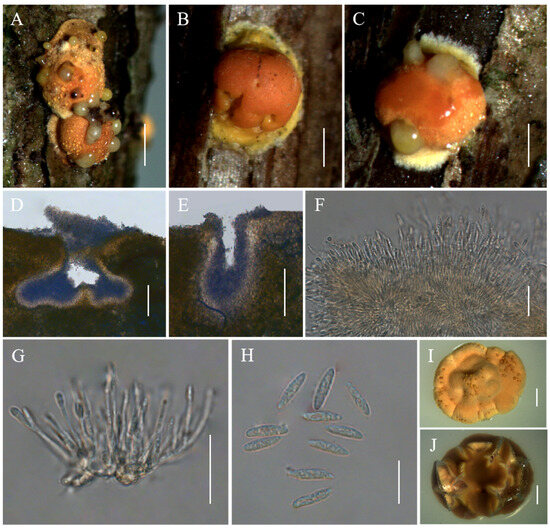
Figure 4.
Morphology of Moelleriella jinuoana. (A–C) Anamorphic stromata containing conidiomata; (D,E) Section of stromata showing conidiomata; (F,G) Phialides with conidia at the tips; (H) Conidia; (I) Obverse of colonies on PDA at 25 °C after 21 days; (J) Reverse of colonies on PDA at 25 °C after 21 days. Scale bars: 1 mm (A); 0.5 mm (B,C); 100 µm (D,E); 20 µm (F,G); 10 µm (H); and 2 mm (I,J).
Mycobank No: 851869.
Etymology. Moelleriella jinuoana was named after the Jinuo nationality, one of the 56 ethnic groups in China.
Type. China, Yunnan Province, Jinghong City, Jinuo Township, Banpo village, 100°98′ E, 22°06′ N, alt. 1046 m, found on trunks of dicotyledonous plants. 26 October 2023, Hong Yu (YHH MJBP2309031, holotype; YFCC MJBP23099451, ex-holotype living culture).
Description. Sexual morph: Not known. Asexual morph: Anamorphic stromata on natural substrate globose, surface smooth, yellow, orangish-yellow to orange, 1.2–2.3 mm diam, often with narrow hypothallus. Hyphae of stromata form compact textura epidermoidea. Conidiomata simple depressions of surface, producing grayish-yellow copious slime, several conidiomata per stroma, U-shaped or irregular shape, 110–373 × 181–286 μm. Phialides formed in a thick, compact palisade or cylindrical shape, 11.8–29.5 × 1–1.9 μm. Conidia unicellular, hyaline, smooth, fusoid with rounded ends, 8–11.5 × 2.1–2.9 μm. No praphyses were observed.
Culture characteristics. Colonies on PDA slow-growing, attaining a diam of 9–11 mm in 21 days at 25 °C. Stromatic colonies compact pulvinate, surface wrinkled, pale orange to orange. Conidial masses usually abundant, orange. Reverse of colony brownish.
Habitat. Parasitic on scale insects (Coccidae, Sternorrhyncha, Hemiptera) or whiteflies (Aleyrodidae, Sternorrhyncha, Hemiptera), on trunks of dicotyledonous plants.
Distribution. China, Yunnan Province, Jinghong City.
Other Material examined. China, Yunnan Province, Jinghong City, Jinuo Township, Bapo village, 100°98′ E, 22°06′ N, alt. 1046 m, found on trunks of dicotyledonous plants. 26 October 2023, Hong Yu and Zhi-Qin Wang (YHH MJBP2309032, paratype; YFCC MJBP23099452, ex-paratype living culture); Ibid., (YHH MJBP2309033, YHH MJBP2309034, YHH MJBP2309035).
Commentary. The three-gene phylogenetic analyses showed that the three samples of M. jinuoana were clustered in the Globe clade and closely related to M. boliviensis P. Chaverri & K.T. Hodge, M. insperata (Rombach, M. Liu, Humber, and K.T. Hodge) P. Chaverri & K.T. Hodge, and M. macrostroma (P. Chaverri and K.T. Hodge) P. Chaverri & K.T. Hodge. Morphologically, the stromata shape, texture, and color of M. jinuoana was significantly different from those of M. boliviensis, M. insperata, and M. macrostroma [3,39]. Ecologically, M. jinuoana was found on the trunk of dicotyledonous plants, while M. boliviensis and M. insperata were found on the underside of leaves, and M. macrostroma was found on the living vines of dicotyledonous plants [3,39].
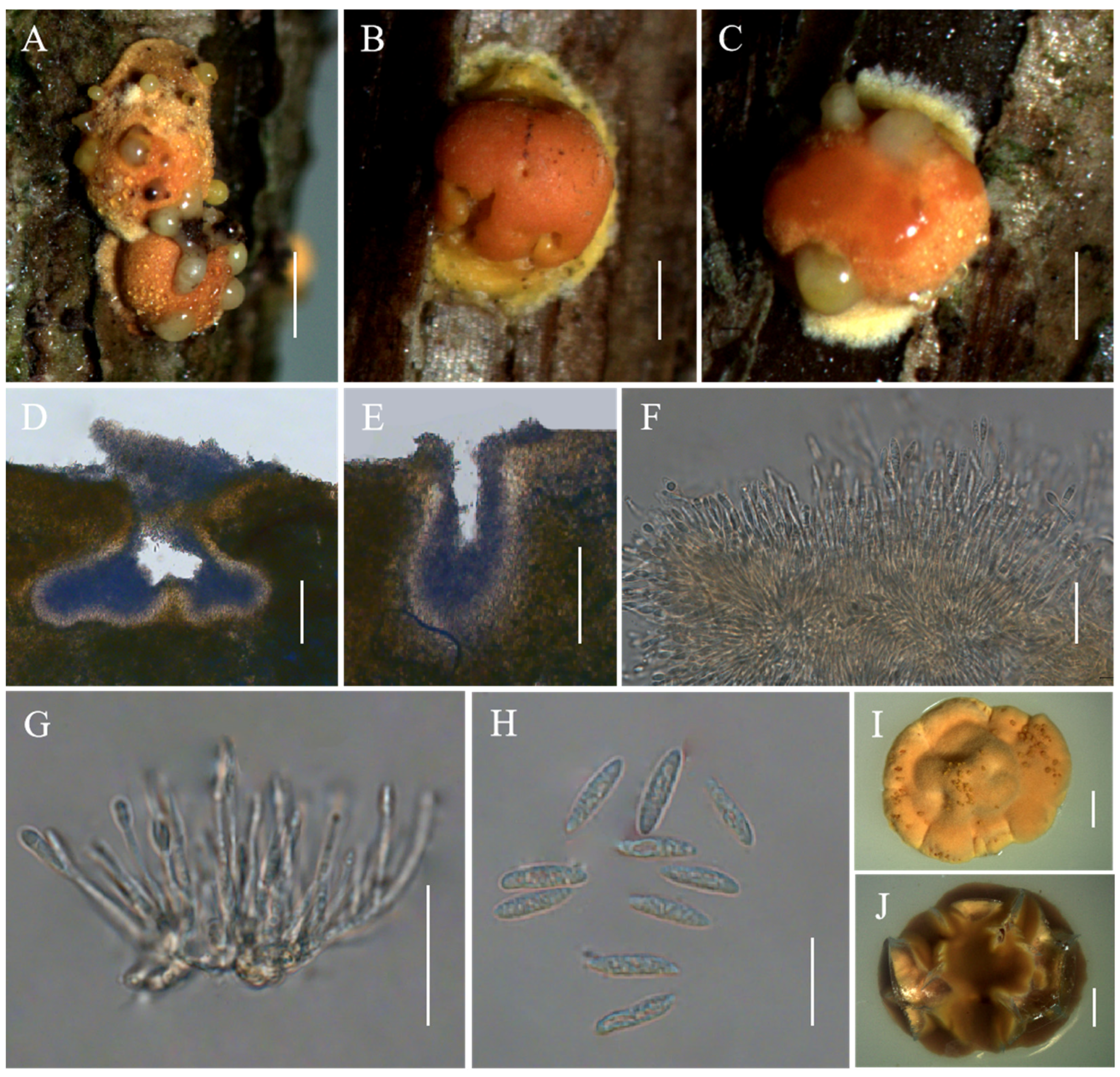
Moelleriella longzhuensis Hong Yu bis, Z.L. Yang & Z.Q. Wang, sp. nov. Figure 5.
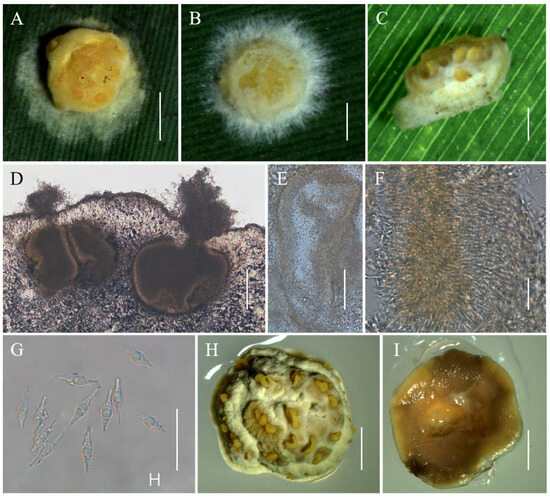
Figure 5.
Morphology of Moelleriella longzhuensis. (A–C) Anamorphic stromata containing conidiomata; (D,E) Section of stromata showing conidiomata; (F) Phialides with conidia at the tips; (G) Conidia; (H) Obverse of colonies on PDA at 25 °C after 21 days; (I) Reverse of colonies on PDA at 25 °C after 21 days. Scale bars: 1 mm (A); 0.5 mm (B,C); 50 µm (D); 100 µm (E); 20 µm (F,G); and 2 mm (H,I).
Mycobank No: 851870.
Etymology. Named after the Chinese name “Longzhu” of the plant Dendrocalamus giganteus Wall. ex Munro, to which the stromata were attached.
Type. China, Yunnan Province, Jinping County, the Fenshuiling National Nature Reserve, 103°22′ E, 22°78′ N, alt. 1436 m, found on the underside of the living leaves of D. giganteus. 22 October 2023, Hong Yu (YHH MLFSL2310012, holotype; YFCC MLFSL23109453, ex-holotype living culture).
Description. Sexual morph: Not known. Asexual morph: Anamorphic stromata with a subglobose head and constricted base (stud-shaped), white to pale yellow when immature becoming pale yellow to yellow when mature, 1–1.9 mm diam, surface pruinose or tomentose, with hypothallus 0.1–1.1 mm. Hyphae of stromata forming loose textura intricata to epidermoidea. Conidiomata simple depressions of surface, 319–481 × 237–448 μm, several conidiomata per stroma, but difficult to count because some of them are confluent with neighboring ones. Conidial masses pale yellow to yellow. In section, the Conidioma subglobose to globose. Phialides not observed. Conidia unicellular, hyaline, smooth, inflated at midpoint and tapering at both ends, 12–16.1 × 2.6–3.9 μm. No paraphyses observed.
Culture characteristics. Colonies on PDA slow-growing, attaining a diam of 6–7 mm in 21 days at 25 °C. Colonies pale yellow to pale yellowish brown, compact, forming a subglobose structure. Conidial masses usually abundant, usually forming several gushing bands, pale yellow to pale orange. Reverse of colony pale yellow to yellowish brown.
Habitat. Parasitic on scale insects (Coccidae, Sternorrhyncha, Hemiptera) and whiteflies (Aleyrodidae, Sternorrhyncha, Hemiptera), found on the underside of the living leaves of D. giganteus.
Distribution. China, Yunnan Province, Jinping County.
Other material examined. China, Yunnan Province, Jinping County, the Fenshuiling National Nature Reserve, 103°22′ E, 22°78′ N, alt. 1436 m, found on the underside of the living leaves of D. giganteus. 22 October 2023, Hong Yu and Zhi-Qin Wang (YHH MLFSL2310013, paratype; YFCC MLFSL23109454, ex-paratype living culture); Ibid., (YHH MLFSL2310014, YHH MLFSL2310015, YHH MLFSL2310016, YHH MLFSL2310017, YHH MLFSL2310018).
Commentary. Phylogenetically, the three samples of M. longzhuensis were grouped together in subclade II of the Effuse clade and formed a monophyletic group. It was sister to M. rhombispora, with a high level of statistical support in terms of the BI posterior probabilities (PP = 1) and the ML bootstrap proportions (BP = 100%). Morphologically, M. longzhuensis was similar to M. rhombispora in that its conidia were inflated at the midpoint and tapered at both ends [3]. However, M. longzhuensis exhibits significant morphological differences from M. rhombispora due to its color and the shape of its anamorphic stromata. The conidiomata of the former could be confluent with their neighbors but the those of the latter were not confluent. The former also had larger conidia (12–16.1 × 2.6–3.9 vs. 9–14 × 2.5–3 μm) and no paraphyses were observed [3].
4. Discussion
The calculation of genetic distances for the three genes showed that the interspecific genetic distances between the new species in this study and other species of the genus were greater than the intraspecific genetic distances (see Tables S1–S6). This study resulted in the discovery of two new species of Moelleriella and one new species of Conoideocrella through phylogenetic analyses, morphological observations, and calculations of inter- and intraspecific genetic distances within genera.
The genus Moelleriella was divided into the Effuse clade and the Globose clade by Chaverri et al. [3]. The Effuse clade is composed of two sister clades, i.e., subclade I and subclade II [6]. Currently, subclade I includes 13 species, and subclade II includes 13 species [15]. The Effuse clade species were characterized as having effuse to thin, pulvinate stromata of loose hyphal tissue. Many species had hypothalli (e.g., M. basicystis P. Chaverri & K.T. Hodge; M. disjuncta (Seaver) P. Chaverri & K.T. Hodge; M. libera (Syd. and P. Syd.) P. Chaverri & M. Liu; M. madidiensis P. Chaverri & K.T. Hodge; M. ochracea (Massee) M. Liu & P. Chaverri; M. phyllogena (Mont.) P. Chaverri & K.T. Hodge; M. pongdueatensis Mongkols., Thanakitp. & Luangsa-ard; M. raciborskii (Zimm.) P. Chaverri, M. Liu & K.T. Hodge; M. rhombispora (M. Liu & K.T. Hodge) M. Liu & P. Chaverri; M. thanathonensis Y.P. Xiao, T.C. Wen & K.D. Hyde; M. umbospora P. Chaverri & K.T. Hodge; and M. zhongdongii (M. Liu & K.T. Hodge) M. Liu & P. Chaverri). These species had mostly whitish coloration and occasionally their stromata were pale yellow to orange (e.g., M. basicystis) [3,7,8,9,10,13,14,15]. The Globose clade species had globose and darker stromata, compact tissue, were hard or coriaceous, did not have hypothalli (except M. sloaneae (Pat.) P. Chaverri & K.T. Hodge), and had stromata that were usually 1–2 mm (except for M. macrostroma (P. Chaverri & K.T. Hodge) P. Chaverri & K.T. Hodge) [3]. However, these characteristics were not unique to each clade, and overlap could be found between the clades [3].
All three reported species of Conoideocrella were present in the field as telemorphic stromata with elongated flask-shaped or elongated conic-shaped perithecia [23,24]. In this way, it could be easily distinguished from Moelleriella, although it was in good agreement with Moelleriella in terms of growing environment and host category. Two genera, Moelleriella and Conoideocrella, were parasitic on whiteflies (Aleyrodidae, Hemiptera) or scale insects (Coccidae and Lecaniidae, Hemiptera) [3,23,24]. The fungus always completely obliterated the host, making it nearly impossible to identify the insect [3,23].
Scale insects and whiteflies are tiny, widely distributed parasites that suck plant sap, and many of these species are serious agricultural pests and act as vectors for viral plant diseases [40,41,42,43]. In the face of severe crop infestation by whiteflies, pesticides have been used mainly to suppress the whitefly population [44]. However, the overuse of pesticides has led to a certain degree of resistance to pesticides and harmful effects on non-target organisms and the environment [45]. The ability of Moelleriella and Conoideocrella species to parasitize large populations of whiteflies and scale insects in the wild gives these species the potential to be developed as green and non-polluting biological control agents. Moelleriella libera (anamorph A. aleyrodis) was the first species of Moelleriella to be applied to control whiteflies in Florida, U.S.A. [46]. Subsequently, there have been an increasing number of studies on the control of pests with M. libera [47,48,49,50,51]. Relatively few studies have been conducted on other species of Molleriella for biocontrol materials.
Hypocrella s. str. (anamorph Aschersonia) and Molleriella species are not only important biocontrol agents, but their metabolites also possess a wide range of biological activities, such as anti-tumor, anti-malarial, anti-bacterial, and insecticidal properties, and have great value within applications for biopesticides and pharmaceuticals [52,53,54,55]. Various compounds have been reported in studies of the metabolites of Moelleriella, such as benzophenones, terpenoids, cyclopeptides, steroids, and carotenoids [52,56,57,58,59,60]. In the studies of the metabolites of Conoideocrella, depsipeptides, bioxanthracenes and their monomers, conoideoxime A, hopane triterpenoid (zeorin), xanthone glycoside and naphthopyrone glycoside, phenolic glucosides, and chromane analogues have been reported [61,62,63,64,65,66,67]. In this paper, three new species of Moelleriella and Conoideocrella have been identified. This discovery provides valuable insights to facilitate further research, exploitation, and utilization of their metabolites and entomopathogenic fungi for effective biological control of scale insects or whiteflies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof10060423/s1, Table S1: Interspecific genetic distance matrix and intragroup genetic distances for nrLSU sequences of Moelleriella. Table S2: Interspecific genetic distance matrix and intragroup genetic distances for tef-1α sequences of Moelleriella. Table S3: Interspecific genetic distance matrix and intragroup genetic distances for rpb1 sequences of Moelleriella. Table S4: Interspecific genetic distance matrix and intragroup genetic distances for nrLSU sequences of Conoideocrella. Table S5: Interspecific genetic distance matrix and intragroup genetic distances for tef-1α sequences of Conoideocrella. Table S6: Interspecific genetic distance matrix and intragroup genetic distances for rpb1 sequences of Conoideocrella.
Author Contributions
Conceptualization, Z.-Q.W. and J.-M.M.; methodology, Z.-Q.W.; software, J.-M.M.; validation, Z.-L.Y. and J.Z.; formal analysis, Z.-Q.W.; investigation, Z.-Q.W., J.-M.M., Z.-L.Y., J.Z., Z.-Y.Y., J.-H.L. and H.Y.; resources, H.Y.; data curation, Z.-L.Y.; writing—original draft preparation, Z.-Q.W.; writing—review and editing, H.Y.; visualization, Z.-Q.W.; supervision, H.Y.; project administration, H.Y.; funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (grant number 31870017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The DNA sequence data obtained in this study have been deposited in GenBank. The accession numbers can be found in the article (Table 1).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sullivan, R.F.; Bills, G.F.; Hywel-Jones, N.L.; White, J.F., Jr. Hyperdermium: A new clavicipitalean genus for some tropical epibionts of dicotyledonous plants. Mycologia 2000, 92, 908–918. [Google Scholar] [CrossRef]
- Chaverri, P.; Bischoff, J.F.; Evan, H.C.; Hodge, K.T. Regiocrella, a newentomopathogenic genus with a pycnidial anamorph and its phylogenetic placement in the Clavicipitaceae. Mycologia 2005, 97, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, P.; Liu, M.; Hodge, K.T. A monograph of the entomopathogenic genera Hypocrella, Moelleriella, and Samuelsia gen. nov. (Ascomycota, Hypocreales, Clavicipitaceae), and their aschersonia-like anamorphs in the Neotropics. Stud. Mycol. 2008, 60, 1–66. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Sung, G.H.; Hywel-Jones, N.L.; Luangsa-ard, J.J.; Bischoff, J.F.; Kepler, R.M.; Spatafora, J.W. Systematics and evolution of the genus Torrubiella (Hypocreales, Ascomycota). Mycol. Res. 2009, 113, 279–289. [Google Scholar] [CrossRef]
- Luangsa-ard, J.J.; Mongkolsamrit, S.; Noisripoom, W.; Thanakitpipattana, D.; Khonsanit, A.; Wutikhun, T. Helicocollum, a new clavicipitalean genus pathogenic to scale insects (Hemiptera) in Thailand. Mycol. Prog. 2017, 16, 419–431. [Google Scholar] [CrossRef]
- Khonsanit, A.; Noisripoom, W.; Mongkolsamrit, S.; Phosrithong, N.; Luangsa-ard, J.J. Five new species of Moelleriella infecting scale insects (Coccidae) in Thailand. Mycol. Prog. 2021, 20, 847–867. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Nguyen, T.T.; Tran, N.L.; Luangsa-ard, J.J. Moelleriella pumatensis, a new entomogenous species from Vietnam. Mycotaxon 2011, 17, 45–51. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Khonsanit, A.; Noisripoom, W.; Luangsa-ard, J.J. Two new entomogenous species of Moelleriella with perithecia in tubercles from Thailand. Mycoscience 2015, 56, 66–74. [Google Scholar] [CrossRef]
- Li, G.J.; Hyde, K.D.; Zhao, R.L.; Hongsanan, S.; Abdel-Aziz, F.A.; Abdel-Wahab, M.A.; Alvarado, P.; Alves-Silva, G.; Ammirati, J.F.; Ariyawansa, H.A.; et al. Fungal diversity notes 253–366: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2016, 78, 1–237. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; Jeewon, R.; Maharachchikumbura, S.S.N.; Liu, J.K.; Bhat, D.J.; Jones, E.B.G.; McKenzie, E.H.C.; Camporesi, E.; Bulgakov, T.S.; et al. Fungal diversity notes 491–602: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2017, 83, 1–261. [Google Scholar] [CrossRef]
- Chen, Y.X.; Xue, Q.L.; Xie, Y.X.; Keyhani, N.O.; Guan, X.Y.; Wei, X.Y.; Zhao, J.; Yan, Z.; Chen, H.; Qiu, J.Z.; et al. Moelleriella sinensis sp. nov. (Clavicipitaceae, Ascomycota) from southeast China. Phytotaxa 2020, 429, 22. [Google Scholar] [CrossRef]
- Crous, P.W.; Cowan, D.A.; Maggs-Kölling, G.; Yilmaz, N.; Larsson, E.; Angelini, C.; Brandrud, T.E.; Dearnaley, J.D.W.; Dima, B.; Dovana, F.; et al. Fungal Planet description sheets: 1112–1181. Persoonia 2020, 45, 251–409. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.S.; Lu, X.; Dai, Y.C.; Hyde, K.D.; Kan, Y.H.; Kušan, I.; He, S.H.; Liu, N.G.; Sarma, V.V.; Zhao, C.L.; et al. Fungal diversity notes 1277–1386: Taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2020, 104, 1–266. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Wang, Y.; Wang, Y.B.; Yu, H. A new species of Moelleriella from Yunnan Province in Southwestern China. Phytotaxa 2022, 555, 187–194. [Google Scholar] [CrossRef]
- Yang, Z.L.; Wang, Z.Q.; Ma, J.M.; Yu, H. Moelleriella simaoensis, a new entomogenous species of Moelleriella (Clavicipitaceae, Hypocreales) from Southwestern China. Phytotaxa 2023, 603, 060–068. [Google Scholar] [CrossRef]
- Ma, H.F. Resource Survey and Phylogenetic Study on Aschersonia. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2008. [Google Scholar]
- Wang, Y.Y. Resource Survey and Genetic Diversity Analysis of Aschersonia spp. and Their Telemorph Stage. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2009. [Google Scholar]
- Qiu, J.Z.; Chen, Y.; Wang, Y.Y.; Guan, X. The finding of Moelleriella ochracea (Ascomycota, Clavicipitaceae) in China. Mycosystema 2009, 28, 148–150. [Google Scholar]
- Qiu, Y.F. Molecular Systematics and DNA Barcode of Aschersonia. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2013. [Google Scholar]
- Kobayasi, Y.; Shimizu, D. Monograph of the genus Torrubiella. Bull. Natl. Sci. Mus. Tokyo 1982, 8, 43–78. [Google Scholar]
- Artjariyasripong, S.; Mitchell, J.I.; Hywel-Jones, N.L.; Jones, E.B.G. Relationship of the genus Cordyceps and related genera, based on parsimony and spectral analysis of partial 18S and 28S ribosomal gene sequences. Mycoscience 2001, 42, 503–517. [Google Scholar] [CrossRef]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classifcation of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef]
- Hywel-Jones, N.L. Torrubiella luteorostrata: A pathogen of scale insects and its association with Paecilomyces cinnamomeus with a note on Torrubiella tenuis. Mycol. Res. 1993, 97, 1126–1130. [Google Scholar] [CrossRef]
- Mongkolsamrit, S.; Thanakitpipattana, D.; Khonsanit, A.; Promharn, R.; Luangsa-ard, J.J. Conoideocrella krungchingensis sp. nov., an entomopathogenic fungus from Thailand. Mycoscience 2016, 57, 264–270. [Google Scholar] [CrossRef]
- Petch, T. Studies in entomogenous fungi. III. Torrubiella. Trans. Br. Mycol. Soc. 1923, 9, 108–128. [Google Scholar] [CrossRef]
- Liu, M.; Hodge, K.T. Hypocrella zhongdongii sp. nov., the teleomorph of Aschersonia incrassata. Mycol. Res. 2005, 109, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identifcation and mapping of enzymatically amplifed ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Bischof, J.F.; Rehner, S.A.; Humber, R.A. Metarhizium frigidum sp. nov.: A cryptic species of M. anisopliae and a member of the M. favoviride Complex. Mycologia 2006, 98, 737–745. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Wang, Y.; Dong, Q.Y.; Fan, Q.; Dao, V.M.; Yu, H. Morphological and Phylogenetic Characterization Reveals Five New Species of Samsoniella (Cordycipitaceae, Hypocreales). J. Fungi 2022, 8, 747. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.L.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the raxml web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efcient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef]
- Liu, M.; Rombach, M. What’s in a name? Aschersonia insperata: A new pleoanamorphic fungus with characteristics of Aschersonia and Hirsutella. Mycologia 2005, 97, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef]
- Xiong, J.; Zheng, W.J.; Yao, S.L. Research situations of occurrence, damage of Pealius mori (Homoptera: Aleyrodidae) and its integrated pest management in China. Guizhou Sci. 2011, 29, 85–91. [Google Scholar]
- Wang, J.R.; Song, Z.Q.; Du, Y.Z. Six New Record Species of Whiteflies (Hemiptera: Aleyrodidae) Infesting Morus alba in China. J. Insect Sci. 2014, 14, 274. [Google Scholar] [CrossRef]
- Arias-Corpuz, S.; Romero-Nápoles, J.; Hernández-Fuentes, L.M.; González-Hernández, H.; Illescas-Riquelme, C.P.; Lomeli-Flores, J.R.; Montalvo-González, E.; Nolasco-González, Y.; Velázquez-Monreal, J.J.; García-Magaña, M.d.L. Scale Insects (Hemiptera: Coccomorpha) on Jackfruit (Moraceae) in Nayarit, Mexico. J. Entomol. Sci. 2021, 57, 82–95. [Google Scholar] [CrossRef]
- Liang, P.; Tian, Y.A.; Biondi, A.; Desneux, N.; Gao, X.W. Short-term and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species. Ecotoxicology 2012, 21, 889–1898. [Google Scholar] [CrossRef]
- Gerling, D.; Alomar, Ò.; Arnò, J. Biological control of Bemisia tabaci using predators and parasitoids. Crop Prot. 2001, 20, 779–799. [Google Scholar] [CrossRef]
- Berger, E.W. Natural enemies of scale insects and whiteflies in Florida. Fla. State Plant Board Q. Bull. 1921, 5, 141–154. [Google Scholar]
- Zhang, C.; Ali, S.; Musa, P.D.; Wang, X.M.; Qiu, B.L. Evaluation of the pathogenicity of Aschersonia aleyrodis on Bemisia tabaci in the laboratory and greenhouse. Biocontrol Sci. Technol. 2016, 27, 210–221. [Google Scholar] [CrossRef]
- Lima, B.M.F.V.; Almeida, J.E.M.; Moreira, J.O.T.; Santos, L.C.; Bittencourt, M.A.L. Entomopathogenic fungi associated with citrus blackfly (Aleurocanthus woglumi Ashby) in southern Bahia. Arq. Inst. Biol. 2017, 84, 1–3. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Shao, Z.F.; Han, Y.Y.; Wang, X.M.; Wang, Z.Q.; Peter, D.M.; Qiu, B.L.; Shaukat, A. Effects of Aschersonia aleyrodis on the life table and demographic parameters of Bemisia tabaci. J. Integr. Agric. 2018, 17, 389–396. [Google Scholar] [CrossRef]
- Ingle, Y.V.; Bhosale, D.N.; Paithankar, D.H.; Mane, S.S.; Lande, G.K. Natural occurrence of Aschersonia aleyrodis on citrus black fly in Vidarbha region of maharashtra. J. Plant Dis. 2019, 14, 147–150. [Google Scholar]
- Qasim, M.; Ronliang, J.; Islam, W.; Ali, H.; Khan, K.A.; Dash, C.K.; Jamal, Z.A.; Wang, L.D. Comparative pathogenicity of four entomopathogenic fungal species against nymphs and adults of citrus red mite on the citrus plantation. Int. J. Trop. Insect Sci. 2020, 41, 737–749. [Google Scholar] [CrossRef]
- Fu, D.L.; Chen, Y.X.; Guo, Q.F.; Mao, L.H.; Fei, J.; Qiu, J.Z. Research progress on metabolites from Aschersonia and its teleomorph (Ascomycota). Mycosystema 2018, 37, 541–554. [Google Scholar] [CrossRef]
- Guo, Q.F.; Dong, L.L.; Zang, X.Y.; Gu, Z.J.; He, X.Y.; Yao, L.D.; Cao, L.P.; Qiu, J.Z.; Guan, X. A new azaphilone from the entomopathogenic fungus Hypocrella sp. Nat. Prod. Res 2015, 29, 2000–2006. [Google Scholar] [CrossRef]
- Sadorn, K.; Saepua, S.; Punyain, W.; Saortep, W.; Choowong, W.; Rachtawee, P.; Pittayakhajonwut, P. Chromanones and aryl glucoside analogs from the entomopathogenic fungus Aschersonia confluens BCC53152. Fitoterapia 2020, 144, 104606. [Google Scholar] [CrossRef] [PubMed]
- Aragão, T.M.S.; dos Santos, J.V.F.C.; Santos, T.S.; Souto, E.B.; Severino, P.; Jain, S.; Mendonça, M.C. Scientific-technological analysis and biological aspects of entomopathogenic fungus Aschersonia. Sustain. Chem. Pharm. 2021, 24, 100562. [Google Scholar] [CrossRef]
- van Eijk, G.W.; Mummery, R.S.; Roeymans, H.J.; Valadon, L.R.G. A comparative study of carotenoids of Aschersonia aleyrodis and Aspergillus giganteus. Antonie Van Leeuwenhoek 1979, 45, 417–422. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, G.W.; Roeijmans, H.J.; Seykens, D. Hopanoins from the entomogenous fungus Aschersonia aleyrodis. Tetrahedron Lett. 1986, 27, 2533–2534. [Google Scholar] [CrossRef]
- Krasnoff, S.B.; Gibson, D.M.; Belofsky, G.N.; Gloer, J.B. New destruxins from the entomopathogenic fungus Aschersonia sp. J. Nat. Prod. 1996, 59, 485–489. [Google Scholar] [CrossRef]
- Boonphong, S.; Kittakoop, P.; Isaka, M.; Palittapongarnpim, P.; Jaturapat, A.; Danwisetkanjana, K.; Tanticharoen, M.; Thebtaranonth, Y. A new antimycobacterial, 3β-acetoxy-15α, 22-dihydroxyhopane, from the insect pathogenic fungus Aschersonia tubulata. Planta Med. 2001, 67, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Hywel-Jones, N.L.; Sappan, M.; Mongkolsamrit, S.; Saidaengkham, S. Hopane triterpenes as chemotaxonomic markers for the scale insect pathogens Hypocrella s. lat. and Aschersonia. Mycol. Res. 2009, 113, 491–497. [Google Scholar] [CrossRef]
- Isaka, M.; Sappan, M.; Luangsa-ard, J.J.; Hywel-Jones, N.L.; Mongkolsamrit, S.; Chunhametha, S. Chemical taxonomy of Torrubiella s. lat.: Zeorin as a marker of Conoideocrella. Fungal Biol. 2011, 115, 401–405. [Google Scholar] [CrossRef]
- Saepua, S.; Kornsakulkarn, J.; Choowong, W.; Supothina, S.; Thongpanchang, C. Bioxanthracenes and monomeric analogues from insect pathogenic fungus Conoideocrella luteorostrata Zimm. BCC 31648. Tetrahedron 2015, 71, 2400–2408. [Google Scholar] [CrossRef]
- Saepua, S.; Kornsakulkarn, J.; Somyong, W.; Laksanacharoen, P.; Isaka, M.; Thongpanchang, C. Bioactive compounds from the scale insect fungus Conoideocrella tenuis BCC 44534. Tetrahedron 2018, 74, 859–866. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Choowong, W.; Mongkolsamrit, S. Conoideoxime A, antibacterial bis-oxime prenyl-tryptophan dimer from the whitefly pathogenic fungus Conoideocrella luteorostrata BCC 76664. Tetrahedron Lett. 2019, 60, 154–156. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Rachtawee, P.; Srichomthong, K.; Mongkolsamrit, S. Paecilodepsipeptide D, a cyclohexadepsipeptide from cultures of the whitefly pathogenic fungus Conoideocrella luteorostrata BCC 76664. Phytochem. Lett. 2019, 34, 65–67. [Google Scholar] [CrossRef]
- Sadorn, K.; Saepua, S.; Boonyuen, N.; Komwijit, S.; Rachtawee, P.; Pittayakhajonwut, P. Phenolic glucosides and chromane analogs from the insect fungus Conoideocrella krungchingensis BCC53666. Tetrahedron 2019, 75, 3463–3471. [Google Scholar] [CrossRef]
- Yoneyama, T.; Iguchi, M.; Yoshii, K.; Elshamy, A.I.; Ban, S.; Noji, M.; Umeyama, A. Xanthone glucoside from an insect pathogenic fungus Conoideocrella luteorostrata NBRC106950. Nat. Prod. Res. 2022, 36, 3701–3704. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).