Trichoderma-Bioenriched Vermicompost Induces Defense Response and Promotes Plant Growth in Thai Rice Variety “Chor Khing”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of “Chor Khing” Rice Variety and Preparation of Trichoderma-Bioenriched Vermicompost
2.2. Antifungal Activity of Trichoderma asperelloides PSU-P1 Against Rhizoctonia solani
2.3. Viability of Trichoderma in Trichoderma-Bioenriched Vermicompost
2.4. Effect of Trichoderma-Bioenriched Vermicompost Water Extracts on Seed Germination
2.5. Effect of Trichoderma-Bioenriched Vermicompost on Rice Seedling Growth
2.6. Inducing Defense Response in Rice Seedlings with Trichoderma-Bioenriched Vermicompost
2.7. Pot Experiment
2.8. DNA Extraction
2.9. PCR Amplification and High-Throughput Sequencing
2.10. Alpha Diversity Analysis
2.11. Statistical Analysis
3. Results
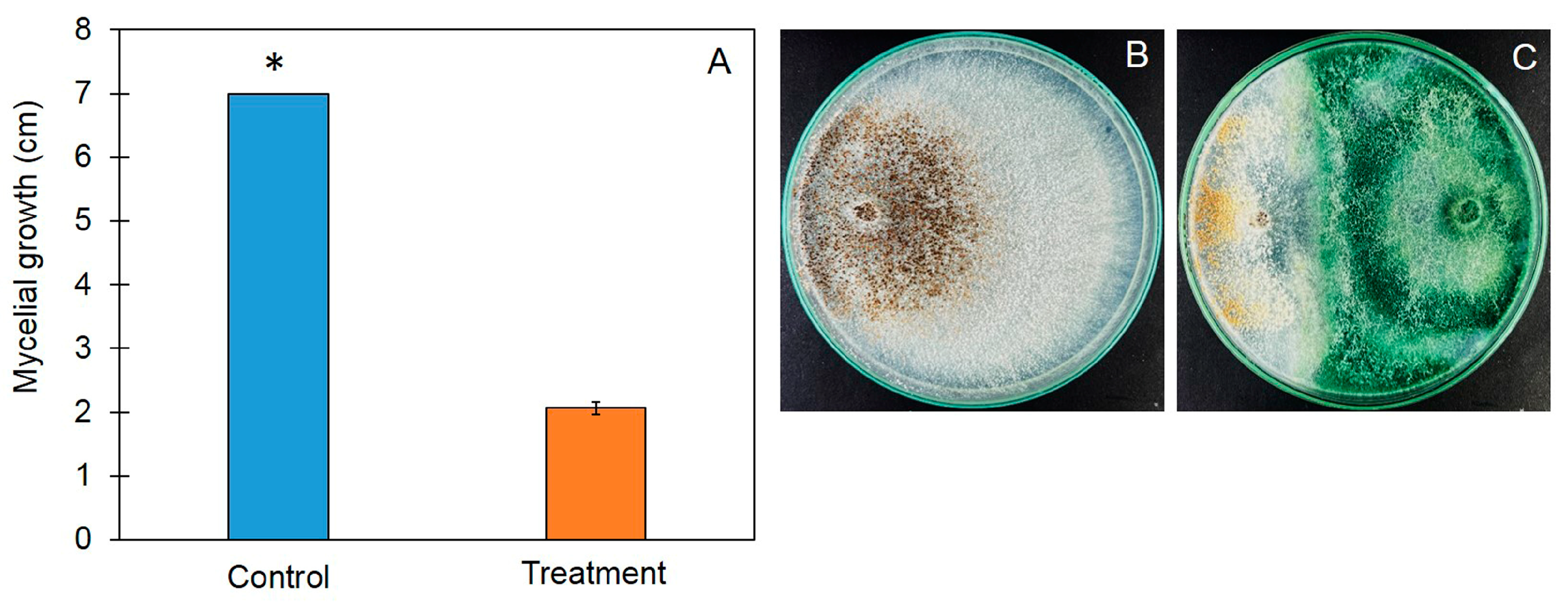
3.1. Antifungal Ability of Trichoderma asperelloides PSU-P1 against Rhizoctonia solani
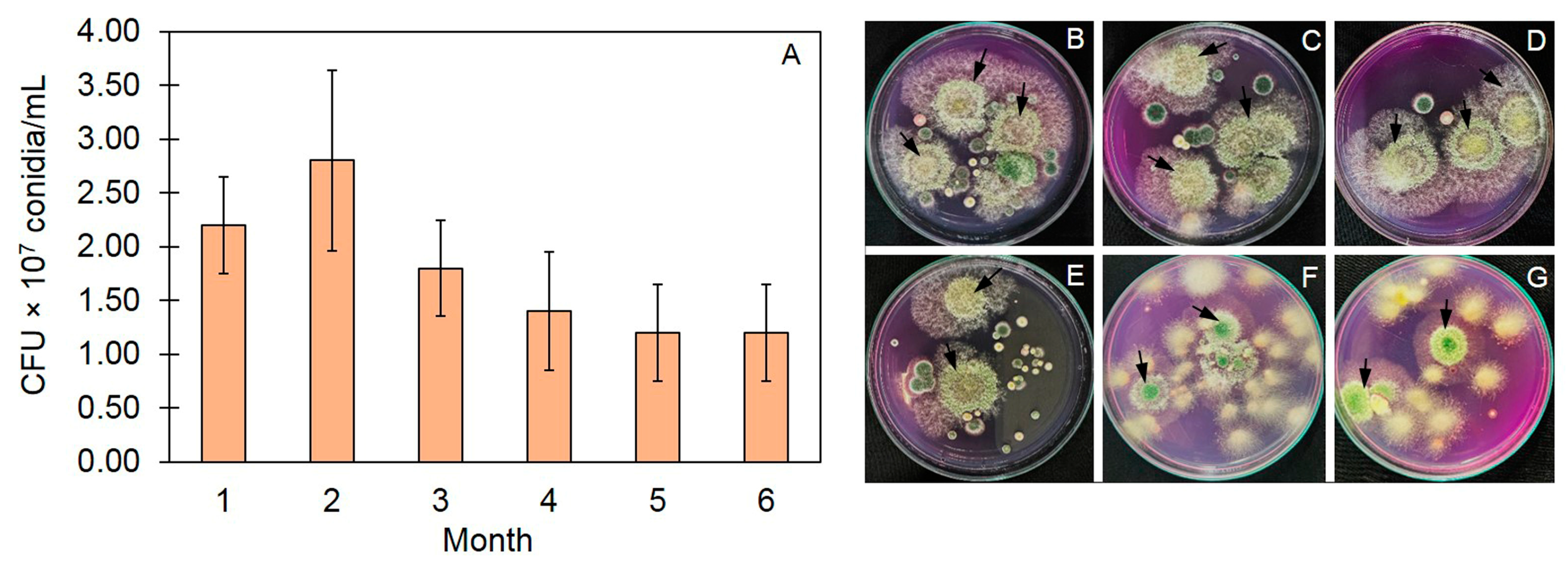
3.2. Survival of Trichoderma in Trichoderma-Bioenriched Vermicompost
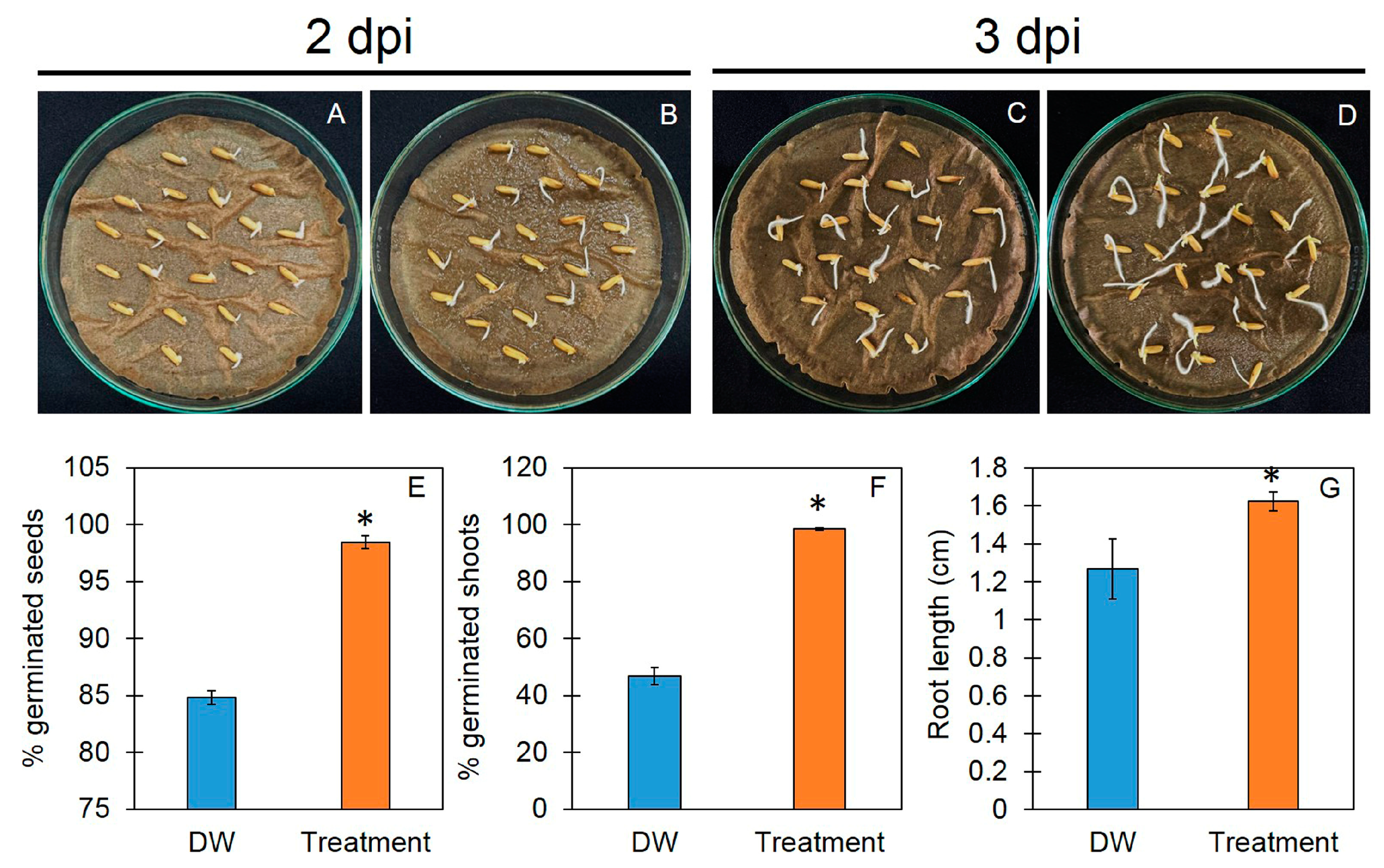
3.3. Trichoderma-Bioenriched Vermicompost Enhances Seed Germination
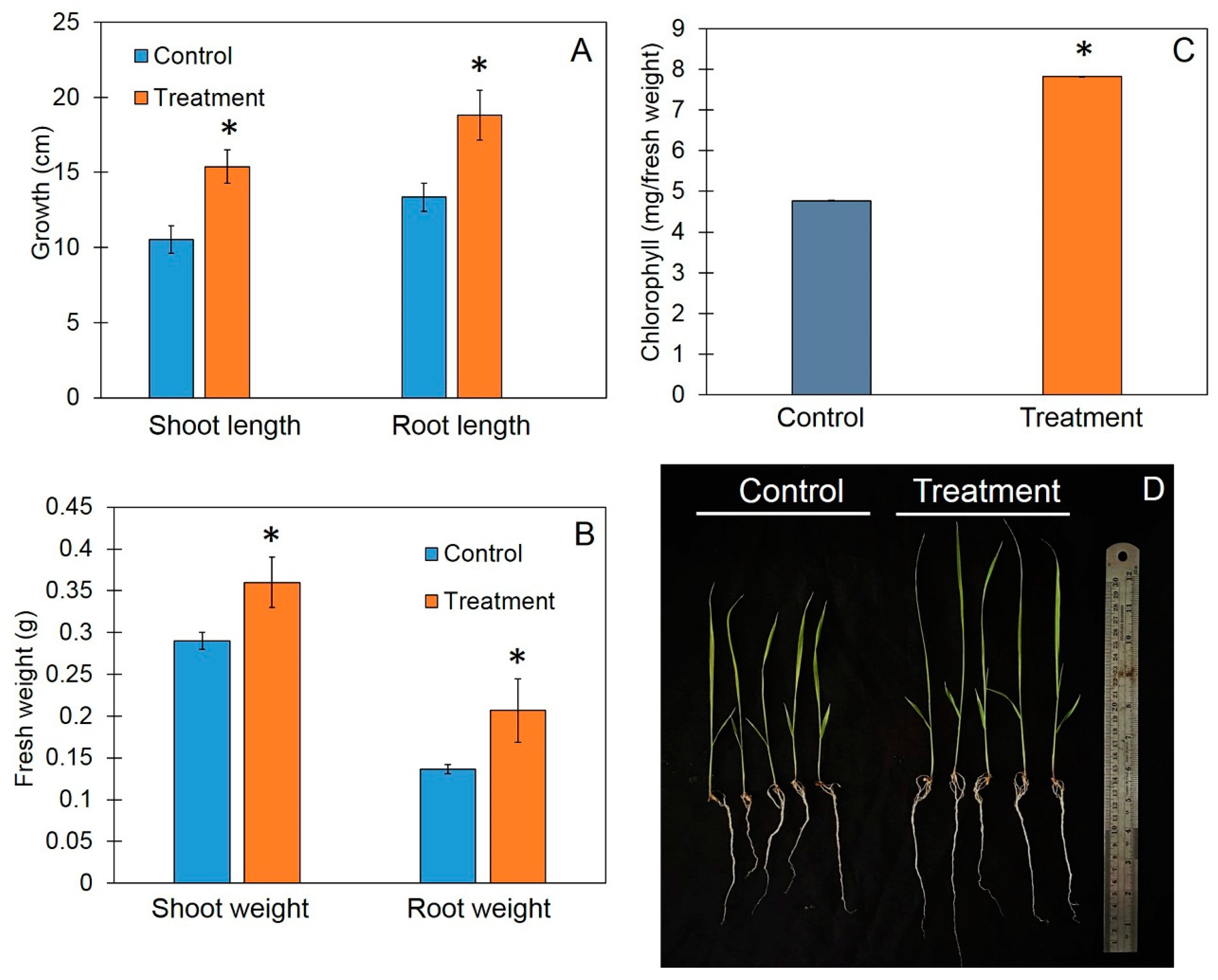
3.4. Trichoderma-Bioenriched Vermicompost Promotes Plant Growth in Rice Seedlings
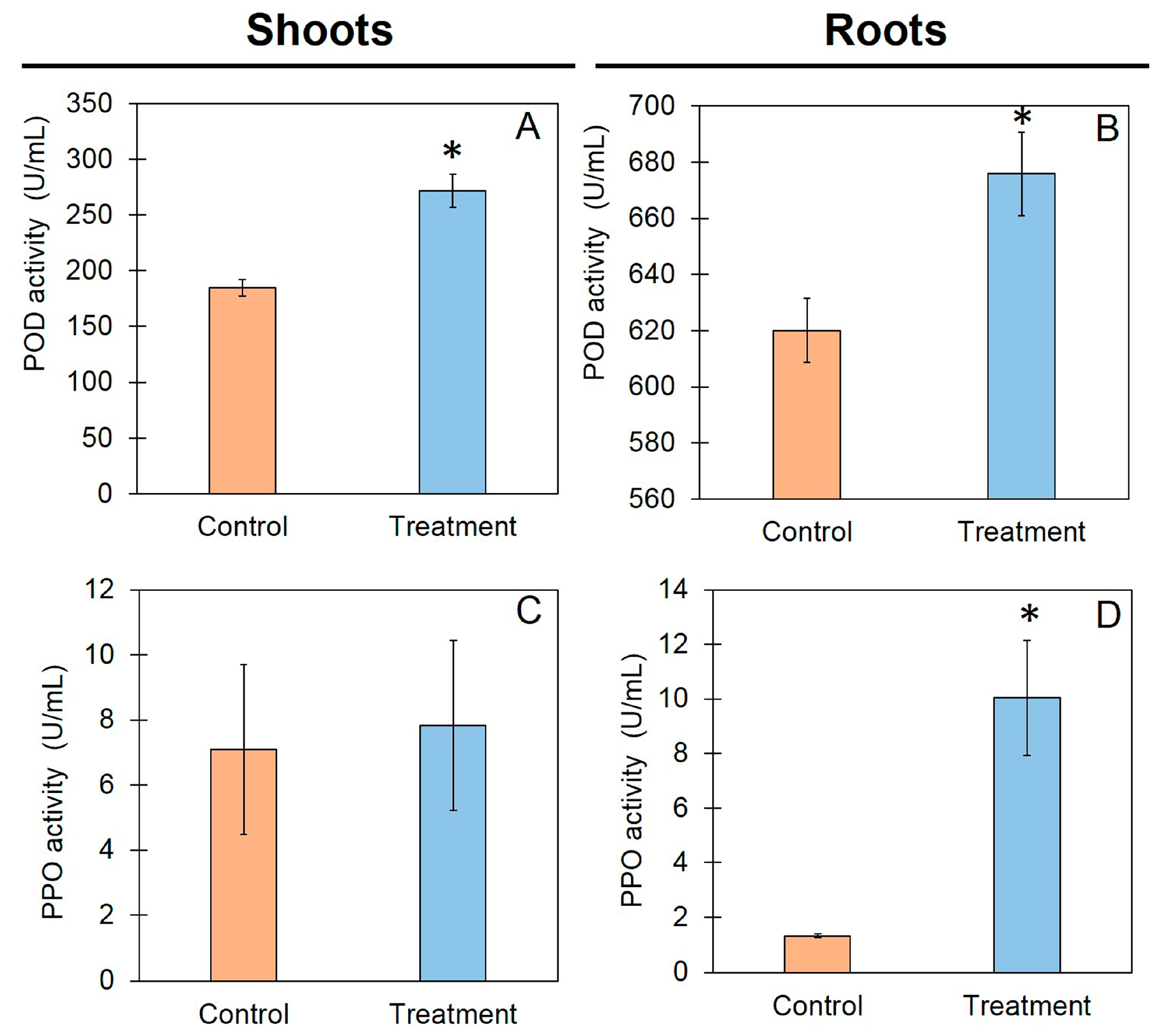
3.5. Defense-Related Enzyme Activities in Rice Seedlings
3.6. Effect of Trichoderma-Bioenriched Vermicompost on Biological Control
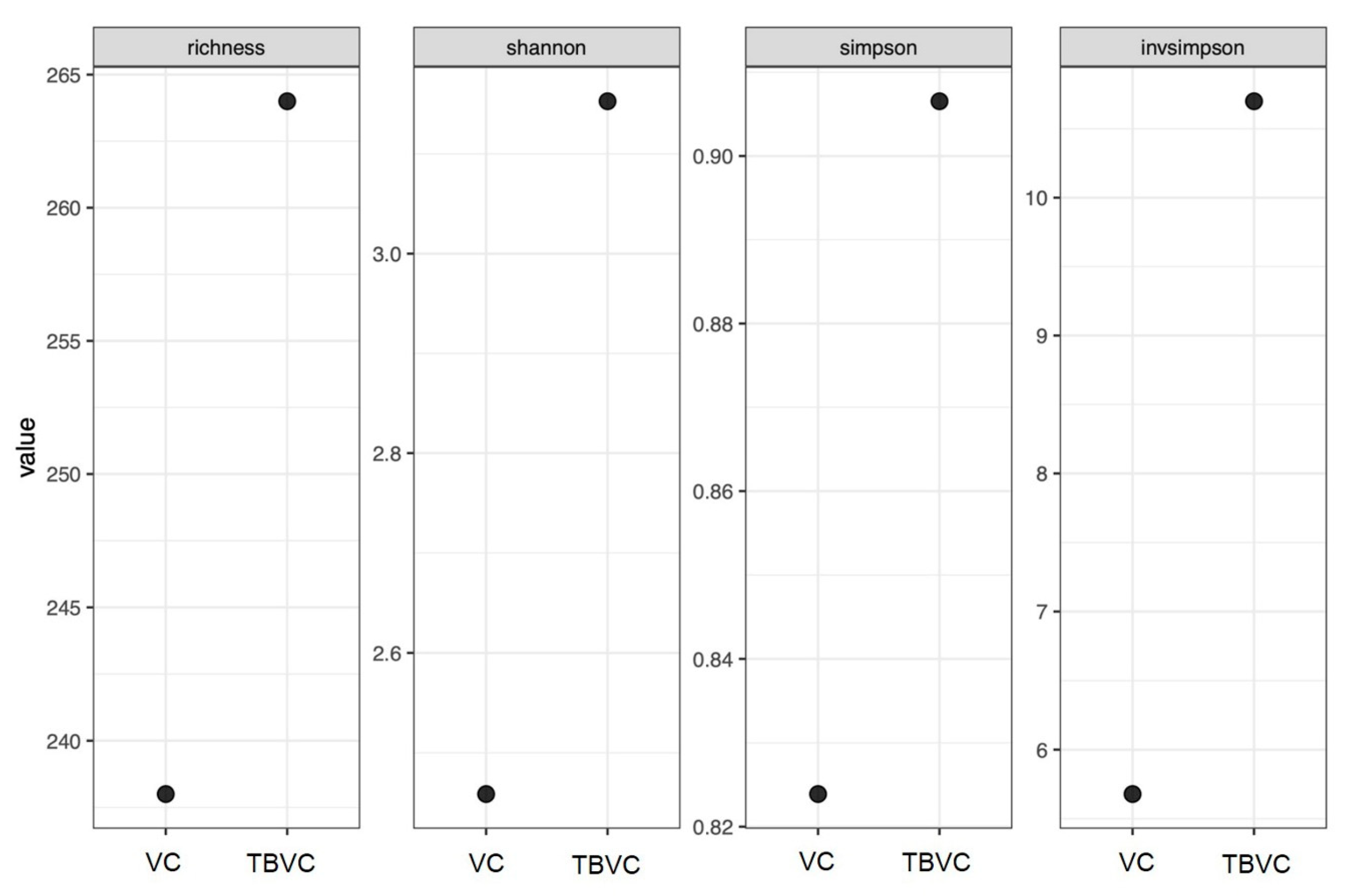
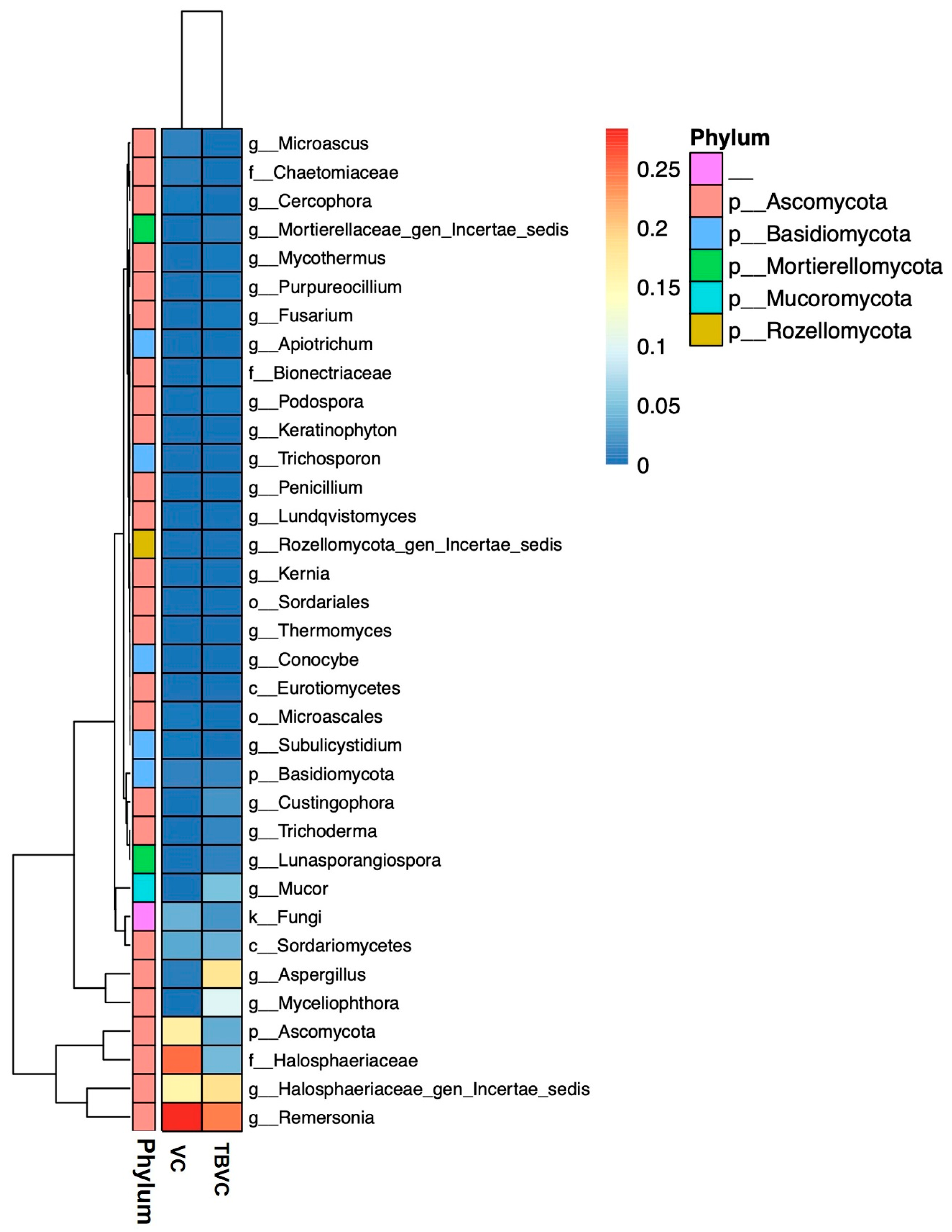
3.7. Fungal Community in Trichoderma-Bioenriched Vermicompost
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaoui, H.I.; Zibilske, L.M.; Ohnot, T. Effects of earthworm casts and compost on soil microbial activity and plant nutrient availability. Soil Biol. Biochem. 2003, 35, 295–302. [Google Scholar] [CrossRef]
- Sinha, R.K.; Agarwal, S.; Chauhan, K.; Valani, D. The wonders of earthworms and its vermicompost in farm production: Charles Darwin’s ‘friends of farmers’, with potential to replace destructive chemical fertilizers from agriculture. Agric. Sci. 2010, 1, 76–94. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ismail, S.A. Role of earthworms in vermitechnology. Int. J. Agric. 2012, 8, 405–415. [Google Scholar]
- Arora, V.K.; Singh, C.B.; Sidhu, A.S.; Thind, S.S. Irrigation, tillage and mulching effects on Soybean yield and water productivity in relation to soil texture. Agric. Water Manag. 2011, 98, 563–568. [Google Scholar] [CrossRef]
- Hoque, T.S.; Hasan, A.K.; Hasan, M.A.; Nahar, N.; Dey, D.K.; Mia, S.; Solaiman, Z.M.; Kader, M.A. Nutrient release from vermicompost under anaerobic conditions in two contrasting soils of Bangladesh and its effect on wetland rice crop. Agriculture 2022, 12, 376. [Google Scholar] [CrossRef]
- Rekha, G.S.; Kaleena, P.K.; Elumalai, D.; Srikumaran, M.P.; Maheswari, V.N. Effects of vermicompost and plant growth enhancers on the exo-morphological features of Capsicum annum (Linn.) Hepper. Int. J. Recycl. Org. Waste Agric. 2018, 7, 83–88. [Google Scholar] [CrossRef]
- Hassan, S.A.M.; Taha, R.A.; Zaied, N.S.M.; Essa, E.M.; Kh, M.A.E. Effect of vermicompost on vegetative growth and nutrient status of acclimatized Grand Naine banana plants. Heliyon 2022, 8, e10914. [Google Scholar] [CrossRef] [PubMed]
- Gudeta, K.; Julka, J.M.; Kumar, A.; Bhagat, A.; Kumari, A. Vermiwash: An agent of disease and pest control in soil, a review. Heliyon 2021, 7, e06434. [Google Scholar] [CrossRef]
- Naidu, Y.; Meon, S.; Siddiqui, Y. In vitro and in vivo evaluation of microbial-enriched compost tea on the development of powdery mildew on melon. BioControl 2012, 57, 827–836. [Google Scholar] [CrossRef]
- Morais, E.M.; Silva, A.A.R.; Sousa, F.W.A.; Azevedo, I.M.B.; Silva, H.F.; Santos, A.M.G.; Beserra Júnior, J.E.A.; Carvalho, C.P.; Eberlin, M.N.; Porcari, A.M.; et al. Endophytic Trichoderma strains isolated from forest species of the Cerrado-Caatinga ecotone are potential biocontrol agents against crop pathogenic fungi. PLoS ONE 2022, 17, e0265824. [Google Scholar] [CrossRef]
- Phoka, N.; Pornsuriya, C.; Sunpapao, A. High-throughput sequencing provides insight into soil fungal community structure and diversity in plant protected areas of Songkhla zoo in southern Thailand. Chiang Mai J. Sci. 2022, 49, 524–537. [Google Scholar] [CrossRef]
- Crozier, J.; Thomas, S.E.; Aime, M.C.; Evans, H.C.; Holmes, K.A. Molecular characterization of fungal endophytic morphospecies isolated from stems and pods of Theobroma cacao. Plant Pathol. 2006, 55, 783–791. [Google Scholar] [CrossRef]
- De Souza, J.T.; Bailey, B.A.; Pomella, A.W.V.; Erbe, E.F.; Murphy, C.A.; Bae, H.; Hebbar, P.K. Colonization of cacao seedlings by Trichoderma stromaticum, a mycoparasite of the witches’ broom pathogen, and its influence on plant growth and resistance. Biol. Control. 2008, 46, 36–45. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Zhang, B.; Yang, X.-Y.; Naicker, O.; Zhao, L. Brown rot disease caused by Trichoderma hamatum on the edible lily, Lilium leichtlinii var. maximowiczii. Chiang Mai J. Sci. 2022, 49, 1500–1508. [Google Scholar] [CrossRef]
- Ruangwong, O.-U.; Pornsuriya, C.; Pitija, K.; Sunpapao, A. Biocontrol mechanisms of Trichoderma koningiopsis PSU3-2 against postharvest anthracnose of chili pepper. J. Fungi 2021, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Wonglom, P.; Daengsuwan, W.; Ito, S.; Sunpapao, A. Biological control of Sclerotium fruit rot of snake fruit and stem rot of lettuce by Trichoderma sp. T76-12/2 and the mechanisms involved. Physiol. Mol. Plant Pathol. 2019, 107, 1–7. [Google Scholar] [CrossRef]
- Intana, W.; Wonglom, P.; Suwannarach, N.; Sunpapao, A. Trichoderma asperelloides PSU-P1 induced expression of pathogenesis-related protein genes against gummy stem blight of muskmelon (Cucumis melo) in field evaluation. J. Fungi 2022, 8, 156. [Google Scholar] [CrossRef]
- Intana, W.; Kumla, J.; Suwannarach, N.; Sunpapao, A. Biological control potential of a soil fungus Trichoderma asperellum K1-02 against Neoscytalidium dimidiatum causing stem canker of dragon fruit. Physiol. Mol. Plant Pathol. 2023, 128, 102151. [Google Scholar] [CrossRef]
- Baiyee, B.; Pornsuriya, C.; Ito, S.-I.; Sunpapao, A. Trichoderma spirale T76-1 displays biocontrol activity against leaf spot on lettuce (Lactuca sativa L.) caused by Corynespora cassiicola or Curvularia aeria. Biol. Control. 2019, 129, 195–200. [Google Scholar]
- Ruangwong, O.U.; Wonglom, P.; Suwannarach, N.; Kumla, J.; Thaochan, N.; Chomnunti, P.; Pitija, K.; Sunpapao, A. Volatile organic compound from Trichoderma asperelloides TSU1: Impact on plant pathogenic fungi. J. Fungi 2021, 7, 187. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Intana, W.; Suwannarach, N.; Kumla, J.; Wonglom, P.; Sunpapao, A. Plant growth promotion and biological control against Rhizoctonia solani in Thai local rice variety “Chor Khing” using Trichoderma breve Z2-03. J. Fungi 2024, 10, 417. [Google Scholar] [CrossRef]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma counteracts the challenge of Phytophthora nicotianae infections on tomato by modulating plant defense mechanisms and the expression of crinkler, necrosis-inducing Phytophthora protein 1, and cellulose-binding elicitor lectin pathogenic effectors. Front. Plant Sci. 2020, 11, 583539. [Google Scholar]
- Degani, O.; Dor, S. Trichoderma biological control to protect sensitive maize hybrids against late wilt disease in the field. J. Fungi 2021, 7, 315. [Google Scholar] [CrossRef]
- Lian, H.; Li, R.; Ma, G.; Zhao, Z.; Zhang, T.; Li, M. The effect of Trichoderma harzianum agents on physiological-biochemical characteristics of cucumber and the control effect against Fusarium wilt. Sci. Rep. 2023, 13, 17606. [Google Scholar] [CrossRef] [PubMed]
- Statista. Principal Rice Exporting Countries Worldwide in 2021/2022. Available online: https://www.statista.com/statistics/255947/top-rice-exporting-countries-worldwide-2011/ (accessed on 17 July 2024).
- Suwannaporn, P.; Linnemann, A. Rice-eating quality among consumers in different rice grain preference countries. J. Sens. Stud. 2008, 23, 1–13. [Google Scholar] [CrossRef]
- Srisompun, O. Rice Economy in Northeast Thailand: Current Status and Challenges. Available online: https://ap.fftc.org.tw/article/1863 (accessed on 5 May 2024).
- Ruangwong, O.-U.; Wonglom, P.; Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.-I.; Sunpapao, A. Biological control activity of Trichoderma asperelloides PSU-P1 against gummy stem blight in muskmelon (Cucumis melo). Physiol. Mol. Plant Pathol. 2021, 115, e101663. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Nagle, N.E.; Haard, N.F. Fractionation and characterization of peroxidase from ripe banana fruit. J. Food Sci. 1975, 40, 576–579. [Google Scholar] [CrossRef]
- Luh, B.S.; Phithakpol, B. Characteristic of polyphenol-oxidase related to browning in cling peaches. J. Food Sci. 1972, 37, 18–25. [Google Scholar] [CrossRef]
- IRRI. Standard Evaluation System for Rice, 4th ed.; INGER Genetic Resource Centre: Manila, Philippines, 1996. [Google Scholar]
- Intana, W.; Wonglom, P.; Dy, K.S.; Sunpapao, A. Development of a novel emulsion formulation of Trichoderma asperelloides PSU-P1 conidia against stem canker on dragon fruit caused by Neoscytalidium dimidiatum. Microbiol. Res. 2023, 14, 1139–1149. [Google Scholar] [CrossRef]
- dos Santos Pereira, T.; Monteiro de Paula, A.; Ferrari, L.H.; da Silva, J.; Borges Pinheiro, J.; Navas Cajamarca, S.M.; Jindo, K.; Pupo Santos, M.; Zandonadi, D.B.; Busato, J.G. Trichoderma-enriched vermicompost extracts reduces nematode biotic stress in tomato and bell pepper crops. Agronomy 2021, 11, 1655. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Pant, A.; Radovich, T.; Hue, N.V.; Potter, J.K.; Converse, C.E. Seed germination and seedling growth of tomato and lettuce as affected by vermicompost water extracts (Teas). HortScience 2012, 47, 1722–1728. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.D.M.; Varanda, C.M.R.; Félix, M.R.F. Induced resistance during the interaction pathogen x plant and the use of resistance inducers. Phytochem. Lett. 2016, 15, 152–158. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Cheng, X.; Zhang, X.; Li, X.; Kong, X. Cloning and expression analysis of the laccase genes CsLAC4 and CsLAC12 from the tea plant. J. Plant Protect. 2018, 45, 1069–1077. [Google Scholar]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2021, 181, 112588. [Google Scholar] [CrossRef]
- Raj, S.N.; Sarosh, B.R.; Shetty, H.S. Induction and accumulation of polyphenol oxidase activities as implicated in development of resistance against pearl millet downy mildew disease. Funct. Plant Biol. 2006, 33, 563–571. [Google Scholar] [CrossRef]
- Afria, B.S.; Mukherjee, D. Peroxidase activity during early seedling growth in light and dark. Plant Physiol. Biochem. 1982, 19, 18–21. [Google Scholar]
- Domínguez, J.; Aira, M.; Crandall, K.A.; Pérez-Losada, M. Earthworms drastically change fungal and bacterial communities during vermicomposting of sewage sludge. Sci. Rep. 2011, 11, 15556. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.; Yoon, S.; Joo, P. Changes in Fungal and Bacterial Diversity during Vermicomposting of Industrial Sludge and Poultry Manure Mixture: Detecting the Mechanism of Plant Growth Promotion by Vermicompost; InTech: London, UK, 2011; pp. 113–124. [Google Scholar] [CrossRef]
- Aslam, Z.; Aljuaid, B.S.; Abbas, R.N.; Bashir, S.; Almas, M.H.; Awan, T.H.; Belliturk, K.; Al-Taisan, W.a.A.; Mahmoud, S.F.; Bashir, S. Reduction in the allelopathic potential of Conocarpus erectus L. through vermicomposting. Sustainability 2022, 14, 12840. [Google Scholar] [CrossRef]
- Simsek Ersahin, Y.; Haktanir, K.; Yanar, Y. Vermicompost suppresses Rhizoctonia solani Kühn in cucumber seedlings. J. Plant Dis. Protect. 2009, 116, 182–188. [Google Scholar] [CrossRef]
- Nonthapa, A.; Pengpan, P.; Chankaew, S.; Boonthai Iwai, C.; Hanboonsong, Y.; Falab, S. Vermicompost in combination with Trichoderma asperellum isolate T13 as bioagent to control sclerotium rot disease on vegetable soybean seedlings. Agr. Nat. Resour. 2022, 56, 877–888. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wonglom, P.; Ruangwong, O.-U.; Poncheewin, W.; Arikit, S.; Riangwong, K.; Sunpapao, A. Trichoderma-Bioenriched Vermicompost Induces Defense Response and Promotes Plant Growth in Thai Rice Variety “Chor Khing”. J. Fungi 2024, 10, 582. https://doi.org/10.3390/jof10080582
Wonglom P, Ruangwong O-U, Poncheewin W, Arikit S, Riangwong K, Sunpapao A. Trichoderma-Bioenriched Vermicompost Induces Defense Response and Promotes Plant Growth in Thai Rice Variety “Chor Khing”. Journal of Fungi. 2024; 10(8):582. https://doi.org/10.3390/jof10080582
Chicago/Turabian StyleWonglom, Prisana, On-Uma Ruangwong, Wasin Poncheewin, Siwaret Arikit, Kanamon Riangwong, and Anurag Sunpapao. 2024. "Trichoderma-Bioenriched Vermicompost Induces Defense Response and Promotes Plant Growth in Thai Rice Variety “Chor Khing”" Journal of Fungi 10, no. 8: 582. https://doi.org/10.3390/jof10080582





