Profiling of the Citrus Leaf Endophytic Mycobiota Reveals Abundant Pathogen-Related Fungal Groups
Abstract
1. Introduction
2. Methods
2.1. Summary of Citrus-Related Fungi
2.2. Sampling of Citrus Leaves
2.3. Molecular Analysis of Leaf Endophytic Fungi
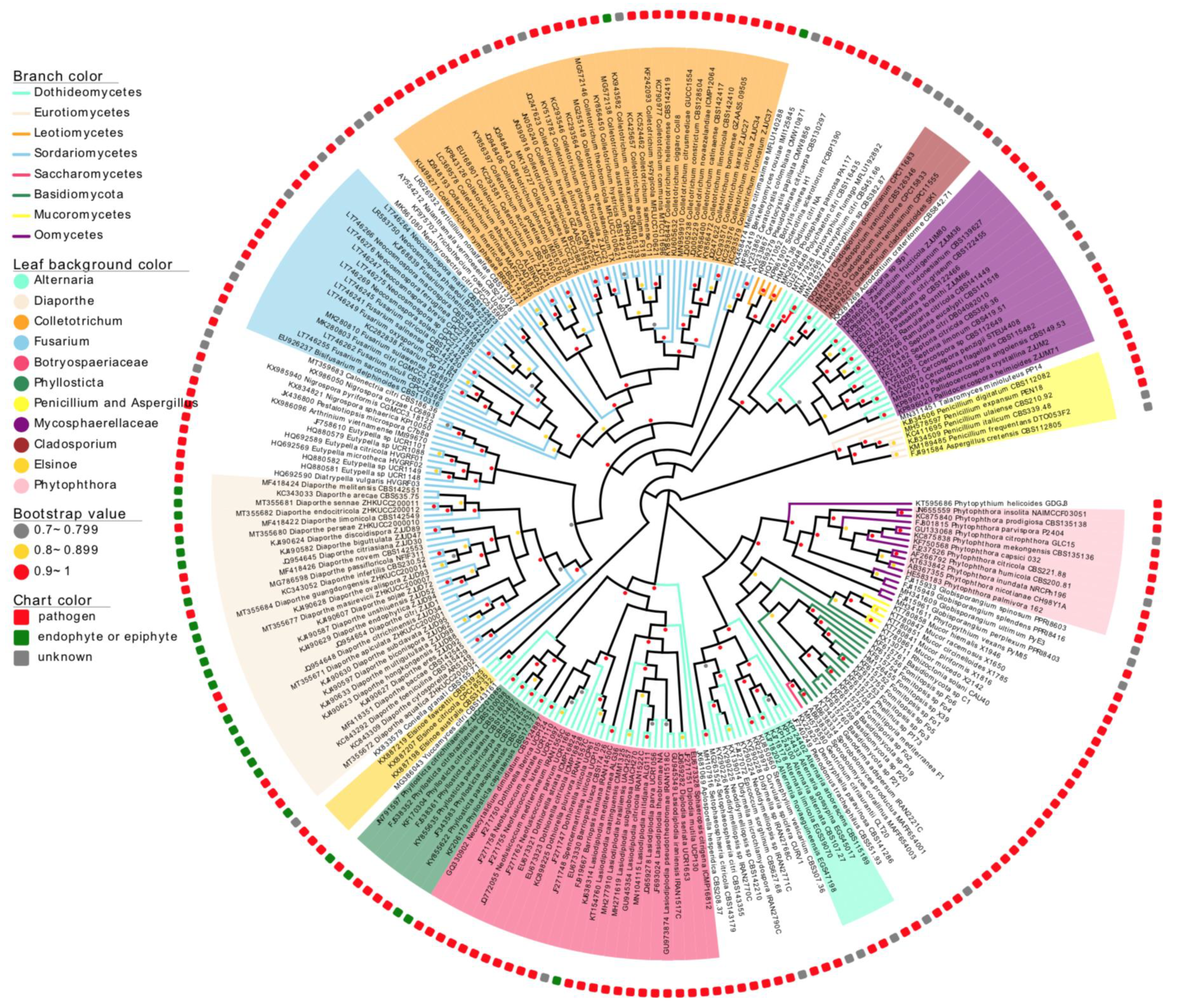
2.4. Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. A Summary of Reported Citrus-Related Fungi
3.2. Diversity and Composition of Citrus Leaf Endophytic Fungal Communities
3.3. Distribution of Pathogens and Potential Pathogens in Citrus Leaf Endophytic Fungal Communities
3.4. Dominance of Latent Pathogens within Their Genus
4. Discussion
4.1. The Composition of the Leaf Endophytic Fungal Community Correlates to the Phylogenetic Relationship of the Citrus
4.2. A Summarized ITS Data Set Is Useful in Studying Citrus Mycobiota
4.3. Leaf Endophytic Community May Be a Reservoir for Citrus Pathogens
4.4. Do Fungal Pathogen-Related Groups Have Competitive Advantages in Colonizing the Leaf Endophytic Space?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, N. The Citrus Huanglongbing crisis and potential solutions. Mol. Plant 2019, 12, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Z.; Fu, Y.; Cheng, J.; Xie, J.; Lin, Y. Identification of Lasiodiplodia pseudotheobromae causing fruit rot of Citrus in China. Plants 2021, 10, 202. [Google Scholar] [CrossRef]
- Wang, L.; He, F.; Huang, Y.; He, J.; Yang, S.; Zeng, J.; Deng, C.; Jiang, X.; Fang, Y.; Wen, S.; et al. Genome of wild mandarin and domestication history of mandarin. Mol. Plant 2018, 11, 1024–1037. [Google Scholar] [CrossRef]
- Penjor, T.; Yamamoto, M.; Uehara, M.; Ide, M.; Matsumoto, N.; Matsumoto, R.; Nagano, Y. Phylogenetic relationships of citrus and its relatives based on matK gene sequences. PLoS ONE 2013, 8, e62574. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Zhang, S.; Cao, L.; Huang, Y.; Cheng, J.; Wu, G.; Tian, S.; Chen, C.; Liu, Y.; et al. Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction. Nat. Genet. 2017, 49, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Adesemoye, A.O.; Mayorquin, J.S.; Wang, D.H.; Twizeyimana, M.; Lynch, S.C.; Eskalen, A. Identification of species of Botryosphaeriaceae causing bot gummosis in Citrus in California. Plant Dis. 2014, 98, 55–61. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Groenewald, J.Z.; Li, H.Y.; Glienke, C.; Carstens, E.; Hattingh, V.; Fourie, P.H.; Crous, P.W. First report of Phyllosticta citricarpa and description of two new species, P. paracapitalensis and P. paracitricarpa, from citrus in Europe. Stud. Mycol. 2017, 87, 161–185. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, V.; Groenewald, J.Z.; Polizzi, G.; Crous, P.W. High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia 2017, 39, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; de Silva, D.D.; Moslemi, A.; Edwards, J.; Ades, P.K.; Crous, P.W.; Taylor, P.W.J. Colletotrichum species causing anthracnose of Citrus in Australia. J. Fungi 2021, 7, 47. [Google Scholar] [CrossRef]
- Huang, F.; Chen, G.Q.; Hou, X.; Fu, Y.S.; Cai, L.; Hyde, K.D.; Li, H.Y. Colletotrichum species associated with cultivated citrus in China. Fungal Divers. 2013, 61, 61–74. [Google Scholar] [CrossRef]
- Peng, L.J.; Yang, Y.L.; Hyde, K.D.; Bahkali, A.; Liu, Z.Y. Colletotrichum species on Citrus leaves in Guizhou and Yunnan provinces, China. Cryptogam. Mycol. 2012, 33, 267–283. [Google Scholar] [CrossRef]
- Abdollahzadeh, J.; Javadi, A.; Zare, R.; Phillips, A.J. A phylogenetic study of Dothiorella and Spencermartinsia species associated with woody plants in Iran, New Zealand, Portugal and Spain. Persoonia 2014, 32, 1–12. [Google Scholar] [CrossRef]
- Bezerra, J.D.P.; Crous, P.W.; Aiello, D.; Gullino, M.L.; Polizzi, G.; Guarnaccia, V. Genetic diversity and pathogenicity of Botryosphaeriaceae species associated with symptomatic Citrus plants in Europe. Plants 2021, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Koringa, P.G.; Singh, S.P. Understanding Host-Microbiome Interactions: An Omics Approach; Singh, R.P., Ed.; Springer: Singapore, 2017. [Google Scholar]
- Grady, K.L.; Sorensen, J.W.; Stopnisek, N.; Guittar, J.; Shade, A. Assembly and seasonality of core phyllosphere microbiota on perennial biofuel crops. Nat. Commun. 2019, 10, 4135. [Google Scholar] [CrossRef]
- Sapkota, R.; Knorr, K.; Jørgensen, L.N.; O’Hanlon, K.A.; Nicolaisen, M. Host genotype is an important determinant of the cereal phyllosphere mycobiome. New Phytol. 2015, 207, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Remus-Emsermann, M.N.P.; Schlechter, R.O. Phyllosphere microbiology: At the interface between microbial individuals and the plant host. New Phytol. 2018, 218, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Amine, P.D.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Stone, B.W.; Weingarten, E.A.; Jackson, C.R. The role of the phyllosphere microbiome in plant health and function. Ann. Plant Rev. 2018, 1, 533–556. [Google Scholar] [CrossRef]
- Vacher, C.; Hampe, A.; Porté, A.J.; Sauer, U.; Compant, S.; Morris, C.E. The phyllosphere: Microbial jungle at the plant-climate interface. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Lagier, J.C.; Dubourg, G.; Million, M.; Cadoret, F.; Bilen, M.; Fenollar, F.; Levasseur, A.; Rolain, J.M.; Fournier, P.E.; Raoult, D. Culturing the human microbiota and culturomics. Nat. Rev. Microbiol. 2018, 16, 540–550. [Google Scholar] [CrossRef]
- Sarhan, M.S.; Hamza, M.A.; Youssef, H.H.; Patz, S.; Becker, M.; ElSawey, H.; Nemr, R.; Daanaa, H.S.A.; Mourad, E.F.; Morsi, A.T.; et al. Culturomics of the plant prokaryotic microbiome and the dawn of plant-based culture media-A review. J. Adv. Res. 2019, 19, 15–27. [Google Scholar] [CrossRef]
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef]
- Herrera Paredes, S.; Gao, T.; Law, T.F.; Finkel, O.M.; Mucyn, T.; Teixeira, P.J.P.L.; Salas González, I.; Feltcher, M.E.; Powers, M.J.; Shank, E.A.; et al. Design of synthetic bacterial communities for predictable plant phenotypes. PLoS Biol. 2018, 16, e2003962. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Groenewald, J.Z.; Zhu, L.; Crous, P.W.; Li, H.Y. Cercosporoid diseases of Citrus. Mycologia 2015, 107, 1151–1171. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Udayanga, D.; Wang, X.; Hou, X.; Mei, X.; Fu, Y.; Hyde, K.D.; Li, H. Endophytic Diaporthe associated with Citrus: A phylogenetic reassessment with seven new species from China. Fungal Biol. 2015, 119, 331–347. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.J.; Sessitsch, A. Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Ann. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Malmierca, M.G.; Izquierdo-Bueno, I.; McCormick, S.P.; Cardoza, R.E.; Alexander, N.J.; Barua, J.; Lindo, L.; Casquero, P.A.; Collado, I.G.; Monte, E.; et al. Trichothecenes and aspinolides produced by Trichoderma arundinaceum regulate expression of Botrytis cinerea genes involved in virulence and growth. Environ. Microbiol. 2016, 18, 3991–4004. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Orozco-Mosqueda, M.; Santoyo, G. Plant-microbial endophytes interactions: Scrutinizing their beneficial mechanisms from genomic explorations. Curr. Plant Biol. 2020, 25, 100189. [Google Scholar] [CrossRef]
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium verticillioides: Managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev. 2008, 27, 411–446. [Google Scholar] [CrossRef]
- Jiménez-Gasco, M.M.; Malcolm, G.M.; Berbegal, M.; Armengol, J.; Jiménez-Díaz, R.M. Complex molecular relationship between vegetative compatibility groups (VCGs) in Verticillium dahliae: VCGs do not always align with clonal lineages. Phytopathology 2014, 104, 650–659. [Google Scholar] [CrossRef]
- Allen, G.C.; Flores-Vergara, M.A.; Krasynanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammoniumbromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum-evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Saito, S.; Michailides, T.; Xiao, C.L. Baseline Sensitivity of Alternaria alternata and A. arborescens to natamycin and control of Alternaria rot on stored mandarin fruit. Plant Dis. 2021, 105, 3653–3656. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; De Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Cruz, M.A.; Almaguer-Vargas, G.; Leyva-Mir, S.G.; Colinas-León, M.T.; Correia, K.C.; Camacho-Tapia, M.; Tovar-Pedraza, J.M. Phylogeny, distribution, and pathogenicity of Lasiodiplodia species associated with cankers and dieback symptoms of Persian lime in Mexico. Plant Dis. 2019, 103, 1156–1165. [Google Scholar] [CrossRef]
- Coutinho, I.B.L.; Freire, F.C.O.; Lima, C.S.; Lima, J.S.; Gonçalves, F.J.T.; Machado, A.R.; Cardoso, J.E. Diversity of genus Lasiodiplodia associated with perennial tropical fruit plants in northeastern Brazil. Plant Pathol. 2017, 66, 90–104. [Google Scholar] [CrossRef]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.Z.; Meijer, M.; Dijksterhuis, J.; Jurjević, Ž.; Andersen, B.; Samson, R.A. Cladosporium species in indoor environments. Stud. Mycol. 2018, 89, 177–301. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef] [PubMed]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Johnston, P.R.; Weir, B.S.; Tan, Y.P.; Crous, P.W. The Colletotrichum boninense species complex. Stud. Mycol. 2012, 73, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, D.; Manamgoda, D.S.; Liu, X.; Chukeatirote, E.; Hyde, K.D. What are the common anthracnose pathogens of tropical fruits? Fungal Divers. 2013, 61, 165–179. [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.Y.; Lin, Y.; Li, J.B.; Xiong, B.; Luo, C.X. Phylogenetic analysis and development of molecular tool for detection of Diaporthe citri causing melanose disease of citrus. Plants 2020, 9, 329. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Crous, P.W. Emerging citrus diseases in Europe caused by species of Diaporthe. IMA Fungus 2017, 8, 317–334. [Google Scholar] [CrossRef]
- Huang, F.; Hou, X.; Dewdney, M.M.; Fu, Y.; Chen, G.; Hyde, K.D.; Li, H.Y. Diaporthe species occurring on citrus in China. Fungal Divers. 2013, 61, 237–250. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Hyde, K.D. Species limits in Diaporthe: Molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 2014, 32, 83–101. [Google Scholar] [CrossRef]
- Fan, X.L.; Barreto, R.W.; Groenewald, J.Z.; Bezerra, J.D.P.; Pereira, O.L.; Cheewangkoon, R.; Crous, P.W. Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales, Dothideomycetes). Stud. Mycol. 2017, 87, 1–41. [Google Scholar] [CrossRef]
- Hou, X.; Huang, F.; Zhang, T.Y.; Xu, J.G.; Kevin, H.D.; Li, H.Y. Pathotypes and genetic diversity of Chinese collections of Elsinoë fawcettii causing citrus scab. J. Integr. Agric. 2014, 13, 1293–1302. [Google Scholar] [CrossRef]
- Miles, A.K.; Tan, Y.P.; Shivas, R.G.; Drenth, A. Novel pathotypes of Elsinoë australis associated with Citrus australasica and Simmondsia chinensis in Australia. Trop. Plant Pathol. 2015, 40, 26–34. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Guarnaccia, V.; Polizzi, G.; Crous, P.W. Symptomatic Citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia 2018, 40, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Fogliata, G.M.; Martínez, C.V.; Acosta, M.E.; Muñoz, M.L.; Ploper, L.D. First report of Fusarium rot caused by Fusarium oxysporum on lemon in Tucumán, Argentina. Plant Dis. 2013, 97, 989. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, I.; Rezgui, S.; Cherif, M. First report of mature citrus trees being affected by Fusarium wilt in Tunisia. Plant Dis. 2014, 98, 566. [Google Scholar] [CrossRef]
- Groenewald, J.Z.; Nakashima, C.; Nishikawa, J.; Shin, H.D.; Park, J.H.; Jama, A.N.; Crous, P.W. Species concepts in Cercospora: Spotting the weeds among the roses. Stud. Mycol. 2013, 75, 115–170. [Google Scholar] [CrossRef]
- Pretorius, M.C.; Crous, P.W.; Groenewald, J.Z.; Braun, U. Phylogeny of some cercosporoid fungi from Citrus. Sydowia 2003, 55, 286–305. Available online: https://www.zobodat.at/pdf/Sydowia_55_0286-0305.pdf (accessed on 31 December 2014).
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Khan, A.; Subhani, M.N.; Chattha, M.B.; Anwar, W.; Nawaz, K. First report of whisker mold of citrus (Citrus sinensis) caused by Penicillium ulaiense in Pakistan. Plant Dis. 2017, 101, 1042. [Google Scholar] [CrossRef]
- Palou, L.; Taberner, V. First report of Penicillium ulaiense causing postharvest whisker mold of oranges (Citrus sinensis) in Spain. Plant Dis. 2019, 103, 153–154. [Google Scholar] [CrossRef]
- Park, J.H.; Hyun, J.W.; Park, M.J.; Choi, Y.J. First report of whisker mold as a postharvest disease caused by Penicillium ulaiense on citrus (Citrus unshiu) in Korea. Plant Dis. 2018, 102, 2643. [Google Scholar] [CrossRef]
- Miles, A.K.; Tan, Y.P.; Tan, M.K.; Donovan, N.J.; Ghalayini, A.; Drenth, A. Phyllosticta spp. on cultivated Citrus in Australia. Australas. Plant Pathol. 2013, 42, 461–467. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.; Huang, F.; Zhang, J.Z.; Hyde, K.D.; Li, H.Y. Phyllosticta species associated with citrus diseases in China. Fungal Divers. 2012, 52, 209–224. [Google Scholar] [CrossRef]
- Wikee, S.; Lombard, L.; Crous, P.W. Phyllosticta capitalensis, a widespread endophyte of plants. Fungal Divers. 2013, 60, 91–105. [Google Scholar] [CrossRef]
- Alvarez, L.A.; Vicent, A.; De la Roca, E.; Bascón, J.; Abad-Campos, P.; Armengol, J.; García-Jiménez, J. Branch cankers on citrus trees in Spain caused by Phytophthora citrophthora. Plant Pathol. 2008, 57, 84–91. [Google Scholar] [CrossRef]
- Meitz-Hopkins, J.C.; Pretorius, M.C.; Spies, C.F.J.; Huisman, L.; Botha, W.J.; Langenhoven, S.D.; McLeod, A. Phytophthora species distribution in South African citrus production regions. Eur. J. Plant Pathol. 2014, 138, 733–749. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Uematsu, S.; Coffey, M.D.; Uzuhashi, S.; Suga, H.; Kageyama, K. Re-evaluation of Japanese Phytophthora isolates based on molecular phylogenetic analyses. Mycoscience 2014, 55, 314–327. [Google Scholar] [CrossRef]
- Bodenhausen, N.; Bortfeld-Miller, M.; Ackermann, M.; Vorholt, J.A. A synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLoS Genet. 2014, 10, e1004283. [Google Scholar] [CrossRef]
- Wagner, M.R.; Lundberg, D.S.; Del Rio, T.G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef]
- Matsuzaki, N.; Wu, J.; Kawaide, M.; Choi, J.H.; Hirai, H.; Kawagishi, H. Plant growth regulatory compounds from the mushroom Russula vinosa. Mycoscience 2016, 57, 404–407. [Google Scholar] [CrossRef][Green Version]
- Osaki-Oka, K.; Suyama, S.; Sakuno, E.; Ushijima, S.; Nagasawa, E.; Maekawa, N.; Ishihara, A. Antifungal activity of the volatile compound isovelleral produced by ectomycorrhizal Russula fungi against plant-pathogenic fungi. J. Gen. Plant Pathol. 2019, 85, 428–435. [Google Scholar] [CrossRef]
- Yao, H.; Song, J.; Liu, C.; Luo, K.; Han, J.; Li, Y.; Pang, X.; Xu, H.; Zhu, Y.; Xiao, P.; et al. Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS ONE 2010, 5, e13102. [Google Scholar] [CrossRef]
- Bazzicalupo, A.L.; Bálint, M.; Schmitt, I. Comparison of ITS1 and ITS2 rDNA in 454 sequencing of hyperdiverse fungal communities. Fungal Ecol. 2013, 6, 102–109. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bödeker, I.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region-evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, E.J.N.; Vogel, C.M.; Ueoka, R.; Schäfer, M.; Ryffel, F.; Müller, D.B.; Probst, S.; Kreuzer, M.; Piel, J.; Vorholt, J.A. Bipartite interactions, antibiotic production and biosynthetic potential of the Arabidopsis leaf microbiome. Nat. Microbiol. 2018, 3, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Kusstatscher, P.; Zachow, C.; Harms, K.; Maier, J.; Eigner, H.; Berg, G.; Cernava, T. Microbiome-driven identification of microbial indicators for postharvest diseases of sugar beets. Microbiome 2019, 7, 112. [Google Scholar] [CrossRef]
- Sukno, S.A.; García, V.M.; Shaw, B.D.; Thon, M.R. Root infection and systemic colonization of maize by Colletotrichum graminicola. Appl. Environ. Microbiol. 2008, 74, 823–832. [Google Scholar] [CrossRef]
- Muñoz-Barrios, A.; Sopeña-Torres, S.; Ramos, B.; López, G.; Del Hierro, I.; Díaz-González, S.; Molina, A. Differential expression of fungal genes determines the lifestyle of Plectosphaerella strains during Arabidopsis thaliana colonization. Mol. Plant-Microbe Interact. 2020, 33, 1299–1314. [Google Scholar] [CrossRef]
- Friesen, T.L.; Stukenbrock, E.H.; Liu, Z.; Meinhardt, S.; Ling, H.; Faris, J.D.; Rasmussen, J.B.; Solomon, P.S.; McDonald, B.A.; Oliver, R.P. Emergence of a new disease as a result of interspecific virulence gene transfer. Nat. Genet. 2006, 38, 953–956. [Google Scholar] [CrossRef]
- Ma, L.J.; Van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Hurek, T. Living inside plants: Bacterial endophytes. Curr. Opin. Plant Biol. 2011, 14, 435–443. [Google Scholar] [CrossRef]



| Variety | Chongqing | Guangdong | Guangxi | Guizhou | Hubei | Jiangxi | Sichuan | Zhejiang | Sum |
|---|---|---|---|---|---|---|---|---|---|
| Lemon | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Mandarin | 8 | 45 | 8 | 1 | 4 | 8 | 0 | 12 | 86 |
| Orange | 8 | 0 | 0 | 0 | 8 | 17 | 8 | 0 | 41 |
| Pomelo | 0 | 20 | 0 | 0 | 0 | 0 | 7 | 0 | 27 |
| Group Name | Genera | No. of Species | Infecting Tissues | Symptoms | Influential Diseases | References |
|---|---|---|---|---|---|---|
| Alternaria | Alternaria (A.) | 5 | leaf, shoot, fruit | spot, fruit rot | Citrus brown spot, Citrus black rot | [36,37] |
| Botryospaeriaceae | Barriopsis, Diplodia, Dothiorella, Lasiodiplodia (L.), Neofusicoccum, Neoscytalidium, Spencermartinsia, Sphaeropsis | 26 | branch, trunk, twig | canker, dieback, gummosis | Bot gummosis | [6,12,38,39] |
| Cladosporium | Cladosporium | 5 | leaf | spot | - | [40,41] |
| Colletotrichum | Colletotrichum (Co.) | 36 | leaf, shoot, flower, fruit | spot, wither-tip | Citrus anthracnose, Postbloom fruit drop | [8,11,42,43,44] |
| Diaporthe | Diaporthe (D.) | 30 | leaf, fruit, twig, fruit | black points, fruit rot, dieback, gummosis | Citrus melanose, Stem-end rot | [45,46,47,48] |
| Elsinoë | Elsinoë (E.) | 3 | leaf, fruit | spot | Citrus scab | [49,50,51] |
| Fusarium | Bisifusarium, Fusarium (F.), Neocosmospora | 17 | leaf, branch, fruit, root | spot, rot, canker | - | [52,53,54] |
| Mycosphaerellaceae | Cercospora, Pallidocercospora, Passalora, Pseudocercospora, Ramularia, Septoria, Zasmidium (Z.) | 18 | leaf, fruit | spot | Citrus greasy spot | [25,55,56] |
| Penicillium and Aspergillus | Aspergillus, Penicillium | 6 | fruit | mold | Citrus green mold, Citrus blue mold | [57,58,59,60] |
| Phyllosticta | Phyllosticta (P.) | 8 | leaf, fruit | spot | Citrus black spot | [7,61,62,63] |
| Phytophthora | Phytophthora | 11 | root, fruit, branch | rot, canker | - | [64,65,66] |
| Group Name | Species | No. of OTUs |
|---|---|---|
| Alternaria alternata.arborescens.gossypina | Alternaria alternata, A. arborescens, A. gossypina | 57 |
| Cercospora.Septoria | Cercospora sp., Septoria sp. | 1 |
| Cladosporium cladosporioides.iranicum.subuliforme.tenuissimum | Cladosporium cladosporioides, Cladosporium iranicum, Cladosporium subuliforme, Cladosporium tenuissimum | 21 |
| Colletotrichum citricola.karstii | Colletotrichum citricola, Co. karstii | 135 |
| Colletotrichum gloeosporioides | Colletotrichum aenigma, Co. australianum, Co. ciggaro, Co. citrimaximae, Co. communis, Co. fructicola, Co. gloeosporioides, Co. helleniense, Co. hystricis, Co. queenslandicum, Co. siamense, Co. syzygicola, Co. theobromicola | 384 |
| Diaporthe citri | Diaporthe citri | 3 |
| Diaporthe endocitricola.sennae | Diaporthe endocitricola, D. sennae | 1 |
| Diaporthe endophytica.masirevicii | Diaporthe endophytica, D. masirevicii | 1 |
| Didymella microchlamydospora | Didymella microchlamydospora | 3 |
| Didymella sp IRAN2768C | Didymella sp. | 4 |
| Fusarium citri.sulawense | Fusarium citri, F. sulawense | 2 |
| Fusarium citricola.salinense | F. citricola, F. salinense | 7 |
| Fusarium oxysporum.siculi | F. oxysporum, F. siculi | 58 |
| Lasiodiplodia brasiliensis.caatinguensis.subglobosa | Lasiodiplodia brasiliensis, L. caatinguensis, L. subglobosa | 1 |
| Lasiodiplodia iraniensis.mediterranea.parva.pseudotheobromae.subglobosa.theobromae | L. iraniensis, L. mediterranea, L. parva, L. pseudotheobromae, L. subglobosa, L. theobromae | 1 |
| Leptoxyphium fumago.citri | Leptoxyphium fumago, Leptoxyphium citri | 1 |
| Neocosmospora ferruginea.macrospora | Neocosmospora ferruginea, Neocosmospora macrospora | 1 |
| Nigrospora oryzae | Nigrospora oryzae | 5 |
| Nigrospora pyriformis | Nigrospora pyriformis | 5 |
| Nigrospora sphaerica | Nigrospora sphaerica | 5 |
| Pallidocercospora crystallina | Pallidocercospora crystallina | 11 |
| Phyllosticta capitalensis.paracapitalensis | Phyllosticta capitalensis, P. paracapitalensis | 1 |
| Phyllosticta citricarpa.paracitricarpa | P. citricarpa, P. paracitricarpa | 1 |
| Zasmidium citri-griseum | Zasmidium citri-griseum | 40 |
| Zasmidium fructicola | Zasmidium fructicola | 20 |
| Zasmidium fructigenum | Zasmidium fructigenum | 130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Ling, J.; Cui, Y.; Guo, B.; Song, X. Profiling of the Citrus Leaf Endophytic Mycobiota Reveals Abundant Pathogen-Related Fungal Groups. J. Fungi 2024, 10, 596. https://doi.org/10.3390/jof10090596
Huang F, Ling J, Cui Y, Guo B, Song X. Profiling of the Citrus Leaf Endophytic Mycobiota Reveals Abundant Pathogen-Related Fungal Groups. Journal of Fungi. 2024; 10(9):596. https://doi.org/10.3390/jof10090596
Chicago/Turabian StyleHuang, Feng, Jinfeng Ling, Yiping Cui, Bin Guo, and Xiaobing Song. 2024. "Profiling of the Citrus Leaf Endophytic Mycobiota Reveals Abundant Pathogen-Related Fungal Groups" Journal of Fungi 10, no. 9: 596. https://doi.org/10.3390/jof10090596
APA StyleHuang, F., Ling, J., Cui, Y., Guo, B., & Song, X. (2024). Profiling of the Citrus Leaf Endophytic Mycobiota Reveals Abundant Pathogen-Related Fungal Groups. Journal of Fungi, 10(9), 596. https://doi.org/10.3390/jof10090596





